Zinc-Modified Nanotransporter of Doxorubicin for Targeted Prostate Cancer Delivery
Abstract
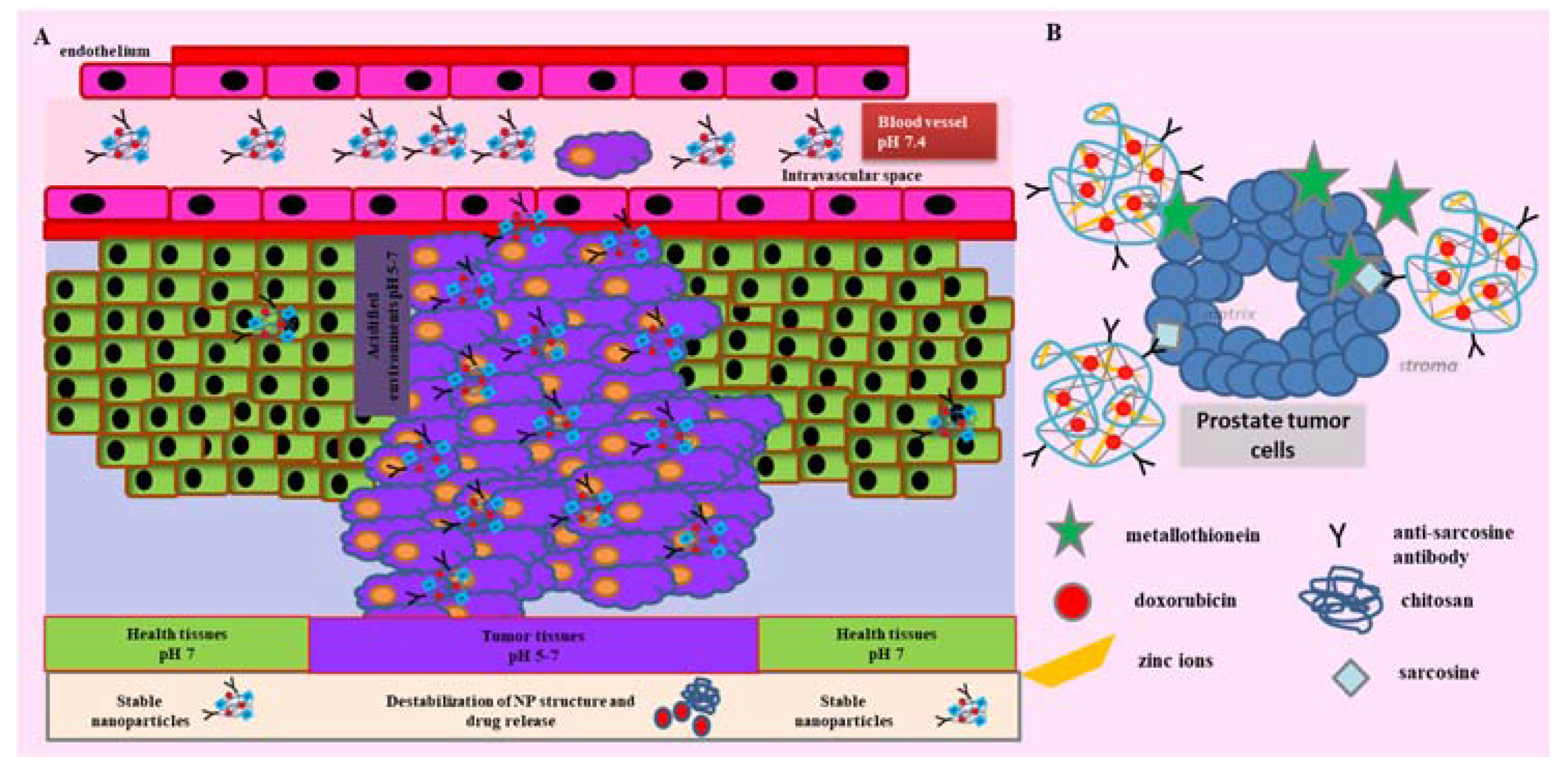
:1. Introduction
2. Results
2.1. Biophysical Characterization of CS Nanoparticles
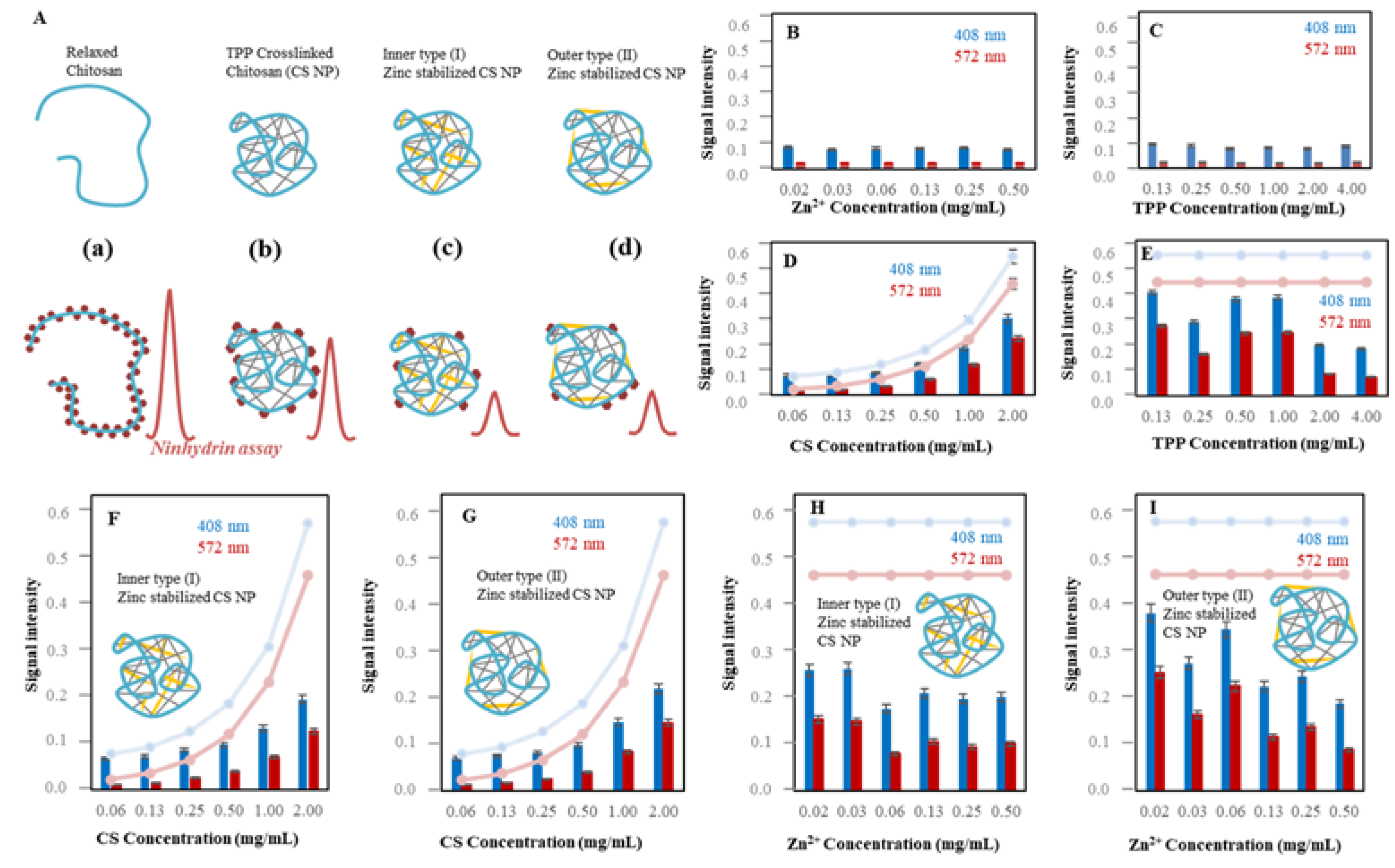
2.1.1. Ninhydrin Assay Optimization
2.1.2. Characterization of Zn-TPP-CS Nanoparticles Using Ninhydrin Assay
2.1.3. Electrochemical Study of CS NPs Formation
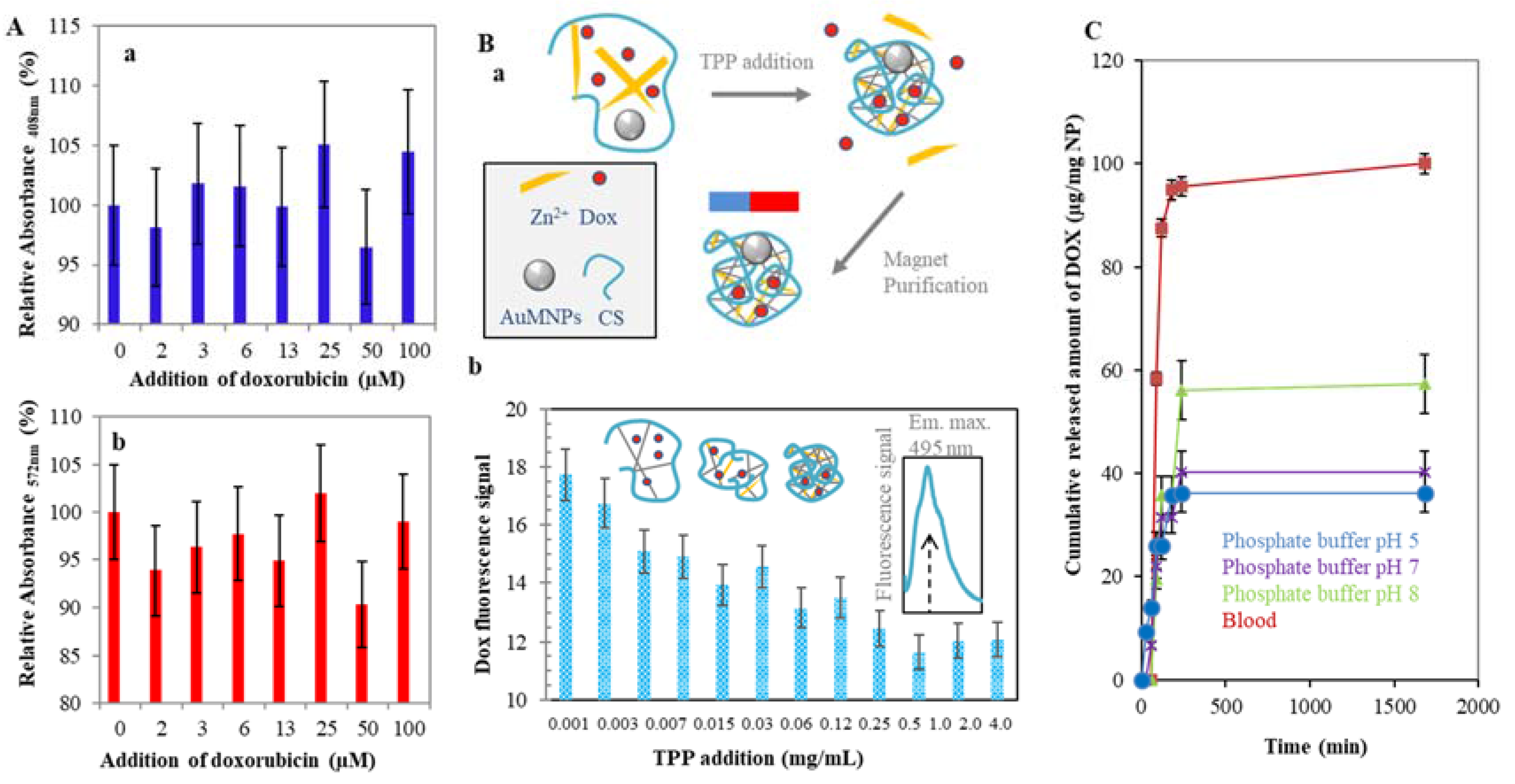
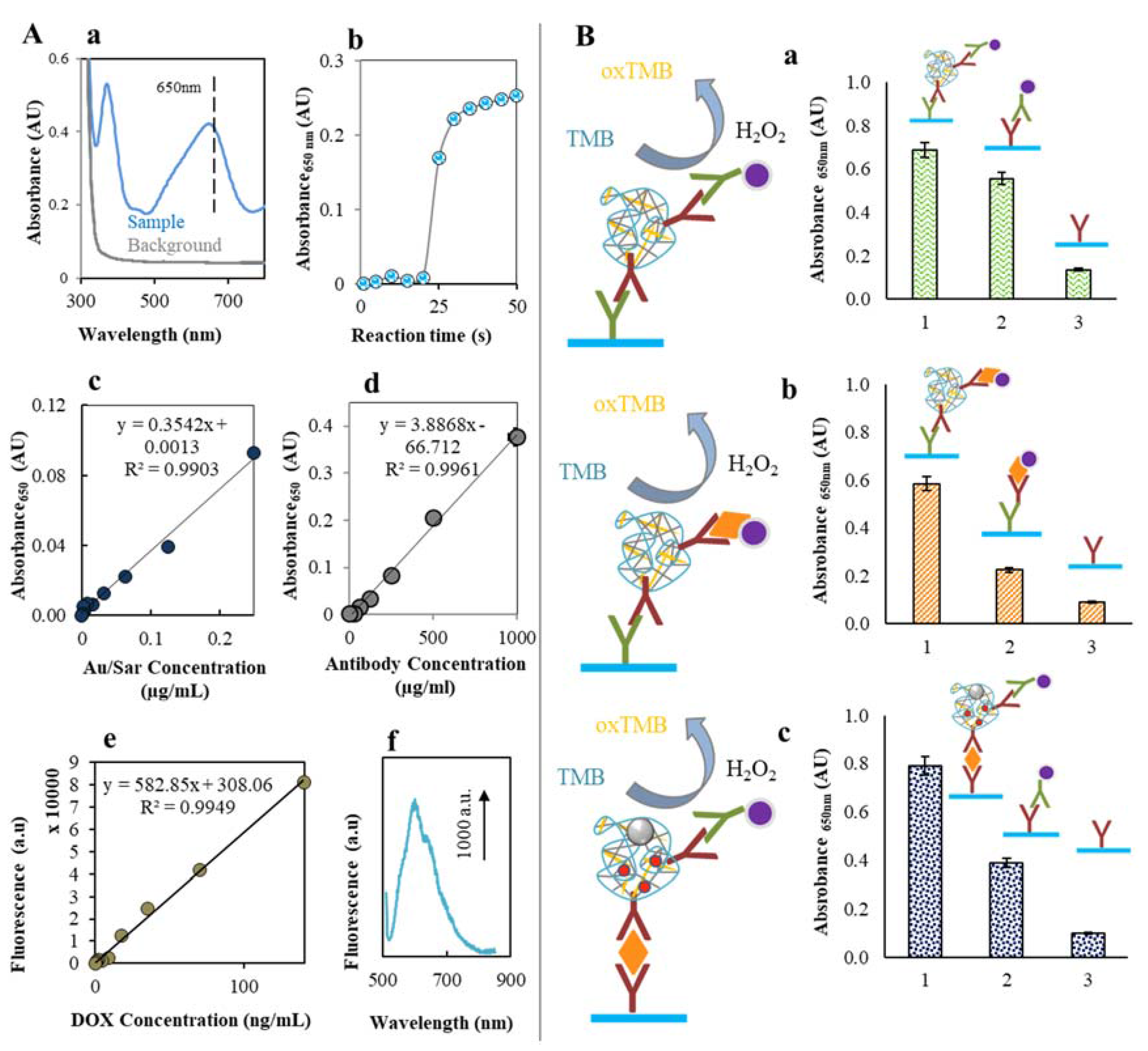
2.2. Doxorubicin Encapsulation to the CS NPs Structure
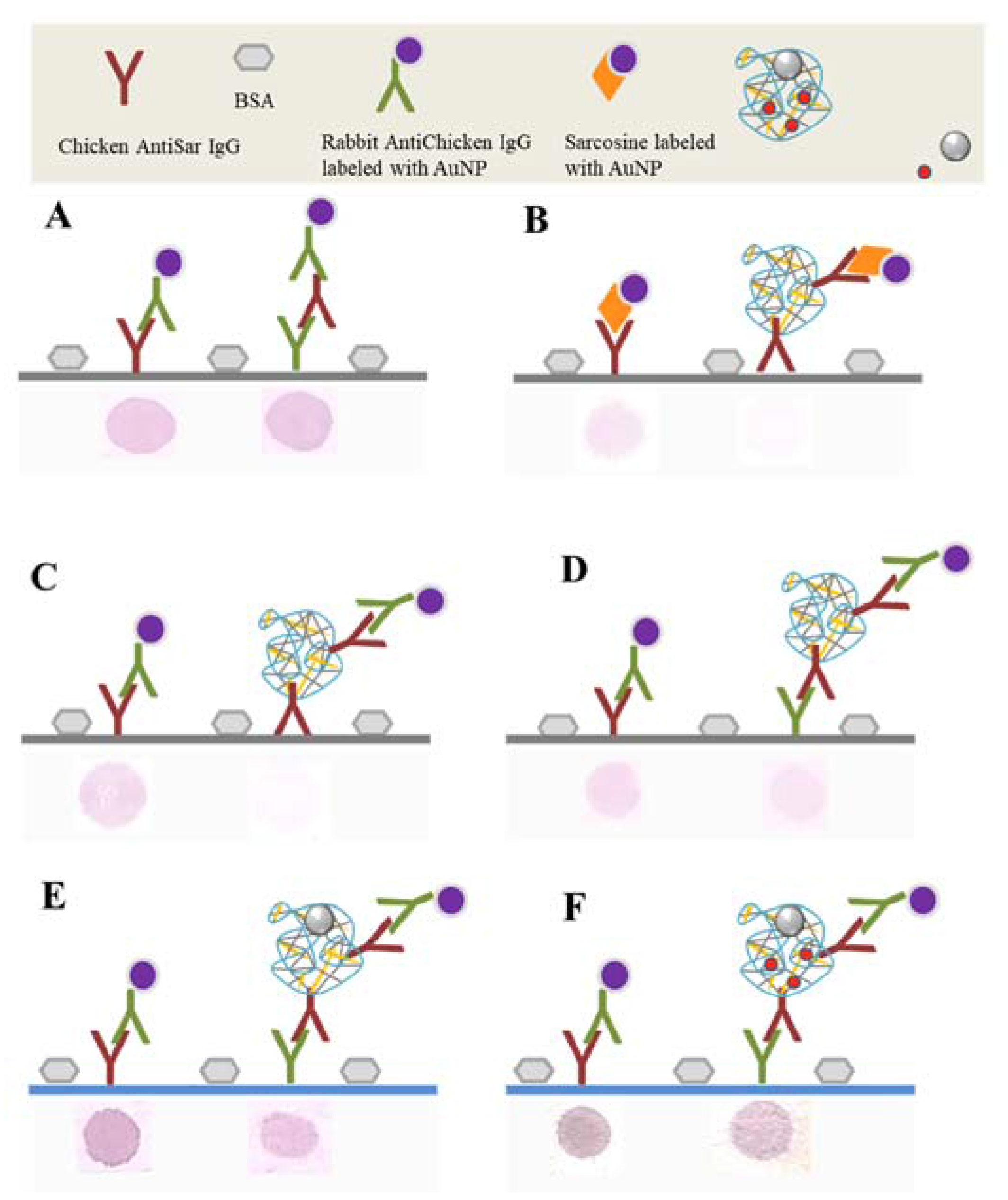
2.3. Labeling of CS NPs by AntiSar Antibodies
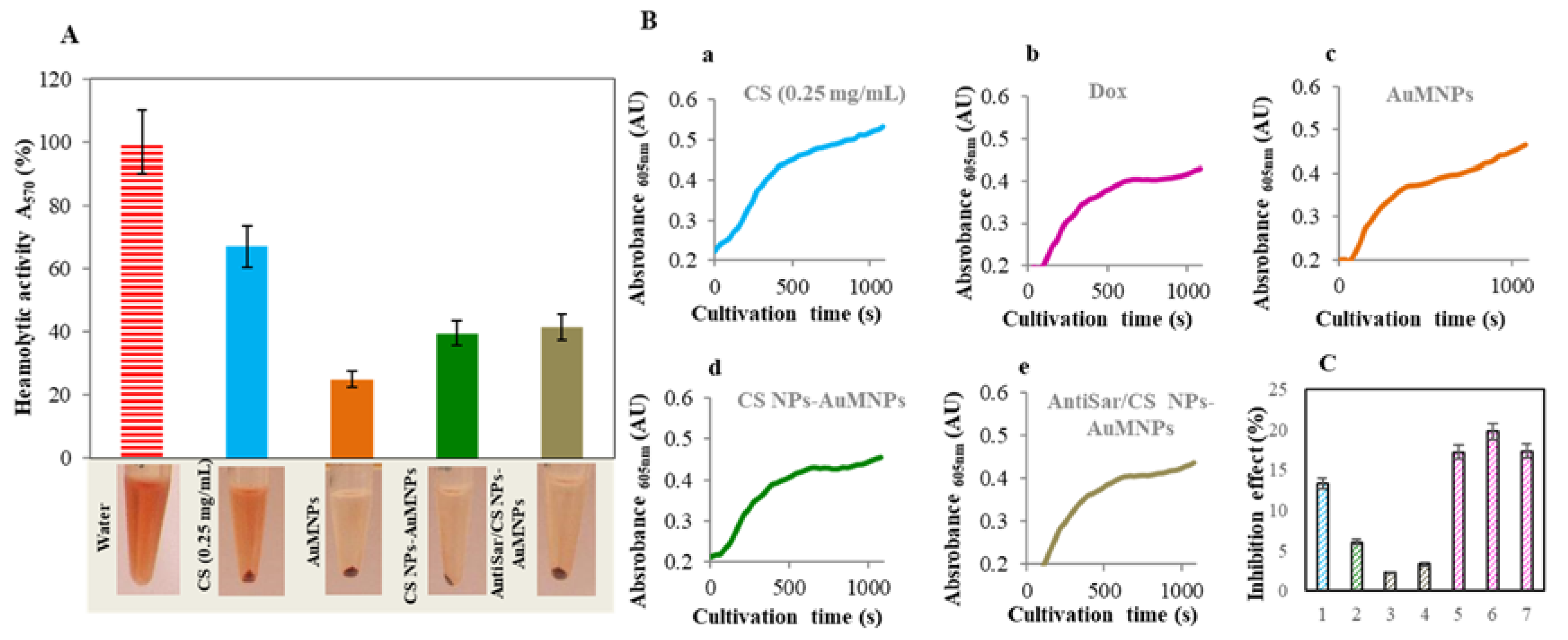
2.4. Toxicity Evaluation of CS NPs
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Chitosan Nanoparticles Preparation
4.3. AuNPs Preparation and Sarcosine/IgG Labeling
4.4. AuMNPs Preparation
4.5. Ninyhdrin Assay for Chitosan Detection
4.6. Absorbance Measurements
4.7. Fluorescence Reading
4.8. Electrochemical Determination of Zinc Ions
4.9. CS NPs Magnetic Separation
4.10. CS NPs-Dox Entrapment Efficiency
4.11. Cumulative Release of Dox from CS NPs
4.12. Dot Blot Assay
4.13. ELISA
4.14. Hemolytic Assay
4.15. Inhibition Assay
4.16. Data Treatment and Descriptive Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AntiSar | Anti-sarcosine Chicken Antibodies |
| AuMNPs | Gold Magnetic Nanoparticles |
| AuNPs | Gold Nanoparticles |
| AuSar | AuNPs Labeled Sarcosine |
| BSA | Bovine Serum Albumin |
| CS | Chitosan |
| MT | Metallothionein |
| NPs | Nanoparticles |
| PC | Prostate Cancer |
| RBCs | Red Blood Cells |
| TPP | Sodium Tripolyphosphate |
References
- Lal, N.; Dubey, J.; Gaur, P.; Verma, N.; Verma, A. Chitosan based in situ forming polyelectrolyte complexes: A potential sustained drug delivery polymeric carrier for high dose drugs. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Sen, V.D.; Sokolova, E.M.; Neshev, N.I.; Kulikov, A.V.; Pliss, E.M. Low molecular chitosan-(poly)nitroxides: Synthesis and evaluation as antioxidants on free radical-induced erythrocyte hemolysis. React. Funct. Polym. 2017, 111, 53–59. [Google Scholar] [CrossRef]
- Niaz, T.; Nasir, H.; Shabbir, S.; Rehman, A.; Imran, M. Polyionic hybrid nano-engineered systems comprising alginate and chitosan for antihypertensive therapeutics. Int. J. Biol. Macromol. 2016, 91, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.E.; Korbutt, G.S. Chitosan-based biomaterials for treatment of diabetes. In Chitosan Based Biomaterials; Volume 2; Tissue Engineering and Therapeutics; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Volume 123, pp. 91–113. [Google Scholar]
- Pangestuti, R.; Kim, S.-K. Neuroprotective properties of chitosan and its derivatives. Mar. Drugs 2010, 8, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z.; Fazli, M. Mechanical properties and antibacterial activities of novel nanobiocomposite films of chitosan and starch. Food Hydrocoll. 2015, 46, 112–124. [Google Scholar] [CrossRef]
- Behera, S.S.; Das, U.; Kumar, A.; Bissoyi, A.; Singh, A.K. Chitosan/TiO2 composite membrane improves proliferation and survival of l929 fibroblast cells: Application in wound dressing and skin regeneration. Int. J. Biol. Macromol. 2017, 98, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Chitosan-gelatin-platelet gel composite scaffold for bone regeneration. J. Controll. Release 2017, 254, 137. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Matysiak, S. Effect of ph on chitosan hydrogel polymer network structure. Chem. Commun. 2017, 53, 7373–7376. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H. Chitosan nanoparticles prepared by ionotropic gelation: An overview of recent advances. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lapitsky, Y. On the kinetics of chitosan/tripolyphosphate micro- and nanogel aggregation and their effects on particle polydispersity. J. Colloid Interface Sci. 2017, 486, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Paoletti, S.; Cok, M.; Asaro, F.; Abrami, M.; Grassi, M.; Donati, I. Insight into the ionotropic gelation of chitosan using tripolyphosphate and pyrophosphate as cross-linkers. Int. J. Biol. Macromol. 2016, 92, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan-tpp nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Yata, V.K.; Ghosh, S.S. Investigating structure and fluorescence properties of green fluorescent protein released from chitosan nanoparticles. Mater. Lett. 2012, 73, 209–211. [Google Scholar] [CrossRef]
- Shanmugam, R.; Priyanka, D.L.; Madhuri, K.; Gowthamarajan, K.; Karri, V.; Kumar, C.K.A.; Murali, P. Formulation and characterization of chitosan encapsulated phytoconstituents of curcumin and rutin nanoparticles. Int. J. Biol. Macromol. 2017, 104, 1807–1812. [Google Scholar]
- Duarte, A.P.; Tavares, E.J.M.; Alves, T.V.G.; de Moura, M.R.; da Costa, C.E.F.; Silva, J.O.C.; Costa, R.M.R. Chitosan nanoparticles as a modified diclofenac drug release system. J. Nanopart. Res. 2017, 19. [Google Scholar] [CrossRef]
- Mendelovits, A.; Prat, T.; Gonen, Y.; Rytwo, G. Improved colorimetric determination of chitosan concentrations by dye binding. Appl. Spectrosc. 2012, 66, 979–982. [Google Scholar] [PubMed]
- Badawy, M.E.I. A new rapid and sensitive spectrophotometric method for determination of a biopolymer chitosan. Int. J. Carbohydr. Chem. 2012, 2012, 7. [Google Scholar] [CrossRef]
- Assa, F.; Jafarizadeh-Malmiri, H.; Ajamein, H.; Vaghari, H.; Anarjan, N.; Ahmadi, O.; Berenjian, A. Chitosan magnetic nanoparticles for drug delivery systems. Crit. Rev. Biotechnol. 2017, 37, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Ramesh, R. Multifaceted applications of chitosan in cancer drug delivery and therapy. Mar. Drugs 2017, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-C.; Yoo, S.-Y.; Kim, H.; Lee, J. Chitosan-based multifunctional platforms for local delivery of therapeutics. Mar. Drugs 2017, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Duttagupta, D.S.; Jadhav, V.M.; Kadam, V.J. Chitosan: A propitious biopolymer for drug delivery. Curr. Drug Deliv. 2015, 12, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Landriscina, A.; Rosen, J.; Friedman, A.J. Biodegradable chitosan nanoparticles in drug delivery for infectious disease. Nanomedicine 2015, 10, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Sarvaiya, J.; Agrawal, Y.K. Chitosan as a suitable nanocarrier material for anti-alzheimer drug delivery. Int. J. Biol. Macromol. 2015, 72, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yun, K.S.; Choi, C.S.; Shin, S.H.; Ban, H.S.; Rhim, T.; Lee, S.K.; Lee, K.Y. T cell-specific sirna delivery using antibody-conjugated chitosan nanoparticles. Bioconjug. Chem. 2012, 23, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Sau, S.; Alsaab, H.O.; Kashaw, S.K.; Tatiparti, K.; Iyer, A.K. Advances in antibody-drug conjugates: A new era of targeted cancer therapy. Drug Discov. Today 2017, 22, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, Y.K.; Koo, J.S. Expression of sarcosine-metabolizing enzymes in thyroid cancer. Int. J. Clin. Exp. Pathol. 2016, 9, 7132–7139. [Google Scholar]
- Cha, Y.J.; Kim, D.H.; Jung, W.H.; Koo, J.S. Expression of sarcosine metabolism-related proteins according to metastatic site in breast cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 7824–7833. [Google Scholar] [PubMed]
- Robinson, D.R.; Wu, Y.M.; Lonigro, R.J.; Vats, P.; Cobain, E.; Everett, J.; Cao, X.H.; Rabban, E.; Kumar-Sinha, C.; Raymond, V.; et al. Integrative clinical genomics of metastatic cancer. Nature 2017, 548, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Bizon, A.; Jedryczko, K.; Milnerowicz, H. The role of metallothionein in oncogenesis and cancer treatment. Postepy Hig. Med. Dosw. 2017, 71, 98–109. [Google Scholar] [CrossRef]
- Krizkova, S.; Ryvolova, M.; Hrabeta, J.; Adam, V.; Stiborova, M.; Eckschlager, T.; Kizek, R. Metallothioneins and zinc in cancer diagnosis and therapy. Drug Metab. Rev. 2012, 44, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.J.; Kraft, J.J.; Schuster, S.M. A specific quantitative colorimetric assay for l-asparagine. Anal. Biochem. 1993, 211, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, S.; Vårum, K.M.; Ostgaard, K. Quantitative determination of chitosans by ninhydrin. Carbohydr. Polym. 1999, 38, 115–122. [Google Scholar] [CrossRef]
- Leane, M.M.; Nankervis, R.; Smith, A.; Illum, L. Use of the ninhydrin assay to measure the release of chitosan from oral solid dosage forms. Int. J. Pharm. 2004, 271, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.A.; Arif, M.; Feng, C.; Zeenat, S.; Liu, C.G. Synthesis and evaluation of ph-sensitive, self-assembled chitosan-based nanoparticles as efficient doxorubicin carriers. J. Biomater. Appl. 2017, 31, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Huh, Y.M.; Yang, J.; Lee, K.; Suh, J.S.; Haam, S. Ph-triggered drug-releasing magnetic nanoparticles for cancer therapy guided by molecular imaging by mri. Adv. Mater. 2011, 23, 2436–2442. [Google Scholar] [CrossRef] [PubMed]
- Bekale, L.; Agudelo, D.; Tajmir-Riahi, H.A. Effect of polymer molecular weight on chitosan-protein interaction. Colloids Surf. B Biointerfaces 2015, 125, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Docekalova, M.; Uhlirova, D.; Stankova, M.; Kepinska, M.; Sochor, J.; Milnerowicz, H.; Babula, P.; Fernandez, C.; Brazdova, M.; Zidkova, J.; et al. Characterisation of peroxidase-like activity of thermally synthesized gold nanoparticles. In Proceedings of the Nanocon 2016, 8th International Conference on Nanomaterials, Brno, Czech Republic, 19–21 October 2016; Tanger Ltd.: Ostrava, Czech Republic, 2017; pp. 429–434. [Google Scholar]
- Wang, Y.B.; Zhou, J.R.; Liu, L.; Huang, C.J.; Zhou, D.Q.; Fu, L.L. Characterization and toxicology evaluation of chitosan nanoparticles on the embryonic development of zebrafish, danio rerio. Carbohydr. Polym. 2016, 141, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; Qi, W.; Han, F.; Shao, J.Z.; Gao, J.Q. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int. J. Nanomed. 2011, 6, 3351–3359. [Google Scholar]
- Choi, Y.J.; Gurunathan, S.; Kim, D.; Jang, H.S.; Park, W.J.; Cho, S.G.; Park, C.; Song, H.; Seo, H.G.; Kim, J.H. Rapamycin ameliorates chitosan nanoparticle-induced developmental defects of preimplantation embryos in mice. Oncotarget 2016, 7, 74658–74677. [Google Scholar] [CrossRef] [PubMed]
- De Salamanca, A.E.; Diebold, Y.; Calonge, M.; Garcia-Vazquez, C.; Callejo, S.; Vila, A.; Alonso, M.J. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: Toxicity, uptake mechanism and in vivo tolerance. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.R.; Asghar, S.; Yang, L.; Chen, Z.P.; Li, H.Y.; Shi, W.W.; Li, Y.B.; Shi, Q.Q.; Ping, Q.N.; Xiao, Y.Y. Nanoparticles based on chitosan hydrochloride/hyaluronic acid/peg containing curcumin: In Vitro evaluation and pharmacokinetics in rats. Int. J. Biol. Macromol. 2017, 102, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, L.; Ye, H.; Chen, H.; Shen, W.; Zhong, Y.; Tian, T.; He, H. From saccharomyces cerevisiae to human: The important gene co-expression modules. Biomed. Rep. 2017, 7, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Park, K. Multicomponent, tumor-homing chitosan nanoparticles for cancer imaging and therapy. Int. J. Mol. Sci. 2017, 18, 594. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Xia, J.; Wu, J. Functional chitosan nanoparticles in cancer treatment. J. Biomed. Nanotechnol. 2016, 12, 1585–1603. [Google Scholar] [CrossRef]
- Bugnicourt, L.; Ladaviere, C. Interests of chitosan nanoparticles conically cross-linked with tripolyphosphate for biomedical applications. Prog. Polym. Sci. 2016, 60, 1–17. [Google Scholar] [CrossRef]
- El-Marakby, E.M.; Hathout, R.M.; Taha, I.; Mansour, S.; Mortada, N.D. A novel serum-stable liver targeted cytotoxic system using valerate-conjugated chitosan nanoparticles surface decorated with glycyrrhizin. Int. J. Pharm. 2017, 525, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.X.; Wang, L.; Su, L.; Ai, X.P.; Yang, H.X. Temperature effects on the electrodeposition of zinc. J. Electrochem. Soc. 2003, 150, C19–C23. [Google Scholar] [CrossRef]
- Kudr, J.; Hoai Viet, N.; Gumulec, J.; Nejdl, L.; Blazkova, I.; Ruttkay-Nedecky, B.; Hynek, D.; Kynicky, J.; Adam, V.; Kizek, R. Simultaneous automatic electrochemical detection of zinc, cadmium, copper and lead ions in environmental samples using a thin-film mercury electrode and an artificial neural network. Sensors 2015, 15, 592–610. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Sedlarik, V. Amphiphilic chitosan-grafted-functionalized polylactic acid based nanoparticles as a delivery system for doxorubicin and temozolomide co-therapy. Int. J. Pharm. 2014, 474, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Li, L.; Wang, Y.; Yu, Y.; Wang, S.; Gao, Y.; Liang, Y.; Zhang, G.; Pan, W.; Yang, X. Design and evaluation of a novel potential carrier for a hydrophilic antitumor drug: Auricularia auricular polysaccharide-chitosan nanoparticles as a delivery system for doxorubicin hydrochloride. Int. J. Pharm. 2016, 511, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Controll. Release 2001, 73, 255–267. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Sousa, A.I.; Silva, J.C.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Deepagan, V.G.; Rani, V.V.D.; Menon, D.; Nair, S.V.; Jayakumar, R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate-chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Esfandiarpour-Boroujeni, S.; Bagheri-Khoulenjani, S.; Mirzadeh, H.; Amanpour, S. Fabrication and study of curcumin loaded nanoparticles based on folate-chitosan for breast cancer therapy application. Carbohydr. Polym. 2017, 168, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Chen, J.P.; Wei, K.C.; Chen, J.Y.; Huang, C.W.; Liao, Z.X. Release of doxorubicin by a folate-grafted, chitosan-coated magnetic nanoparticle. Nanomaterials 2017, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, X.Y.; Li, L.; Xian, G.J.; Wang, W.; Ma, D.W.; Xie, L. Investigation on novel chitosan nanoparticle-aptamer complexes targeting tgf-beta receptor II. Int. J. Pharm. 2013, 456, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Arya, G.; Vandana, M.; Acharya, S.; Sahoo, S.K. Enhanced antiproliferative activity of herceptin (HER2)-conjugated gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhang, C.-G.; Liu, Y.; Yuan, Z.-Q.; Chen, W.-L.; Yang, S.-D.; Li, J.-Z.; Zhu, W.-J.; Zhou, X.-F.; You, B.-G.; et al. Cd147 monoclonal antibody mediated by chitosan nanoparticles loaded with α-hederin enhances antineoplastic activity and cellular uptake in liver cancer cells. Sci. Rep. 2015, 5, 17904. [Google Scholar] [CrossRef] [PubMed]
- Yousefpour, P.; Atyabi, F.; Vasheghani-Farahani, E.; Movahedi, A.-A.M.; Dinarvand, R. Targeted delivery of doxorubicin-utilizing chitosan nanoparticles surface-functionalized with anti-her2 trastuzumab. Int. J. Nanomed. 2011, 6, 1977–1990. [Google Scholar]
- Zhao, L.; Yang, G.; Shi, Y.; Su, C.; Chang, J. Co-delivery of gefitinib and chloroquine by chitosan nanoparticles for overcoming the drug acquired resistance. J. Nanobiotechnol. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Shargh, V.H.; Hondermarck, H.; Liang, M. Antibody-targeted biodegradable nanoparticles for cancer therapy. Nanomedicine 2016, 11, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Goodall, S.; Jones, M.L.; Mahler, S. Monoclonal antibody-targeted polymeric nanoparticles for cancer therapy-future prospects. J. Chem. Technol. Biotechnol. 2015, 90, 1169–1176. [Google Scholar] [CrossRef]
- Zhu, Y.; Choi, S.H.; Shah, K. Multifunctional receptor-targeting antibodies for cancer therapy. Lancet Oncol. 2015, 16, e543–e554. [Google Scholar] [CrossRef]
- Svirshchevskaya, E.V.; Zubareva, A.A.; Boyko, A.A.; Shustova, O.A.; Grechikhina, M.V.; Shagdarova, B.T.; Varlamov, V.P. Analysis of toxicity and biocompatibility of chitosan derivatives with different physico-chemical properties. Appl. Biochem. Microbiol. 2016, 52, 483–490. [Google Scholar] [CrossRef]
- Zubareva, A.; Shagdarova, B.; Varlamov, V.; Kashirina, E.; Svirshchevskaya, E. Penetration and toxicity of chitosan and its derivatives. Eur. Polym. J. 2017, 93, 743–749. [Google Scholar] [CrossRef]
- Saenko, Y.V.; Shutov, A.M.; Rastorgueva, E.V. Doxorubicin and menadione decrease cell proliferation of saccharomyces cerevisiae by different mechanisms. Cell Tissue Biol. 2010, 4, 332–336. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Lim, Y.J.; Fan, M.H.M.; Jackson, R.A.; Lim, K.K.; Ang, W.H.; Ban, K.H.K.; Chen, E.S. Calcium modulation of doxorubicin cytotoxicity in yeast and human cells. Genes Cells 2016, 21, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Westmoreland, T.J.; Wickramasekara, S.M.; Guo, A.Y.; Selim, A.L.; Winsor, T.S.; Greenleaf, A.L.; Blackwell, K.L.; Olson, J.A.; Marks, J.R.; Bennett, C.B. Comparative genome-wide screening identifies a conserved doxorubicin repair network that is diploid specific in saccharomyces cerevisiae. PLoS ONE 2009, 4, e5830. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.B.; Koc, A. High-copy overexpression screening reveals pdr5 as the main doxorubicin resistance gene in yeast. PLoS ONE 2015, 10, e0148108. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Jaafar, L.; Cashikar, A.; Flores-Rozas, H. Identification of genes required for protection from doxorubicin by a genome-wide screen in saccharomyces cerevisiae. Cancer Res. 2007, 67, 11411–11418. [Google Scholar] [CrossRef] [PubMed]
- Hooda, V.; Archita. Enzymes loaded chitosan/coconut fibre/zinc oxide nanoparticles strip for polyamine determination. Food Chem. 2018, 239, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.; Dapkekar, A.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc complexed chitosan/tpp nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydr. Polym. 2017, 165, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R.R.; Hashemi, M.; Coma, V. Preparation and characterization of active emulsified films based on chitosan-carboxymethyl cellulose containing zinc oxide nano particles. Int. J. Biol. Macromol. 2017, 99, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Liu, S.L.; Zhang, A.K.; Li, K.W.; Oderinde, O.; Yao, F.; Fu, G.D. Zinc ion-induced formation of hierarchical N-succinyl chitosan film. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B.; Zou, J.; Feng, P.; Bok, R.; Swanson, M.G.; Kurhanewicz, J. Human prostate cancer zip1/zinc/citrate genetic/metabolic relationship in the tramp prostate cancer animal model. Cancer Biol. Ther. 2011, 12, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Dambal, S.; Baumann, B.; McCray, T.; Williams, L.; Richards, Z.; Deaton, R.; Prins, G.S.; Nonn, L. The mir-183 family cluster alters zinc homeostasis in benign prostate cells, organoids and prostate cancer xenografts. Sci. Rep. 2017, 7, 7704. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Li, L.Z.; Zhao, J.; Sun, Z.W.; Peng, S.Q. Zinc-induced metallothionein overexpression prevents doxorubicin toxicity in cardiomyocytes by regulating the peroxiredoxins. Xenobiotica 2016, 46, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Costello, L.C. The important role of the apoptotic effects of zinc in the development of cancers. J. Cell. Biochem. 2009, 106, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.-T.; Lin, F.-W.; Chuang, C.-K.; Yang, H.-W. Co-delivery of docetaxel and p44/42 mapk sirna using PSMA antibody-conjugated BSA-PEI layer-by-layer nanoparticles for prostate cancer target therapy. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Daniels-Wells, T.R.; Helguera, G.; Leuchter, R.K.; Quintero, R.; Kozman, M.; Rodriguez, J.A.; Ortiz-Sanchez, E.; Martinez-Maza, O.; Schultes, B.C.; Nicodemus, C.F.; et al. A novel ige antibody targeting the prostate-specific antigen as a potential prostate cancer therapy. BMC Cancer 2013, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, P.K.B.; Johnson, N.; Boya, V.K.N.; Chowdhury, P.; Ganju, A.; Hafeez, B.; Khan, S.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. PSMA antibody functionalized docetaxel-loaded magnetic nanoparticles for prostate cancer therapy. Cancer Res. 2016, 76. [Google Scholar] [CrossRef]
- Lukey, M.J.; Katt, W.P.; Cerione, R.A. Targeting amino acid metabolism for cancer therapy. Drug Discov. Today 2017, 22, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Sheng, G.Y.; Herve, S.; Carvalho, E.; Mahanty, A.; Yuan, S.T.; Sun, L. Interplay between cancer cell cycle and metabolism: Challenges, targets and therapeutic opportunities. Biomed. Pharmacother. 2017, 89, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Sidaway, P. Prostate cancer: Targeting lipid metabolism. Nat. Rev. Urol. 2017, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Amelio, I.; Cutruzzola, F.; Antonov, A.; Agostini, M.; Melino, G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014, 39, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Heger, Z.; Polanska, H.; Rodrigo, M.A.M.; Guran, R.; Kulich, P.; Kopel, P.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Kizek, R.; et al. Prostate tumor attenuation in the nu/nu murine model due to anti-sarcosine antibodies in folate-targeted liposomes. Sci. Rep. 2016, 6, 33379. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, S.; Block, L.H. Chitosan as an enabling excipient for drug delivery systems. I. Molecular modifications. Int. J. Biol. Macromol. 2000, 27, 181–186. [Google Scholar] [CrossRef]


| Substance | Regression Equation | Linear Dynamic Range (µg/mL) | R2 | LOD (µg/mL) | LOQ (µg/mL) | RSD * (%) |
|---|---|---|---|---|---|---|
| Chitosan A408 | y = 1.7469x + 0.1037 | 8–500 | 0.9965 | 2 | 17 | 2 |
| Chitosan A572 | y = 1.5443x + 0.0384 | 8–500 | 0.9969 | 2 | 19 | 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalickova, S.; Loffelmann, M.; Gargulak, M.; Kepinska, M.; Docekalova, M.; Uhlirova, D.; Stankova, M.; Fernandez, C.; Milnerowicz, H.; Ruttkay-Nedecky, B.; et al. Zinc-Modified Nanotransporter of Doxorubicin for Targeted Prostate Cancer Delivery. Nanomaterials 2017, 7, 435. https://doi.org/10.3390/nano7120435
Skalickova S, Loffelmann M, Gargulak M, Kepinska M, Docekalova M, Uhlirova D, Stankova M, Fernandez C, Milnerowicz H, Ruttkay-Nedecky B, et al. Zinc-Modified Nanotransporter of Doxorubicin for Targeted Prostate Cancer Delivery. Nanomaterials. 2017; 7(12):435. https://doi.org/10.3390/nano7120435
Chicago/Turabian StyleSkalickova, Sylvie, Martin Loffelmann, Michael Gargulak, Marta Kepinska, Michaela Docekalova, Dagmar Uhlirova, Martina Stankova, Carlos Fernandez, Halina Milnerowicz, Branislav Ruttkay-Nedecky, and et al. 2017. "Zinc-Modified Nanotransporter of Doxorubicin for Targeted Prostate Cancer Delivery" Nanomaterials 7, no. 12: 435. https://doi.org/10.3390/nano7120435





