The Effect of Different Coupling Agents on Nano-ZnO Materials Obtained via the Sol–Gel Process
Abstract
:1. Introduction
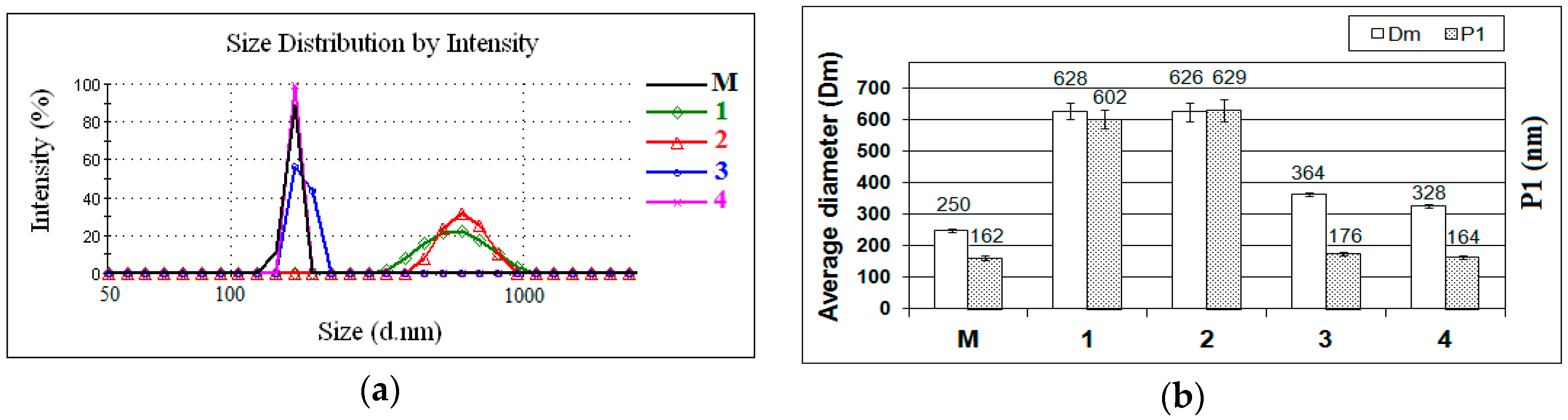
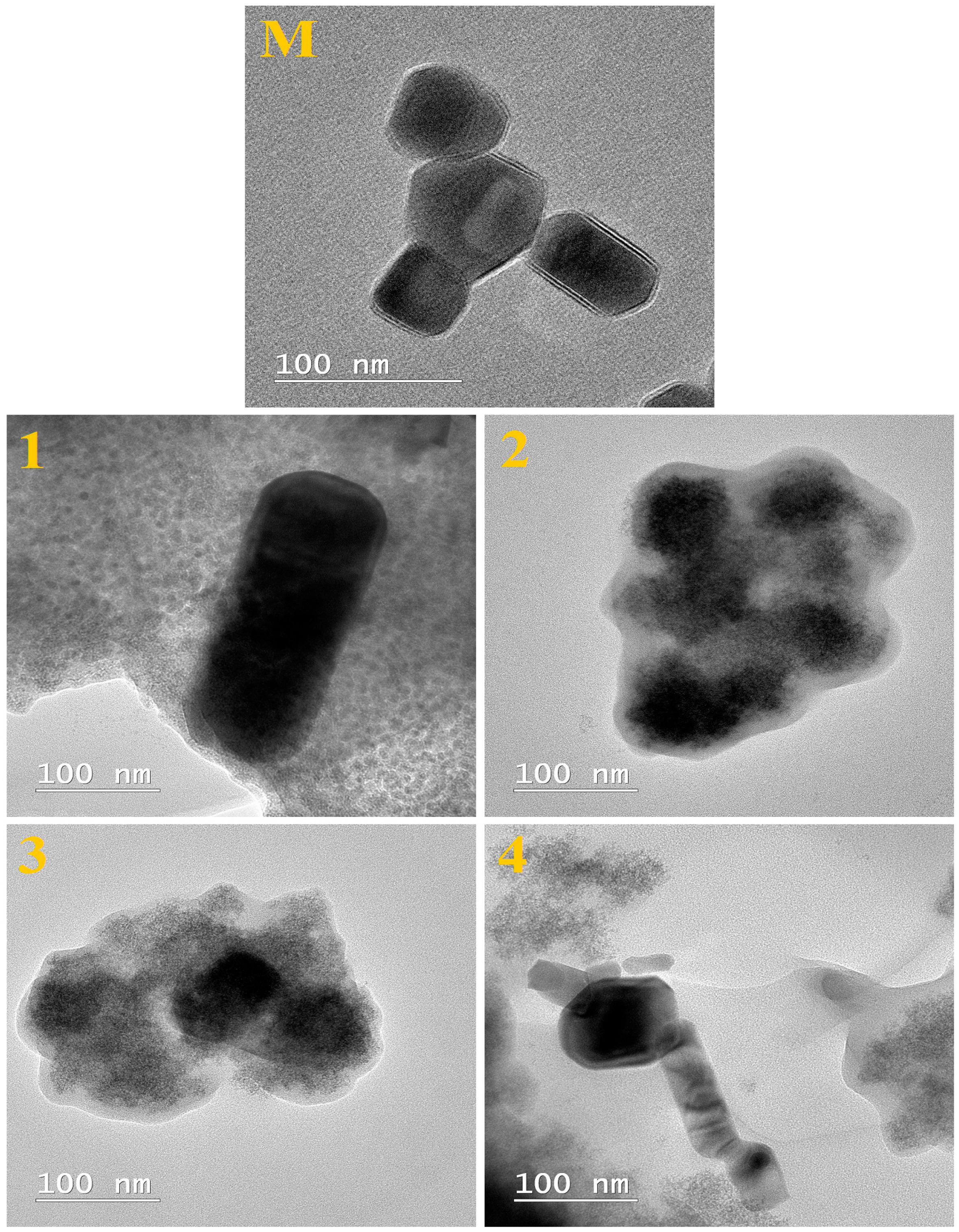
2. Results and Discussion
3. Experimental Section
3.1. Materials
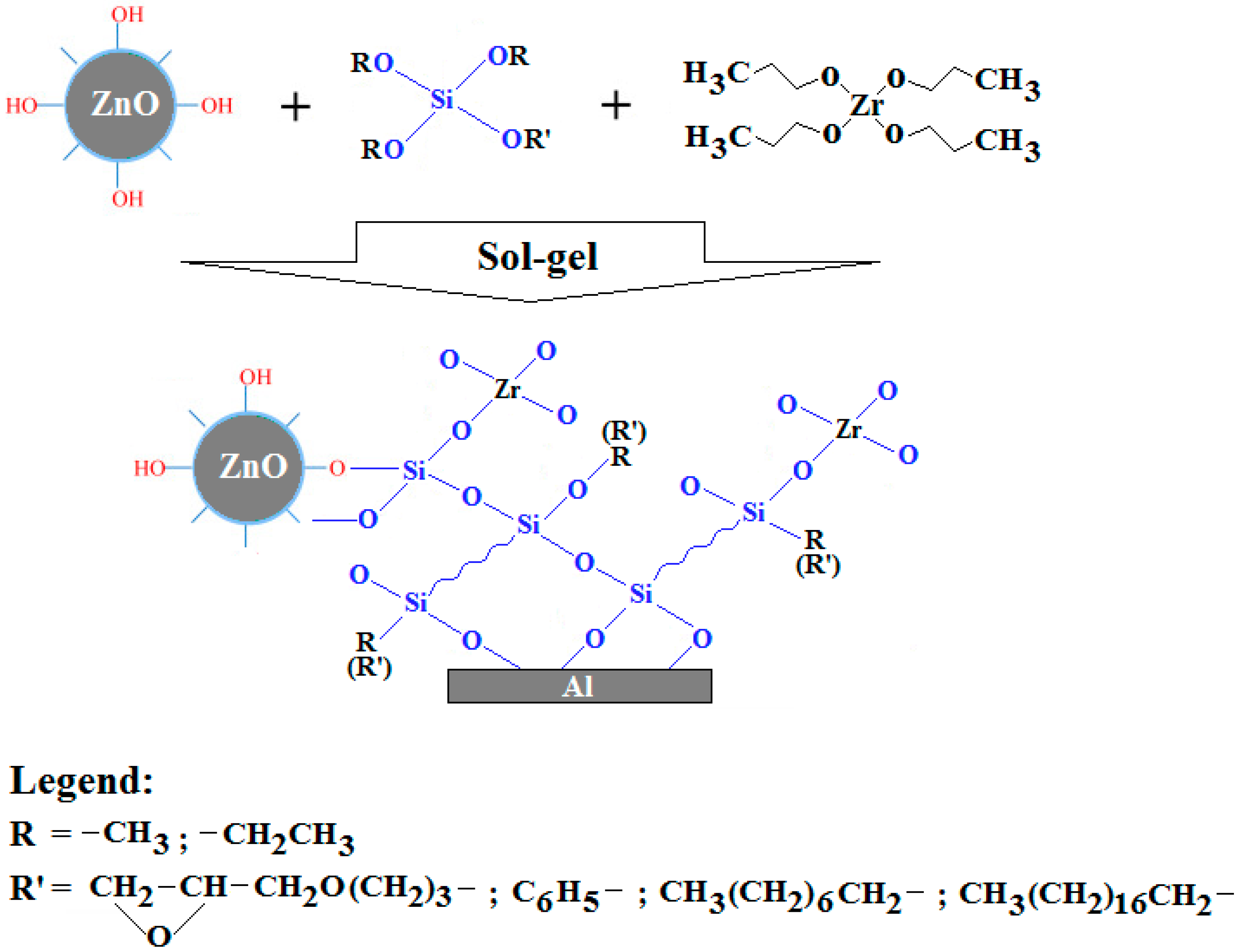
3.2. Synthesis of Modified ZnO-Nanoparticles
3.3. Characterization Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bacaksiz, E.; Parlak, M.; Tomakin, M.; Özcelik, A.; Karakiz, M.; Altunbas, M. The effect of zinc nitrate, zinc acetate and zinc chloride precursors on investigation of structural and optical properties of ZnO thin films. J. Alloys Compd. 2008, 466, 447–450. [Google Scholar] [CrossRef]
- Ludi, B.; Niederberger, M. Zinc oxide nanoparticles: Chemical mechanism and classical and non-classical crystallization. Dalton Trans. 2013, 42, 12554–12568. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Li, Y.; Wu, S.; Lu, P.; Liu, J.; Dong, F. Sol–gel preparation and enhanced photocatalytic performance of Cu doped ZnO nanoparticles. Appl. Surf. Sci. 2011, 258, 1587–1591. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Gopalakrishnan, R. Structural, FTIR and photoluminescence studies of Cu doped ZnO nanopowders by co-precipitation method. Opt. Mater. 2012, 34, 1946–1953. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Markiewicz, E.; Jesionowski, T. Structural characterization of ZnO particles obtained by the emulsion precipitation method. J. Nanomater. 2012, 2012, 15. [Google Scholar] [CrossRef]
- Lanje, A.S.; Sharma, S.J.; Ningthoujam, R.S.; Ahn, J.S.; Pode, R.B. Low temperature dielectric studies of zinc oxide (ZnO) nanoparticles prepared by precipitation method. Adv. Powder Technol. 2013, 24, 331–335. [Google Scholar] [CrossRef]
- Benhebal, H.; Chaib, M.; Salomon, T.; Geens, J.; Leonard, A.; Lambert, S.D.; Crine, M.; Heinrichs, B. Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by sol–gel process. Alex. Eng. J. 2013, 52, 517–523. [Google Scholar] [CrossRef]
- Petzold, F.G.; Jasinski, J.; Clark, E.L.; Kim, J.H.; Absher, J.; Toufar, H.; Sunkara, M.K. Nickel supported on zinc oxide nanowires as advanced hydrodesulfurization catalyst. Catal. Today 2012, 198, 219–227. [Google Scholar] [CrossRef]
- Akgul, F.A.; Attenkofer, K.; Winterer, M. Structural properties of zinc oxide and titanium dioxide nanoparticles prepared by chemical vapor synthesis. J. Alloys Compd. 2013, 554, 177–181. [Google Scholar] [CrossRef]
- Murugadhoss, G. Synthesis and Characterization of Transition metals Doped Zno Nanorods. J. Mater. Sci. Technol. 2012, 28, 587–593. [Google Scholar] [CrossRef]
- Sakai, R.T.; Di Da Cruz, F.M.L.; De Melo, H.G.; Benedetti, A.V.; Santilli, C.V.; Suegama, P.H. Electrochemical study of TEOS, TEOS/MPTS, MPTS/MMA and TEOS/MPTS/MMA films on tin coated steel in 3.5% NaCl solution. Prog. Org. Coat. 2012, 74, 288–301. [Google Scholar] [CrossRef]
- Kunst, S.R.; Cardoso, H.R.P.; Oliveira, C.T.; Santana, J.A.; Sarmento, V.H.V.; Muller, I.L.; Malfatti, C.F. Corrosion resistance of siloxane-poly(methyl methacrylate)hybrid films modified with acetic acid on tin plate substrates: Influence of tetraethoxysilane addition. Appl. Surf. Sci. 2014, 298, 1–11. [Google Scholar] [CrossRef]
- Liu, J.G.; Xu, L.; Fang, Y.Q. Hybrid organic–inorganic sol–gel coatings with interpenetrating network for corrosion protection of tinplate. J. Sol–Gel Sci. Technol. 2014, 71, 246–253. [Google Scholar] [CrossRef]
- Wang, J.F.; Tsuzuki, T.; Sun, L.; Wang, X.G. Reducing the photocatalytic activity of zinc oxide quantum dots by surface modification. J. Am. Ceram. Soc. 2009, 922, 2083–2088. [Google Scholar] [CrossRef]
- Yi, D.K. A study of optothermal and cytotoxic properties of silica coated Au nanorods. Mater. Lett. 2011, 65, 2319–2321. [Google Scholar] [CrossRef]
- Laurenti, M.; Stassi, S.; Canavese, G.; Cauda, V. Surface Engineering of Nanostructured ZnO Surfaces. Adv. Mater. Interfaces 2017, 4, 1600758. [Google Scholar] [CrossRef]
- Huang, H.C.; Hsieh, T.E. Highly stable precursor solution containing ZnO nanoparticles for the preparation of ZnO thin film transistors. Nanotechology 2010, 21, 295707. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.S.; Li, C.Z.; Zhang, L.; Du, H.L.; Burnell-Gray, J.S. Effects of surface modification of fumed silica on interfacial structures and mechanical properties of poly (vinyl chloride) composites. Eur. Polym. J. 2006, 42, 1643–1652. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. Use of silane coupling agent for surface modification of zinc oxide as inorganic filler and preparation of poly(amide–imide)/zinc oxide nanocomposite containing phenylalanine moieties. Bull. Mater. Sci. 2012, 35, 333–339. [Google Scholar] [CrossRef]
- Allen, C.G.; Baker, D.J.; Albin, J.M.; Oertli, H.E.; Gillaspie, D.T.; Olson, D.C.; Furtak, T.E.; Collins, R.T. Surface Modification of ZnO Using Triethoxysilane-Based Molecules. Langmuir 2008, 24, 13393–13398. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, D.; Collazo, A.; Nóvoa, X.R.; Pérez, C. Assessment of ZnO nanoparticles as anticorrosive pigment in hybridsol–gel films. Prog. Org. Coat. 2016, 96, 3–12. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Wu, L.K.; Hu, J.M.; Zhang, J.Q. Silane-incorporated epoxy coatings on aluminum alloy (AA2024). Part 1: Improved corrosion performance. Corros. Sci. 2015, 92, 118–126. [Google Scholar] [CrossRef]
- Chen, H.; Guo, Z.; Jia, L. Preparation and surface modification of highly dispersed nano-ZnO with stearic acid activated by N,N′-carbonyldiimidazole. Mater. Lett. 2012, 82, 167–170. [Google Scholar] [CrossRef]
- Li, Y.N.; Xu, W.M.; Zhang, G.Q. Effect of coupling agent on nano-ZnO modification and antibacterial activity of ZnO/HDPE nanocomposite films. In Proceedings of the Global Conference on Polymer and Composite Materials (PCM 2015), Beijing, China, 16–18 May 2015. [Google Scholar]
- Grasset, F.; Saito, N.; Li, D.; Park, D.; Sakaguchi, I.; Ohashi, N.; Haneda, H. Surface modification of zinc oxide nanoparticles by aminopropyltriethoxysilane. J. Alloys Compd. 2003, 360, 298–311. [Google Scholar] [CrossRef]
- Farzi, G.A.; Tayebee, R.; Naghibinasab, S. Surface modification of ZnO nano-particles with Trimetoxyvinyl Silane and Oleic Acid and studying their dispersion in organic media. Int. J. Nano Dimens. 2015, 6, 67–75. [Google Scholar] [CrossRef]
- Tang, E.; Cheng, G.; Ma, X.; Pang, X.; Zhao, Q. Surface modification of zinc oxide nanoparticle by PMAA and its dispersion in aqueous system. Appl. Surf. Sci. 2006, 252, 5227–5232. [Google Scholar] [CrossRef]
- Dugas, V.; Chevalier, Y. Surface hydroxylation and silane grafting on fumed and thermal silica. J. Colloid Interface Sci. 2003, 264, 354–361. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Li, X. Surface modification of nano-SiO2 particles using polyaniline. Surf. Coat. Technol. 2005, 197, 56–60. [Google Scholar] [CrossRef]
- Navarre, S.; Choplin, F.; Bousbaa, J.; Bennetau, B.; Nony, L.; Aimé, J.P. Structural Characterization of Self-Assembled Monolayers of Organosilanes Chemically Bonded onto Silica Wafers by Dynamical Force Microscopy. Langmuir 2001, 17, 4844–4850. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R’’Si(OR’)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Petcu, C.; Purcar, V.; Spataru, C.I.; Alexandrescu, E.; Somoghi, R.; Trica, B.; Nitu, S.G.; Panaitescu, D.M.; Donescu, D.; Jecu, M.L. The influence of new hydrophobic silica nanoparticles on the surface properties of the films obtained from bilayer hybrids. Nanomaterials 2017, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Howland, M.C.; Johal, M.S.; Parikh, A.N. Transition from Homogeneous Langmuir−Blodgett Monolayers to Striped Bilayers Driven by a Wetting Instability in Octadecylsiloxane Monolayers. Langmuir 2005, 21, 10468–10474. [Google Scholar] [CrossRef] [PubMed]
- Gopal, N.O.; Narasimhulu, K.V.; Rao, J.L. EPR, optical, infrared and Raman spectral studies of Actinolite mineral. Spectrochim. Acta Mol. Biomol. Spectrosc. 2004, 60, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Amaria, A.; Nuryono, N.; Suyanta, S. Preparation of L-Arginine-Modified Silica-Coated Magnetite Nanoparticles for Au(III) Adsorption. Orient. J. Chem. 2017, 33, 384–395. [Google Scholar] [CrossRef]
- Petcu, C.; Nistor, C.L.; Purcar, V.; Cinteza, L.O.; Spataru, C.-I.; Ghiurea, M.; Ianchis, R.; Anastasescu, M.; Stoica, M. Facile preparation in two steps of highly hydrophobic coatings on polypropylene surface. Appl. Surf. Sci. 2015, 347, 359–367. [Google Scholar] [CrossRef]
- Wen, X.-F.; Wang, K.; Pi, P.-H.; Yang, J.-X.; Cai, Z.-Q.; Zhang, L.; Qian, Y.; Yang, Z.-R.; Zheng, D.; Cheng, J. Organic–Inorganic Hybrid Superhydrophobic Surfaces Using Methyltriethoxysilane and Tetraethoxysilane Sol–Gel Derived Materials in Emulsion. Appl. Surf. Sci. 2011, 258, 991–998. [Google Scholar] [CrossRef]
- Nocun, M.; Cholewa-Kowalska, K.; Łączka, M. Structure of Hybrids Based on TEOS-Cyclic Forms of Siloxane System. J. Mol. Struct. 2009, 938, 24–28. [Google Scholar] [CrossRef]
- Jurablu, S.; Farahmandjou, M.; Firoozabadi, T.P. Sol–gel Synthesis of Zinc Oxide (ZnO) Nanoparticles: Study of Structural and Optical Properties. J. Sci. Islam. Repub. Iran 2015, 26, 281–285. [Google Scholar] [CrossRef]
- Spori, D.M.; Venkataraman, N.V.; Tosatti, S.G.P.; Durmaz, F.; Spencer, N.D.; Zurcher, S. Influence of alkyl chain length on phosphate self-assembled monolayers. Langmuir 2007, 23, 8053–8060. [Google Scholar] [CrossRef] [PubMed]
- Ooij, W.J.V.; Zhu, D.; Stacy, M.; Seth, A.; Mugada, T.; Gandhi, J. Corrosion protection properties of organofunctional silanes: An overview. Tsinghua Sci. Technol. 2005, 10, 639–664. [Google Scholar] [CrossRef]
- Rao, A.V.; Kalesh, R.R.; Pajonk, G.M. Hydrophobicity and physical properties of TEOS based silica aerogels using phenyltriethoxysilane as a synthesis component. J. Mater. Sci. 2003, 38, 4407–4413. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V.; Gazia, R.; Fontana, M.; Rivera, V.F.; Bianco, S.; Canavese, G. Wettability Control on ZnO Nanowires Driven by Seed Layer Properties. Eur. J. Inorg. Chem. 2013, 14, 2520–2527. [Google Scholar] [CrossRef]
- Laurenti, M.; Verna, A.; Fontana, M.; Stassi, S.; Canavese, G.; Marasso, S.L.; Cauda, V. How Micropatterning and Surface Functionalization Affect the Wetting Behavior of ZnO Nanostructured Surfaces. Adv. Mater. Interfaces 2016, 13, 1600110. [Google Scholar] [CrossRef]

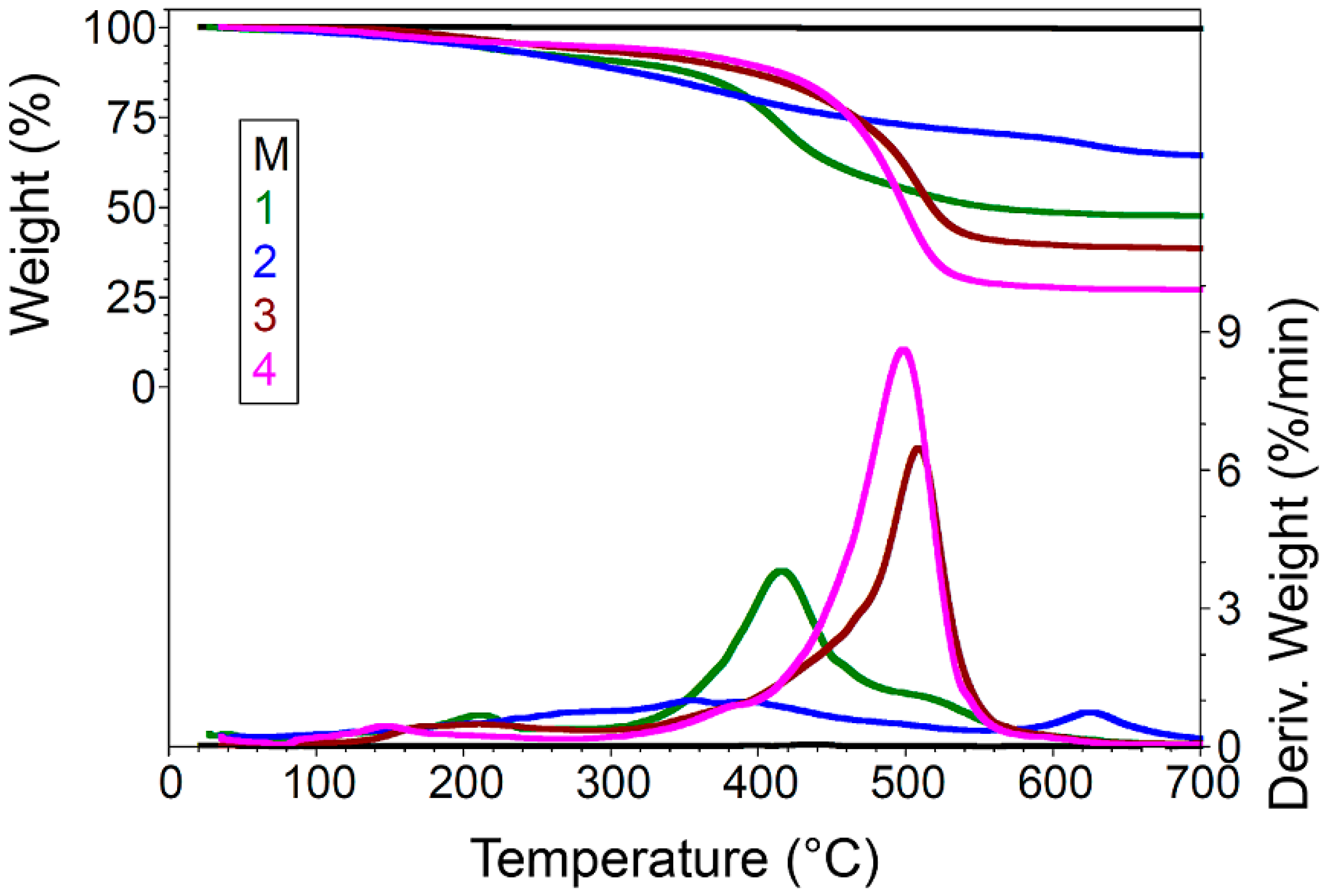



| Sample No. | 40–275 °C | 275–575 °C | 575–700 °C | Residue at 700 °C | |||||
|---|---|---|---|---|---|---|---|---|---|
| Wt. Loss % | Tmax1 1 °C | Wt. Loss % | Tmax2 °C | Tmax3 °C | Tmax4 °C | Wt. Loss % | Tmax5 °C | N2 | |
| M | 0.11 | 216.80 | 0.22 | - | 436.90 | - | 0.02 | - | 99.65 |
| 1 | 8.40 | 212.00 | 42.46 | 358.20 | 415.80 | 518.60 | 1.70 | 624.60 | 47.41 |
| 2 | 9.51 | 270.20 | 20.60 | 356.60 | 390.90 | 492.80 | 5.68 | 623.50 | 64.22 |
| 3 | 6.02 | 211.40 | 53.91 | 369.90 | 429.20 | 508.80 | 1.65 | 600.50 | 38.42 |
| 4 | 5.18 | 146.40 | 66.70 | 379.20 | 417.10 | 498.20 | 1.23 | 609.60 | 26.88 |
| Sample No. | Sample Roughness (nm) | MSE |
|---|---|---|
| M | 2.69 | 0.55 |
| 1 | 7.35 | 1.43 |
| 2 | 27.06 | 1.18 |
| 3 | 13.31 | 1.21 |
| 4 | 54.13 | 1.29 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purcar, V.; Şomoghi, R.; Niţu, S.G.; Nicolae, C.-A.; Alexandrescu, E.; Gîfu, I.C.; Gabor, A.R.; Stroescu, H.; Ianchiş, R.; Căprărescu, S.; et al. The Effect of Different Coupling Agents on Nano-ZnO Materials Obtained via the Sol–Gel Process. Nanomaterials 2017, 7, 439. https://doi.org/10.3390/nano7120439
Purcar V, Şomoghi R, Niţu SG, Nicolae C-A, Alexandrescu E, Gîfu IC, Gabor AR, Stroescu H, Ianchiş R, Căprărescu S, et al. The Effect of Different Coupling Agents on Nano-ZnO Materials Obtained via the Sol–Gel Process. Nanomaterials. 2017; 7(12):439. https://doi.org/10.3390/nano7120439
Chicago/Turabian StylePurcar, Violeta, Raluca Şomoghi, Sabina Georgiana Niţu, Cristian-Andi Nicolae, Elvira Alexandrescu, Ioana Cătălina Gîfu, Augusta Raluca Gabor, Hermine Stroescu, Raluca Ianchiş, Simona Căprărescu, and et al. 2017. "The Effect of Different Coupling Agents on Nano-ZnO Materials Obtained via the Sol–Gel Process" Nanomaterials 7, no. 12: 439. https://doi.org/10.3390/nano7120439






