Impedance Spectroscopy Analysis and Equivalent Circuit Modeling of Graphene Oxide Solutions
Abstract
:1. Introduction
2. Experiment
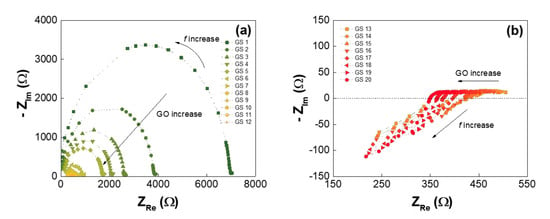
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Neto, A.C.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Mkhoyan, K.A.; Contryman, A.W.; Silcox, J.; Stewart, D.A.; Eda, G.; Mattevi, C.; Chhowalla, M. Atomic and electronic structure of graphene-oxide. Nano Lett. 2009, 9, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Han, K.I.; Kim, S.D.; Yang, W.S.; Kim, H.S.; Shin, M.; Kim, J.P.; Hwang, W.S. Material characteristics and equivalent circuit models of stacked graphene oxide for capacitive humidity sensors. AIP Adv. 2016, 6, 035203. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J. Graphene oxide sheets at interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Müller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically strong, electrically conductive, and biocompatible graphene paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Su, D. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Tu, K.H.; Lin, C.C.; Chen, C.W.; Chhowalla, M. Solution-processable graphene oxide as an efficient hole transport layer in polymer solar cells. ACS Nano 2010, 4, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Mi, B. Layer-by-layer assembly of graphene oxide membranes via electrostatic interaction. J. Membr. Sci. 2014, 469, 80–87. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.C.; Kim, K.S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Tour, J.M. Mechanism of graphene oxide formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Park, W.K.; Kim, H.; Kim, T.Y.; Kim, Y.; Yoo, S.; Kim, S.; Yoon, D.H.; Yang, W.S. Facile synthesis of graphene oxide in a Couette–Taylor flow reactor. Carbon 2015, 83, 217–223. [Google Scholar] [CrossRef]
- Shih, C.J.; Lin, S.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the pH-dependent behavior of graphene oxide aqueous solutions: A comparative experimental and molecular dynamics simulation study. Langmuir 2011, 28, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Sluyters-Rehbach, M. Impedances of electrochemical systems: Terminology, nomenclature and representation-Part I: Cells with metal electrodes and liquid solutions (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 1831–1891. [Google Scholar] [CrossRef]
- Graf, K.; Kappl, M. Physics and Chemistry of Interfaces, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 978-3-527-60640-5. [Google Scholar]
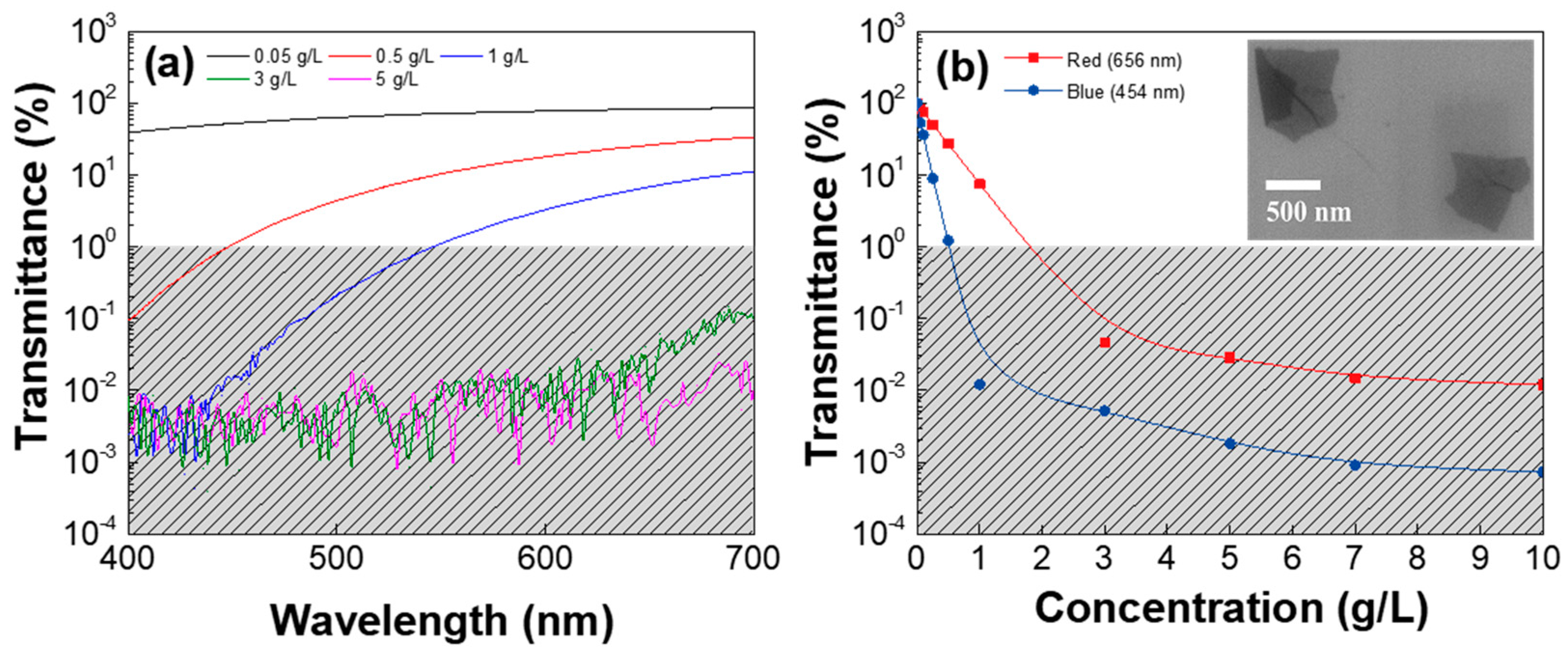
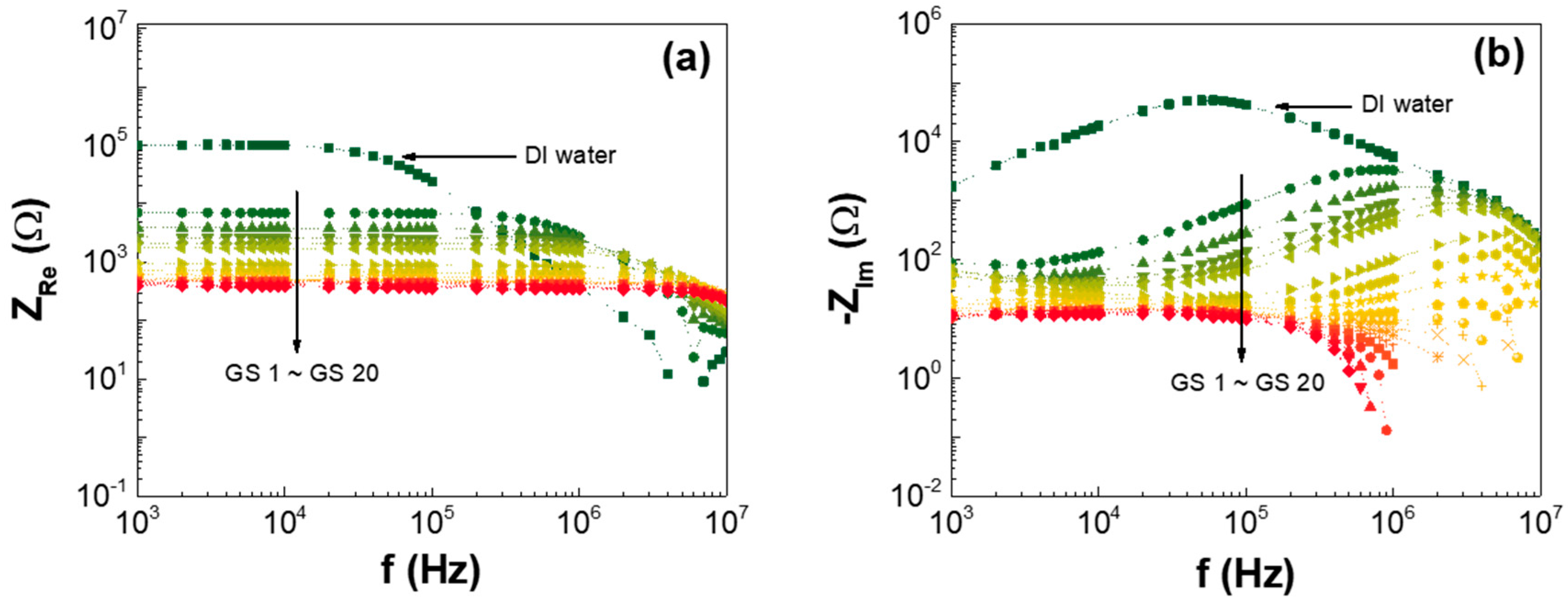
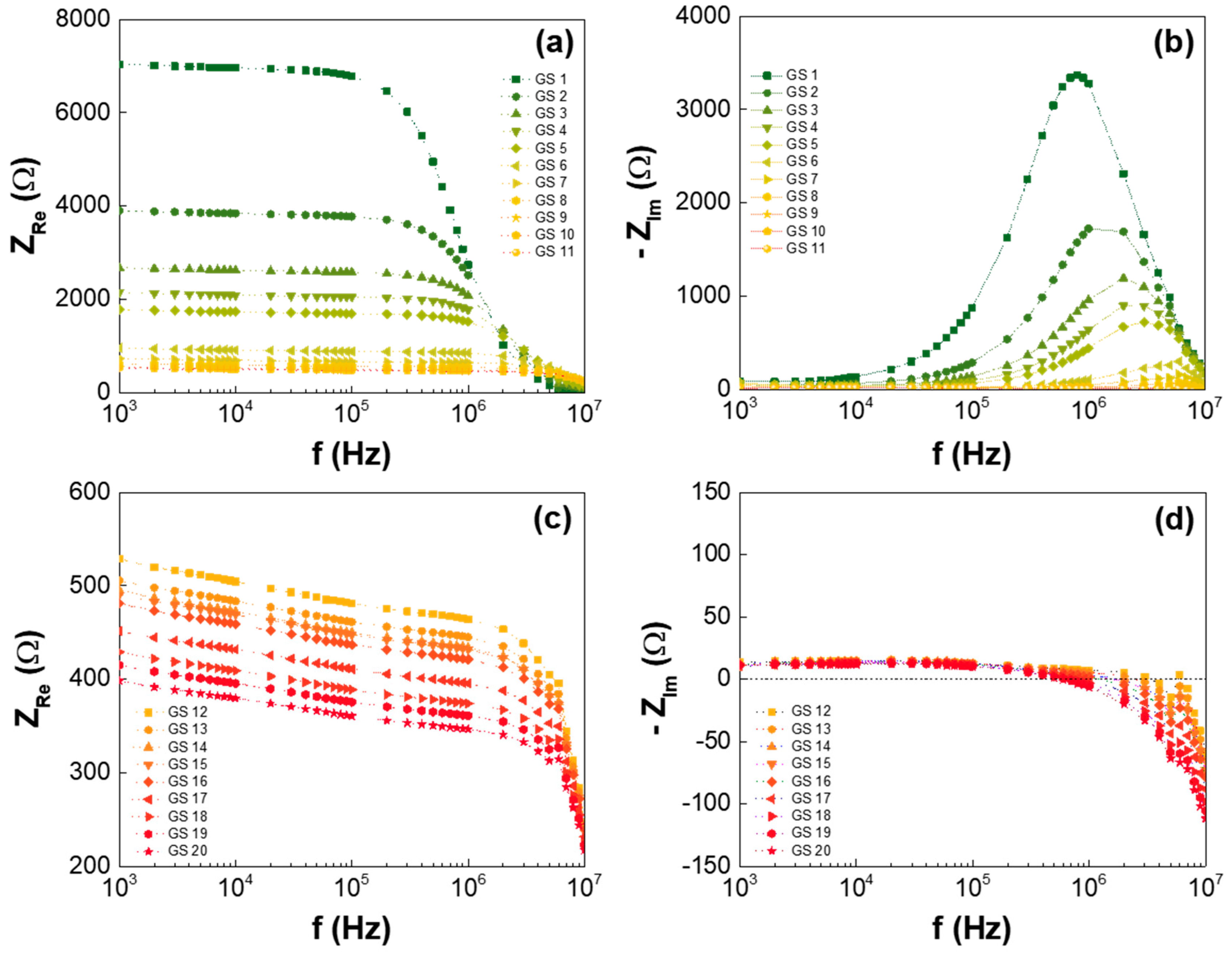
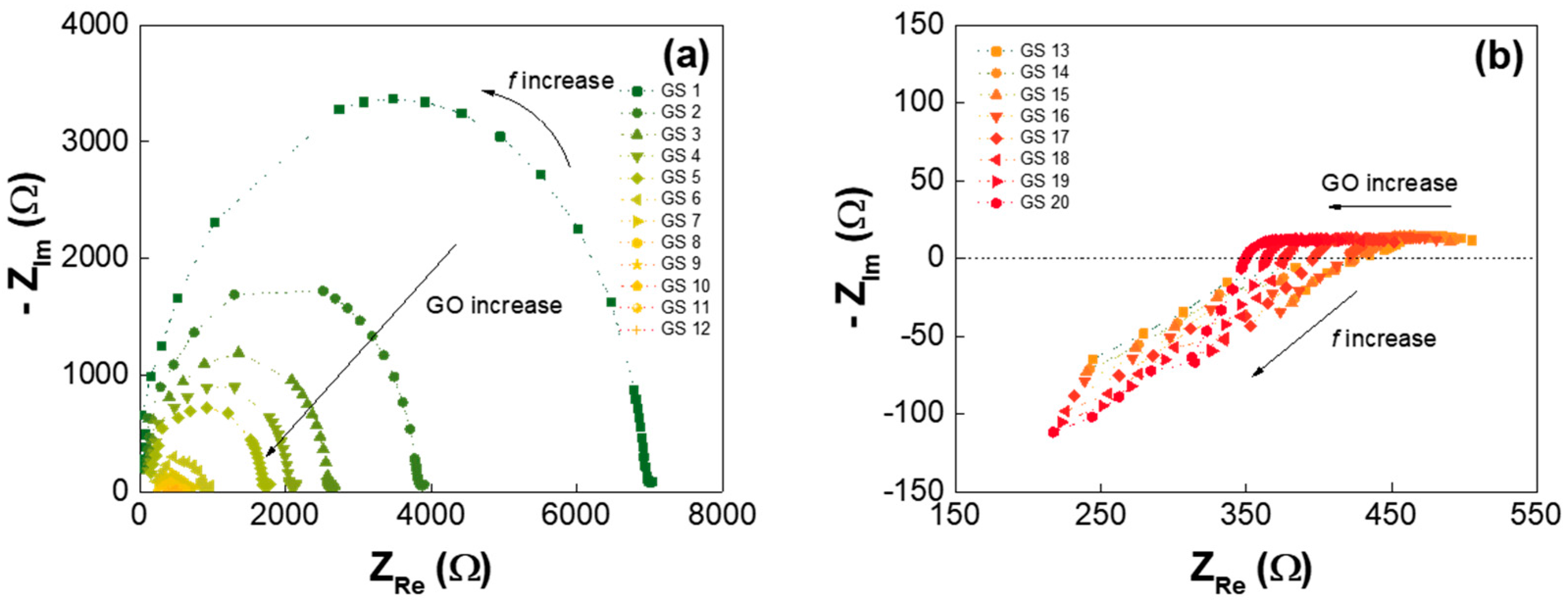
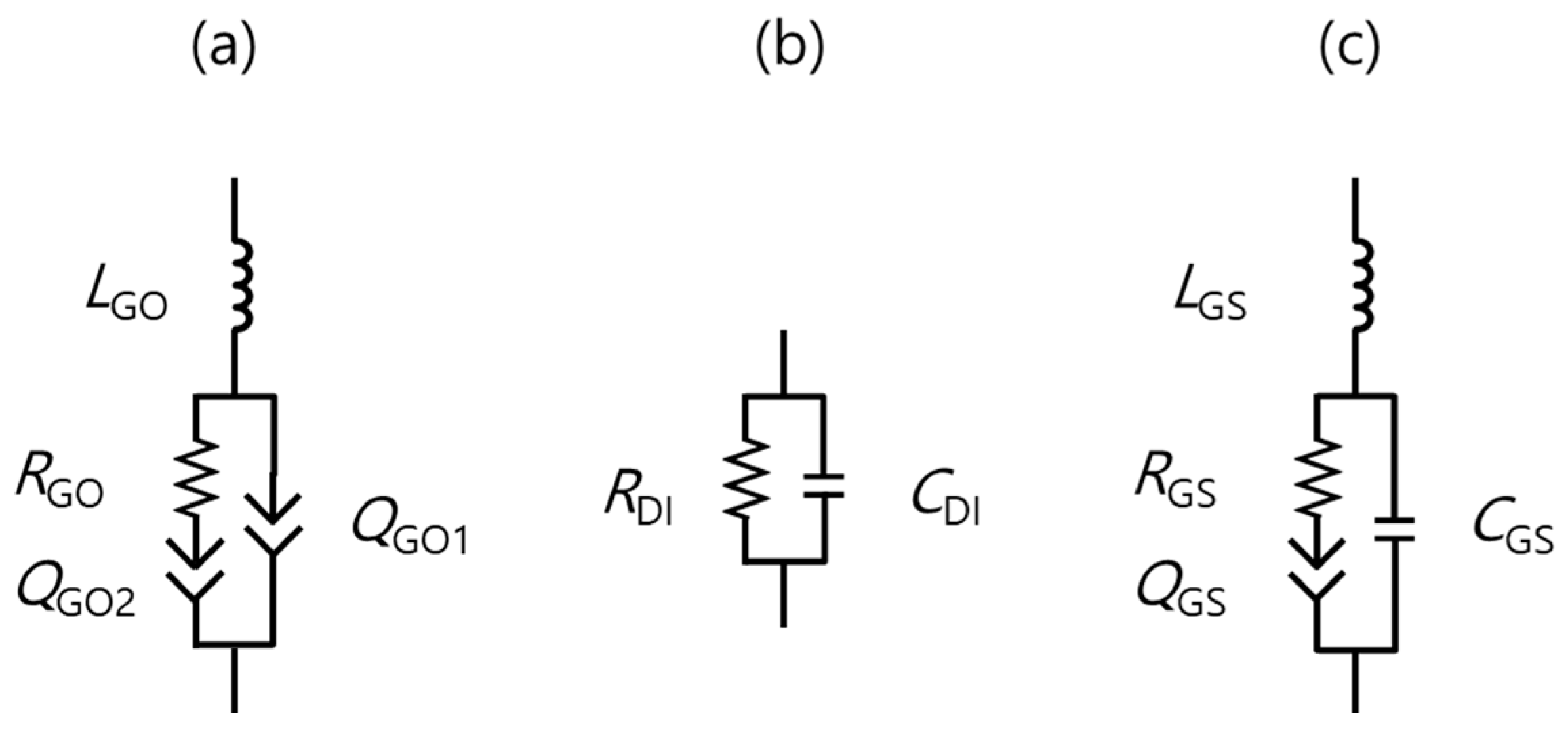
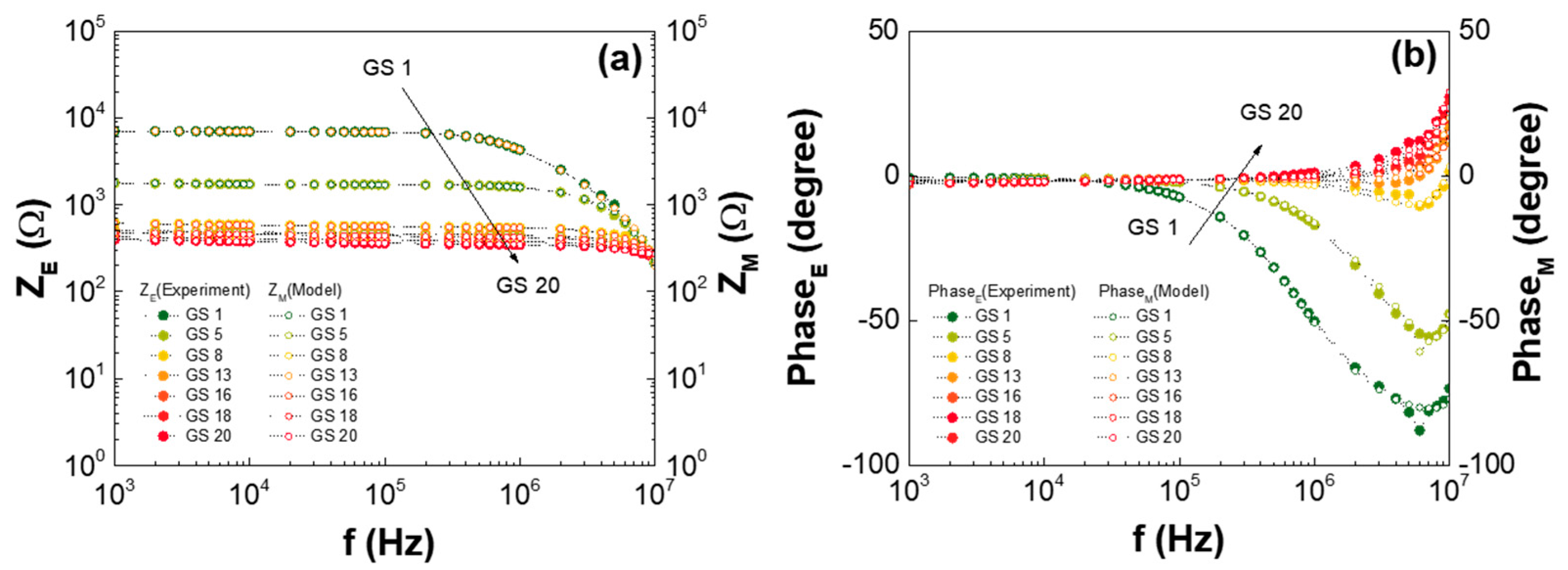
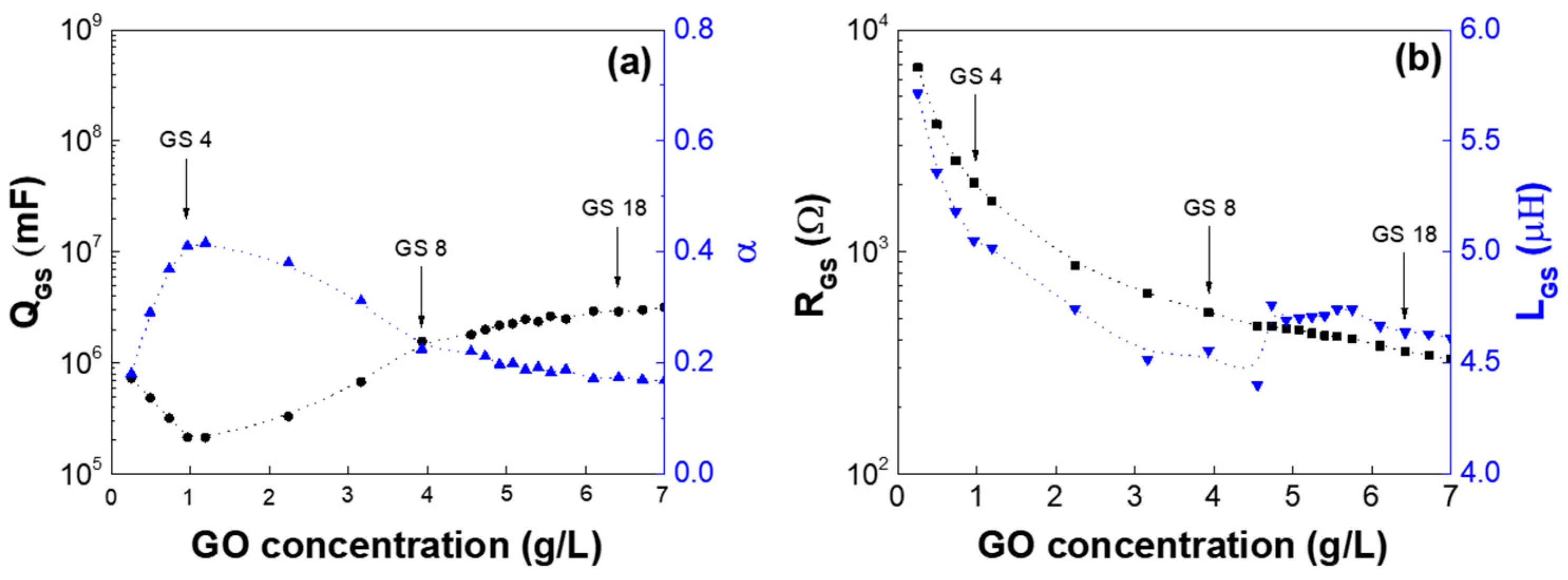
| Low-GO Samples | GS1 | GS2 | GS3 | GS4 | GS5 | GS6 | GS7 | GS8 | GS9 | GS10 | GS11 |
| Concentration(g/L) | 0.25 | 0.49 | 0.73 | 0.96 | 1.19 | 2.24 | 3.16 | 3.93 | 4.55 | 4.73 | 4.91 |
| High-GO samples | GS12 | GS13 | GS14 | GS15 | GS16 | GS17 | GS18 | GS19 | GS20 | n/a | n/a |
| Concentration (g/L) | 5.08 | 5.24 | 5.40 | 5.56 | 5.75 | 6.10 | 6.42 | 6.72 | 7.00 |
| Circuit Component | DI Water | GS4 | GS8 | GS18 |
|---|---|---|---|---|
| LGS (µH) | n/a | 5.05 | 4.56 | 4.64 |
| RGS (kΩ) | 102 | 2.05 | 0.53 | 0.36 |
| QGS (mF·S(α−1)) | n/a | 0.22 | 1.56 | 2.91 |
| α | n/a | 0.41 | 0.23 | 0.17 |
| CGS (pF) | 28.8 | 30.4 | 28.9 | 30.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, Y.; Jo, J.; Kim, S.; Lee, I.G.; Cho, B.J.; Shin, M.; Hwang, W.S. Impedance Spectroscopy Analysis and Equivalent Circuit Modeling of Graphene Oxide Solutions. Nanomaterials 2017, 7, 446. https://doi.org/10.3390/nano7120446
Yoon Y, Jo J, Kim S, Lee IG, Cho BJ, Shin M, Hwang WS. Impedance Spectroscopy Analysis and Equivalent Circuit Modeling of Graphene Oxide Solutions. Nanomaterials. 2017; 7(12):446. https://doi.org/10.3390/nano7120446
Chicago/Turabian StyleYoon, Youngbin, Jeonghoo Jo, Seungdu Kim, In Gyu Lee, Byung Jin Cho, Myunghun Shin, and Wan Sik Hwang. 2017. "Impedance Spectroscopy Analysis and Equivalent Circuit Modeling of Graphene Oxide Solutions" Nanomaterials 7, no. 12: 446. https://doi.org/10.3390/nano7120446