Influence of Sterilization and Preservation Procedures on the Integrity of Serum Protein-Coated Magnetic Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nanoparticle Properties
2.2. Freezing
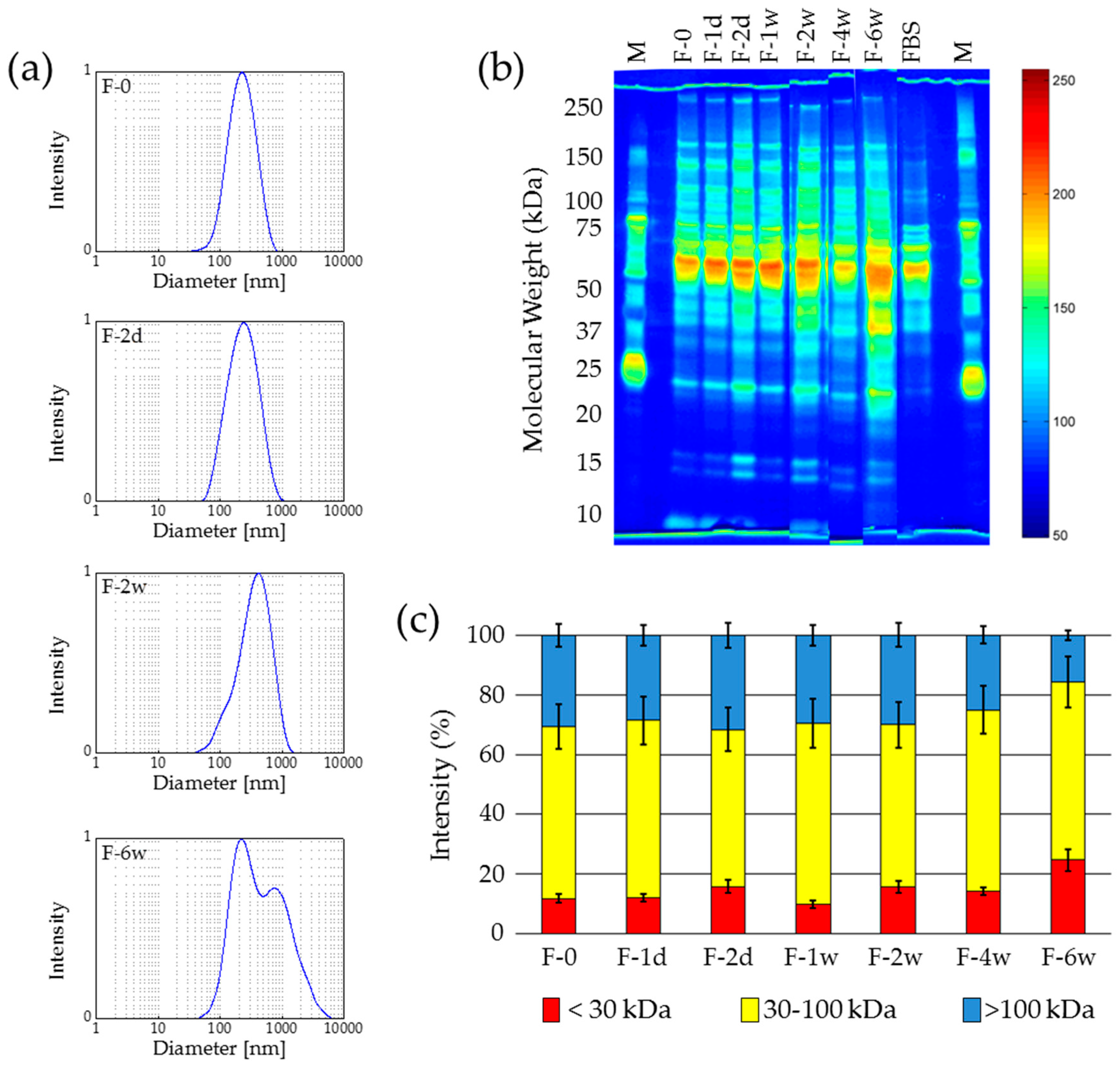
2.2.1. Freezing at −15 °C
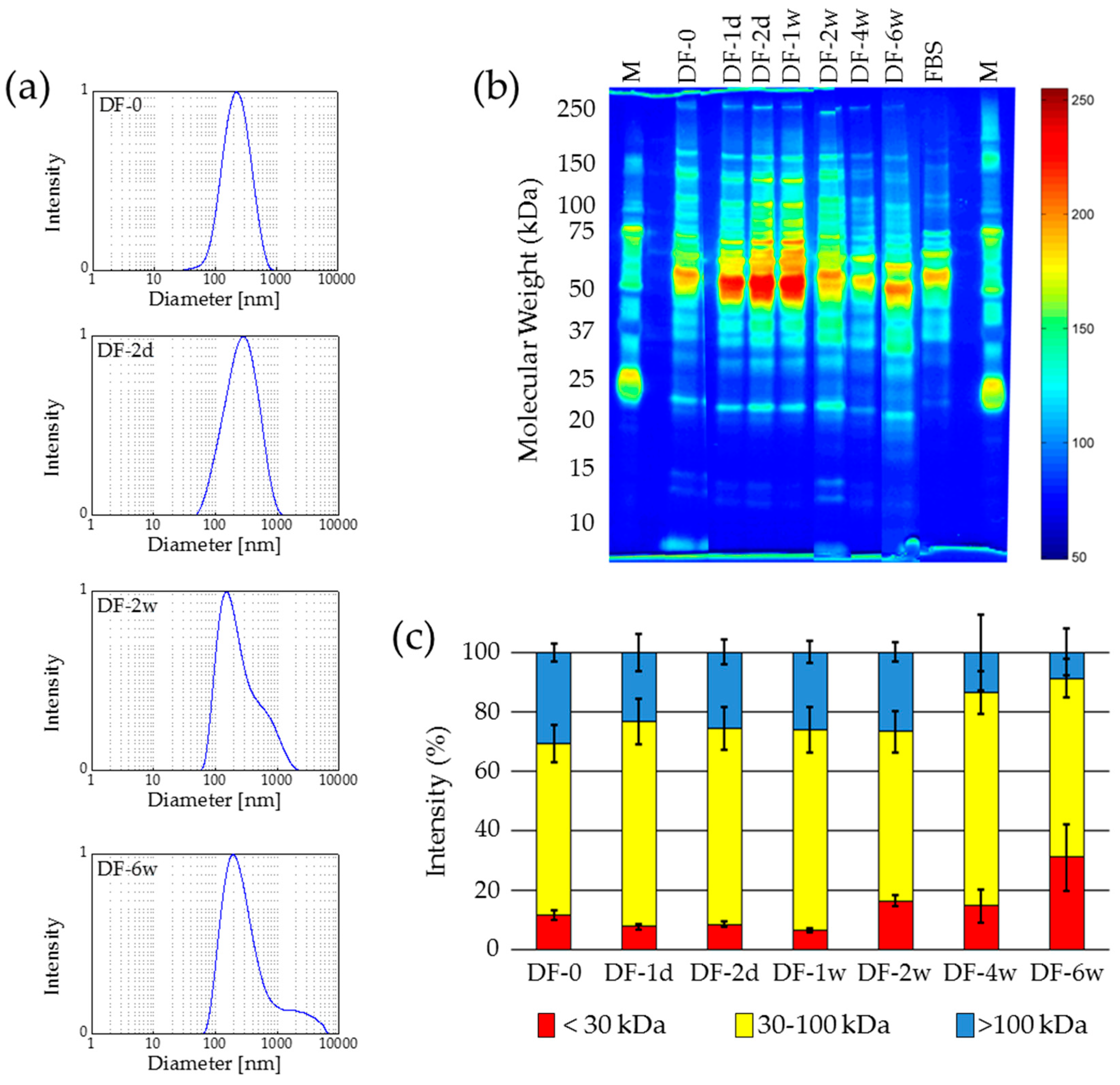
2.2.2. Deep-Freezing at −80 °C
2.3. Lyophilization
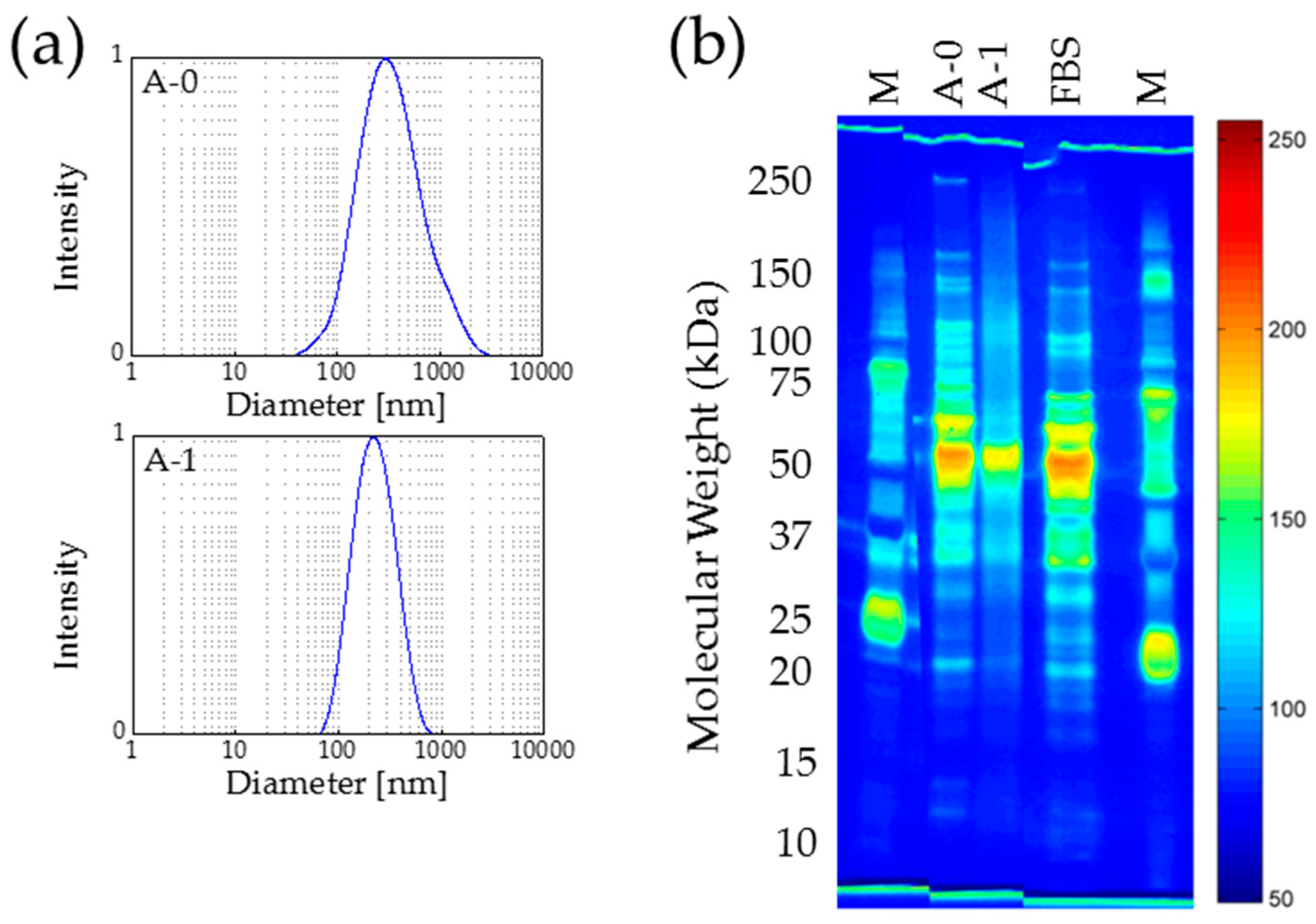
2.4. Autoclaving
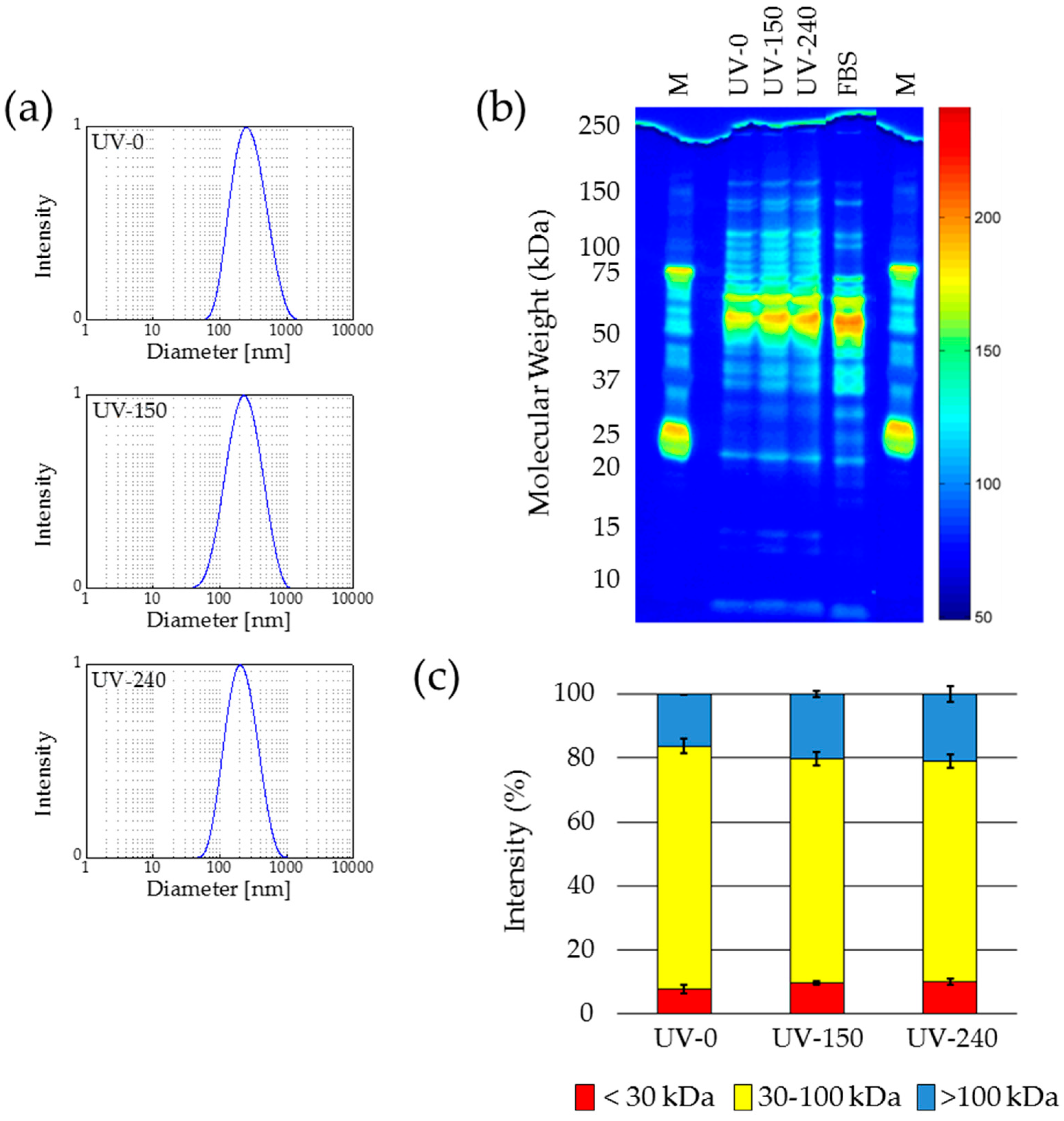
2.5. UV Sterilization
3. Materials and Methods
3.1. Particle Preparation
3.2. Sterilization and Preservation
3.2.1. Freezing
3.2.2. Lyophilization
3.2.3. Autoclaving
3.2.4. UV Sterilization
3.3. Particle Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krishnan, K.M. Biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef]
- Alexiou, C.; Arnold, W.; Klein, R.J.; Parak, F.G.; Hulin, P.; Bergemann, C.; Erhardt, W.; Wagenpfeil, S.; Lubbe, A.S. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000, 60, 6641–6648. [Google Scholar] [PubMed]
- Tietze, R.; Lyer, S.; Durr, S.; Struffert, T.; Engelhorn, T.; Schwarz, M.; Eckert, E.; Goen, T.; Vasylyev, S.; Peukert, W.; et al. Efficient drug-delivery using magnetic nanoparticles—Biodistribution and therapeutic effects in tumour bearing rabbits. Nanomed.-Nanotechnol. Biol. Med. 2013, 9, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia-a promising tumour therapy? Nanotechnology 2014, 25. [Google Scholar] [CrossRef] [PubMed]
- Perigo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef]
- Gleich, B.; Weizenecker, R. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; von See, M.P.; Yu, E.; Gunel, B.; Lu, K.; Vazin, T.; Schaffer, D.V.; Goodwill, P.W.; Conolly, S.M. Quantitative magnetic particle imaging monitors the transplantation, biodistribution, and clearance of stem cells in vivo. Theranostics 2016, 6, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Z.; Mei, S.L.; Wang, W.; Chu, P.K.; Wu, Z.F.; Zhang, Y.M. The role of sterilization in the cytocompatibility of titania nanotubes. Biomaterials 2010, 31, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Del Pino, P.; Pelaz, B.; Zhang, Q.; Maffre, P.; Nienhaus, G.U.; Parak, W.J. Protein corona formation around nanoparticles - from the past to the future. Mater. Horiz. 2014, 1, 301–313. [Google Scholar] [CrossRef]
- Vroman, L. Effect of adsorbed proteins on the wettability of hydrophilic and hydrophobic solids. Nature 1962, 196, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Roecker, C.; Poetzl, M.; Zhang, F.; Parak, W.J.; Nienhaus, G.U. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat. Nanotechnol. 2009, 4, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggard, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Winzen, S.; Schoettler, S.; Baier, G.; Rosenauer, C.; Mailaender, V.; Landfester, K.; Mohr, K. Complementary analysis of the hard and soft protein corona: Sample preparation critically effects corona composition. Nanoscale 2015, 7, 2992–3001. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Abdelmonem, A.M.; Behzadi, S.; Clement, J.H.; Dutz, S.; Ejtehadi, M.R.; Hartmann, R.; Kantner, K.; Linne, U.; Maffre, P.; et al. Temperature: The “ignored” factor at the nanobio interface. ACS Nano 2013, 7, 6555–6562. [Google Scholar] [CrossRef] [PubMed]
- Weidner, A.; Gräfe, C.; Von der Luhe, M.; Remmer, H.; Clement, J.H.; Eberbeck, D.; Ludwig, F.; Müller, R.; Schacher, F.H.; Dutz, S. Preparation of core-shell hybrid materials by producing a protein corona around magnetic nanoparticles. Nanoscale Res. Lett. 2015, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Chanteau, B.; Fresnais, J.; Berret, J.F. Electrosteric enhanced stability of functional sub-10 nm cerium and iron oxide particles in cell culture medium. Langmuir 2009, 25, 9064–9070. [Google Scholar] [CrossRef] [PubMed]
- Safi, M.; Courtois, J.; Seigneuret, M.; Conjeaud, H.; Berret, J.F. The effects of aggregation and protein corona on the cellular internalization of iron oxide nanoparticles. Biomaterials 2011, 32, 9353–9363. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, V.; Graillot, A.; Vitorazi, L.; Crouzet, Q.; Marletta, G.; Loubat, C.; Berret, J.F. Preventing corona effects: Multiphosphonic acid poly(ethylene glycol) copolymers for stable stealth iron oxide nanoparticles. Biomacromolecules 2014, 15, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Huehn, D.; Kantner, K.; Geidel, C.; Brandholt, S.; De Cock, I.; Soenen, S.J.H.; Gil, P.R.; Montenegro, J.-M.; Braeckmans, K.; Muellen, K.; et al. Polymer-coated nanoparticles interacting with proteins and cells: Focusing on the sign of the net charge. ACS Nano 2013, 7, 3253–3263. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Sheibani, S.; Milani, A.S.; Rezaee, F.; Gauberti, M.; Dinarvand, R.; Vali, H. Crucial role of the protein corona for the specific targeting of nanoparticles. Nanomedicine 2015, 10, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Maedler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Choi, E.-J.; Webster, T.J.; Kim, S.-H.; Khang, D. Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. Int. J. Nanomed. 2015, 10, 97–112. [Google Scholar]
- Lesniak, A.; Fenaroli, F.; Monopoli, M.R.; Aberg, C.; Dawson, K.A.; Salvati, A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 2012, 6, 5845–5857. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Bombelli, F.B.; Dawson, K.A. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, L.Y.; Lin, R.; Wang, A.Y.; Yang, L.; Kuang, M.; Qian, W.P.; Mao, H. Casein-coated iron oxide nanoparticles for high mri contrast enhancement and efficient cell targeting. ACS Appl. Mater. Interfaces 2013, 5, 4632–4639. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Neves-Petersen, M.T.; Jeppesen, P.B.; Gregersen, S.; Petersen, S.B. Uv-light exposure of insulin: Pharmaceutical implications upon covalent insulin dityrosine dimerization and disulphide bond photolysis. PLoS ONE 2012, 7, e50733. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S. Are magnetic multicore nanoparticles promising candidates for biomedical applications? IEEE Trans. Magn. 2016, 52. [Google Scholar] [CrossRef]
- Gräfe, C.; Weidner, A.; von der Lühe, M.; Bergemann, C.; Schacher, F.H.; Clement, J.H.; Dutz, S. Intentional formation of a protein corona on nanoparticles: Serum concentration affects protein corona mass, surface charge, and nanoparticle-cell interaction. Int. J. Biochem. Cell Biol. 2016, 75, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, J.S.; Malissek, M.; Simon, S.; Knauer, S.K.; Maskos, M.; Stauber, R.H.; Peukert, W.; Treuel, L. Impact of the nanoparticle-protein corona on colloidal stability and protein structure. Langmuir 2012, 28, 9673–9679. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, T.; Padley, D.; Keever-Taylor, C.; Niederwieser, D.; Teshima, T.; Lanza, F.; Chabannon, C.; Szabolcs, P.; Bazarbachi, A.; Koh, M.B.C.; et al. Essential requirements for setting up a stem cell processing laboratory. Bone Marrow Transplant. 2014, 49, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Prestrelski, S.J.; Arakawa, T.; Carpenter, J.F. Separation of freezing-induced and drying-induced denaturation of lyophilized proteins using stress-specific stabilization. II. Structural studies using infrared spectroscopy. Arch. Biochem. Biophys. 1993, 303, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Cho, T.; Murakami, Y. Alkali digestion method using tmah for determination of trace elements contained in biological materials. In Trace Element Analytical Chemistry in Medicine and Biology, Proceedings of the International Workshop, Neuherberg, Germany, 15–18 April 1988; Braetter, P., Schramel, P., Eds.; Walter de Gruyter: Berlin, Germany, 1988; Volume 5, pp. 72–77. [Google Scholar]
- Bhatnagar, B.S.; Pikal, M.J.; Bogner, R.H. Study of the individual contributions of ice formation and freeze-concentration on isothermal stability of lactate dehydrogenase during freezing. J. Pharm. Sci. 2008, 97, 798–814. [Google Scholar] [CrossRef] [PubMed]
- Logie, J.; Owen, S.C.; McLaughlin, C.K.; Shoichet, M.S. Peg-graft density controls polymeric nanoparticle micelle stability. Chem. Mater. 2014, 26, 2847–2855. [Google Scholar] [CrossRef]
- Vaz, C.M.; De Graaf, L.A.; Reis, R.L.; Cunha, A.M. Effect of crosslinking, thermal treatment and uv irradiation on the mechanical properties and in vitro degradation behavior of several natural proteins aimed to be used in the biomedical field. J. Mater. Sci.-Mater. Med. 2003, 14, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Durchschlag, H.; Fochler, C.; Feser, B.; Hausmann, S.; Seroneit, T.; Swientek, M.; Swoboda, E.; Winklmair, A.; Wlcek, C.; Zipper, P. Effects of x- and uv-irradiation on proteins. Radiat. Phys. Chem. 1996, 47, 501–505. [Google Scholar] [CrossRef]
- Mahmoudi, M. Nanoparticle and protein corona. In Protein-Nanoparticle Interactions; Rahman, M., Laurent, S., Tawil, N., Yahia, L., Mahmoudi, M., Eds.; Springer: Berlin, Germany, 2013. [Google Scholar]
- Khalafalla, S.E.; Reimers, G.W. Preparation of dilution-stable aqueous magnetic fluids. IEEE Trans. Magn. 1980, 16, 178–183. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous ferrofluids without using surfactant - behavior as a function of the ph and the counterions. C. R. Hebd. Seances Acad. Sci. Ser. C 1980, 291, 1–3. [Google Scholar]
- Dutz, S.; Clement, J.H.; Eberbeck, D.; Gelbrich, T.; Hergt, R.; Mueller, R.; Wotschadlo, J.; Zeisberger, M. Ferrofluids of magnetic multicore nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2009, 321, 1501–1504. [Google Scholar] [CrossRef]
- Kurzhals, H. Kühlen und Gefrieren von Lebensmitteln; BEHRS Verlag: Hamburg, Germany, 2007. [Google Scholar]
- Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000, 203, 1–60. [Google Scholar] [CrossRef]
- Bodenschatz, W. Handbuch für den Desinfektor in Ausbildung und Praxis; Elsevier: München, Germany, 1993. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to imageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]

| Treatment Condition | Duration | Analysis | ||
|---|---|---|---|---|
| DLS | ZETA | SDS-PAGE | ||
| Preservation | ||||
| Freezing (−15 °C) | up to 6 weeks | X | X | X |
| Deep-Freezing (−80 °C) | up to 6 weeks | X | X | X |
| Lyophilization | up to 6 weeks | X | X | X |
| Sterilization | ||||
| Autoclaving | before/after | X | X | X |
| UV-Sterilization | before/after | X | X | X |
| Method | Suitable for Application | Remarks |
|---|---|---|
| UV-Sterilisation | yes | no major changes |
| Lyophilization | yes, with PEG as additive | degradation of large proteins with TMAH |
| Freezing (−15 °C) | only for short term storage | agglomeration of MNP, degradation of large proteins |
| Deep-freezing (−80 °C) | only for short term storage | agglomeration of MNP, degradation of large proteins |
| Autoclaving | no | degradation of proteins |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutz, S.; Wojahn, S.; Gräfe, C.; Weidner, A.; Clement, J.H. Influence of Sterilization and Preservation Procedures on the Integrity of Serum Protein-Coated Magnetic Nanoparticles. Nanomaterials 2017, 7, 453. https://doi.org/10.3390/nano7120453
Dutz S, Wojahn S, Gräfe C, Weidner A, Clement JH. Influence of Sterilization and Preservation Procedures on the Integrity of Serum Protein-Coated Magnetic Nanoparticles. Nanomaterials. 2017; 7(12):453. https://doi.org/10.3390/nano7120453
Chicago/Turabian StyleDutz, Silvio, Stephanie Wojahn, Christine Gräfe, Andreas Weidner, and Joachim H. Clement. 2017. "Influence of Sterilization and Preservation Procedures on the Integrity of Serum Protein-Coated Magnetic Nanoparticles" Nanomaterials 7, no. 12: 453. https://doi.org/10.3390/nano7120453





