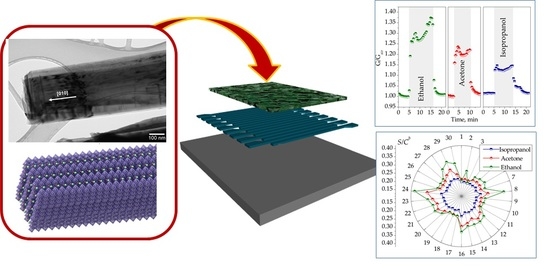
The Room-Temperature Chemiresistive Properties of Potassium Titanate Whiskers versus Organic Vapors
Abstract
:1. Introduction
2. Results and Discussion
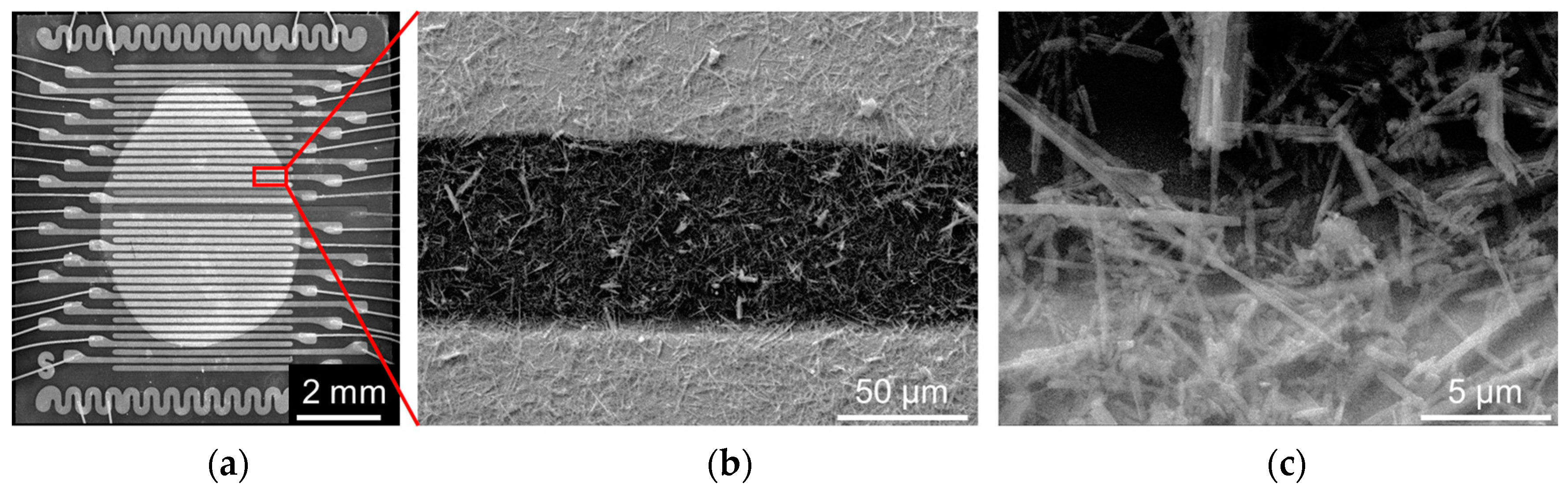
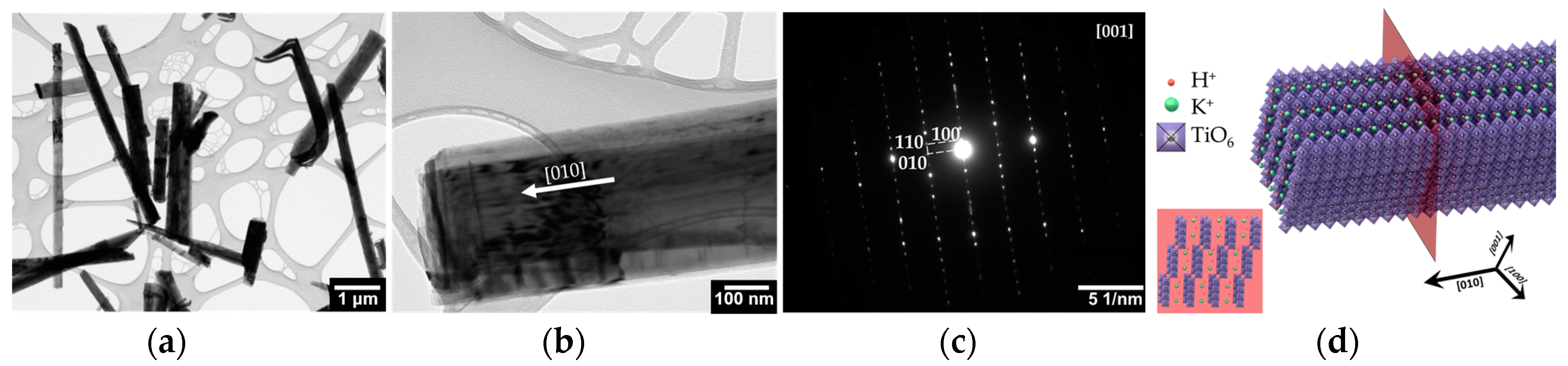
2.1. Composition and Structure of Potasium Titanate Whiskers
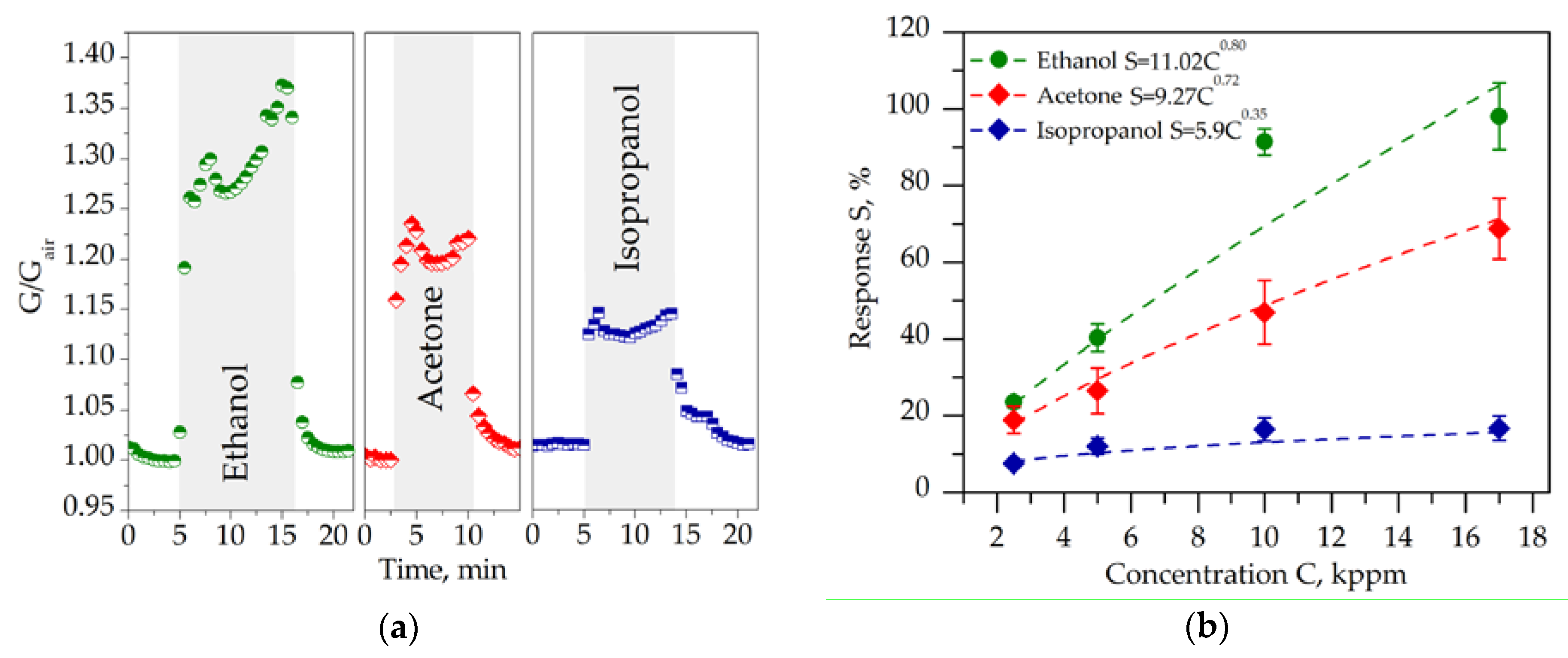
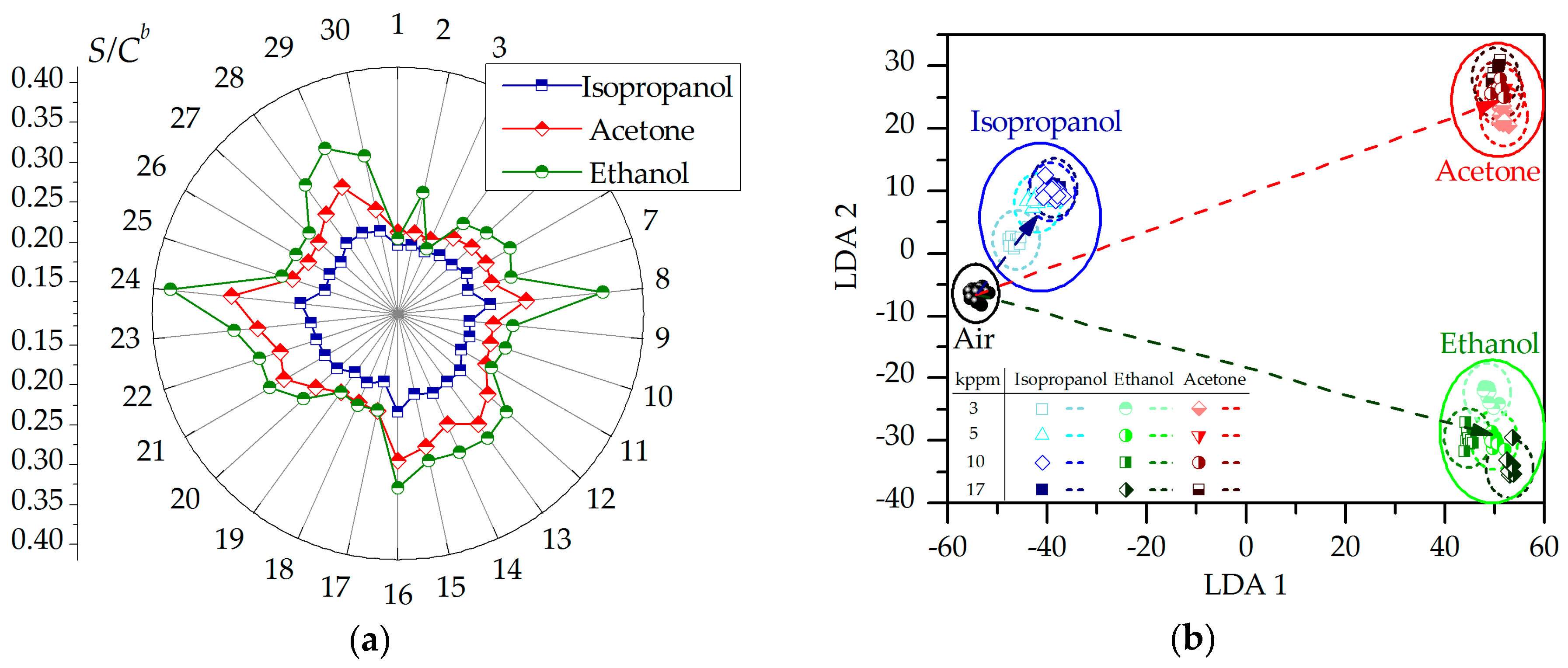
2.2. Chemiresistive Properties of Potassium Titanate Whiskers
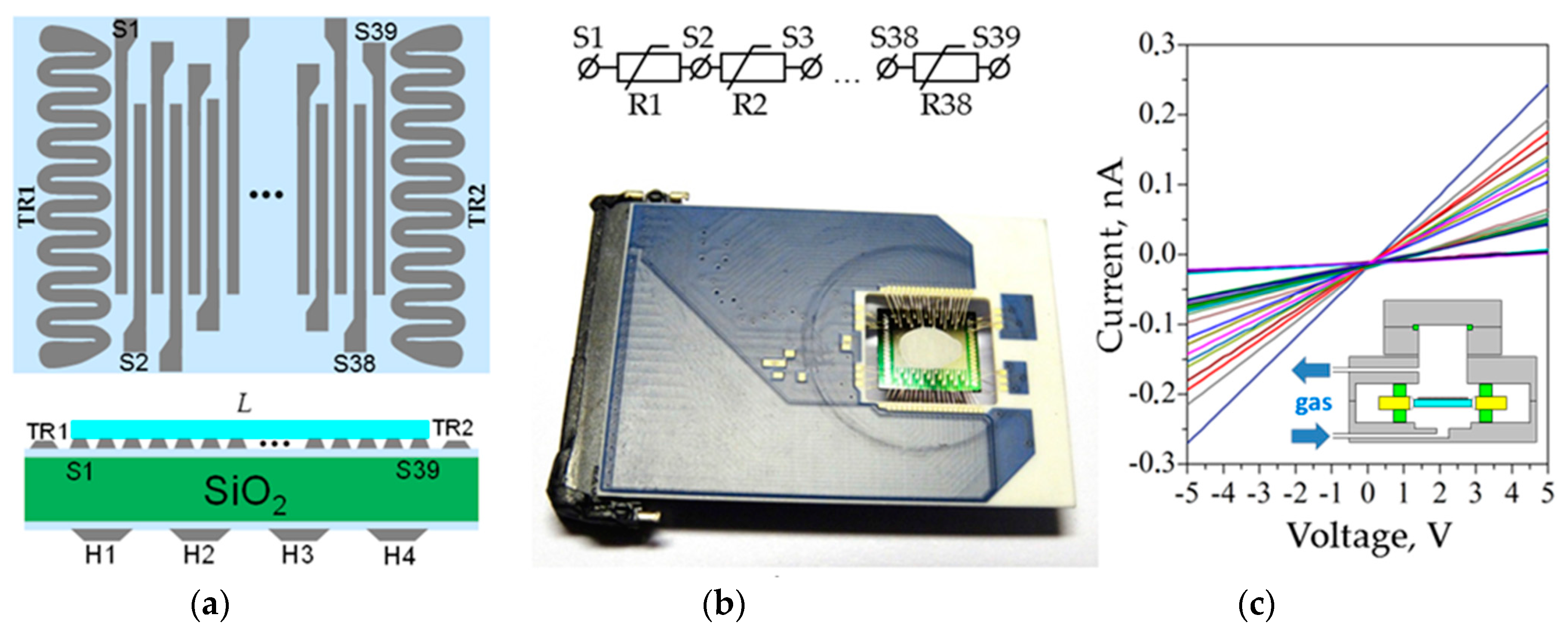
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, J.; Qin, Z.; Zeng, D.; Xie, C. Metal-oxide-semiconductor based gas sensors: Screening, preparation, and integration. Phys. Chem. Chem. Phys. 2017, 19, 6313–6329. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Skouloudis, A.N.; Bell, M.; Viana, M.; Carotta, M.C.; Biskos, G.; Morawska, L. Real-time sensors for indoor air monitoring and challenges ahead in deploying them to urban buildings. Sci. Total Environ. 2016, 560, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Capelli, L.; Sironi, S.; Del Rosso, R. Electronic noses for environmental monitoring applications. Sensors 2014, 14, 19979–20007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korotchenkov, G.; Sysoev, V.V. Conductometric metal oxide gas sensors: Principles of operation and technological approaches to fabrication. In Chemical Sensors: Comprehensive Sensor Technologies; Korotchenkov, G., Ed.; Momentum Press: New York, NY, USA, 2011; Volume 4, pp. 53–186. [Google Scholar]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Semancik, S.; Cavicchi, R.E.; Wheeler, M.C.; Tiffany, J.E.; Poirier, G.E.; Walton, R.M.; Suehle, J.S.; Panchapakesan, B.; Devoe, D.L. Microhotplate platforms for chemical sensor research. Sens. Actuators B 2001, 77, 579–591. [Google Scholar] [CrossRef]
- Rumyantseva, M.; Makeeva, E.; Gaskov, A.; Shepel, N.; Peregudova, S.; Khoroshutin, A.; Tokarev, S.; Fedorova, O. H2S sensing by hybrids based on nanocrystalline SnO2 functionalized with Cu(II) organometallic complexes: The role of the ligand platform. Nanomaterials 2017, 7, 384. [Google Scholar] [CrossRef] [PubMed]
- Comini, E.; Faglia, G.; Sberveglieri, G. UV light activation of tin oxide thin films for NO2 sensing at low temperatures. Sens. Actuators B 2001, 78, 73–77. [Google Scholar] [CrossRef]
- Rackauskas, S.; Barbero, N.; Barolo, C.; Viscardi, G. ZnO nanowire application in chemoresistive sensing: A review. Nanomaterials 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Bayat, B.; Crasta, N.; Crespi, A.; Pascoal, A.M.; Ijspeert, A. Environmental monitoring using autonomous vehicles: A survey of recent searching techniques. Curr. Opin. Biotechnol. 2017, 45, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Meyyappan, M. Carbon nanotube-based chemical sensors. Small 2016, 12, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Chen, G.; Li, Q.; McCreary, A.; Botello-Mendez, A.; Morozov, S.V.; Liang, L.; Declerck, X.; Perea-Lopez, N.; Cullen, D.A.; et al. Ultrasensitive gas detection of large-area boron-doped graphene. Proc. Natl. Acad. Sci. USA 2015, 112, 14527–14532. [Google Scholar] [CrossRef] [PubMed]
- Lipatov, A.; Varezhnikov, A.; Augustin, M.; Bruns, M.; Sommer, M.; Sysoev, V.; Kolmakov, A.; Sinitskii, A. Intrinsic device-to-device variation in graphene field-effect transistors on a Si/SiO2 substrate as a platform for discriminative gas sensing. Appl. Phys. Lett. 2014, 104, 013114. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, H.; Zhang, M.; Zhao, Y.; Qu, L.; Shi, G. Graphene-based smart materials. Nat. Rev. Mater. 2017, 2, 17046. [Google Scholar] [CrossRef]
- Pour, M.M.; Lashkov, A.; Radocea, A.; Liu, X.; Sun, T.; Lipatov, A.; Korlacki, R.A.; Shekhirev, M.; Aluru, N.R.; Lyding, J.W.; et al. Laterally extended atomically precise graphene nanoribbons with improved electrical conductivity for efficient gas sensing. Nat. Commun. 2017, 8, 820. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, Z.; He, Q.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of single-and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small 2012, 8, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.G.; Patel, P.D.; Vaishnav, V.S. Indium tin oxide (ITO) thin film gas sensor for detection of methanol at room temperature. Sens. Actuators B 2003, 96, 180–189. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Li, W.; Gou, X. α-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Law, M.; Kind, H.; Messer, B.; Kim, F.; Yang, P. Photochemical sensing of NO2 with SnO2 nanoribbon nanosensors at room temperature. Angew. Chem. 2002, 114, 2511–2514. [Google Scholar] [CrossRef]
- Wang, J.X.; Sun, X.W.; Yang, Y.; Huang, H.; Lee, Y.C.; Tan, O.K.; Vayssieres, L. Hydrothermally grown oriented ZnO nanorod arrays for gas sensing applications. Nanotechnology 2006, 17, 4995–4998. [Google Scholar] [CrossRef]
- Du, N.; Zhang, H.; Chen, B.; Xiangyang, M.; Liu, Z.; Wu, J.; Yang, D. Porous indium oxide nanotubes: Layer-by-layer assembly on carbon-nanotube templates and application for room-temperature NH3 gas sensors. Adv. Mater. 2007, 19, 1641–1645. [Google Scholar] [CrossRef]
- Buzzeo, M.C.; Hardacre, C.; Compton, R.G. Use of room temperature ionic liquids in gas sensor design. Anal. Chem. 2004, 76, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Harada, T.; Shimizu, Y.; Yamazoe, N. Cordless solid-state hydrogen sensor using proton-conductor thick film. Sens. Actuators B 1990, 1, 125–129. [Google Scholar] [CrossRef]
- Tomita, A.; Namekata, Y.; Nagao, M.; Hibino, T. Room-temperature hydrogen sensors based on an In3+-doped SnP2O7 proton conductor. J. Electrochem. Soc. 2007, 154, J172–J176. [Google Scholar] [CrossRef]
- Strelcov, E.; Dmitriev, S.; Button, B.; Cothren, J.; Sysoev, V.; Kolmakov, A. Evidence of the self-heating effect on surface reactivity and gas sensing of metal oxide nanowire chemiresistors. Nanotechnology 2008, 19, 355502. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.; Hoffmann, M.W.G.; Casals, O.; Mayrhofer, L.; Fabrega, C.; Caccamo, L.; Hernandez-Ramirez, F.; Mohajerani, M.S.; Moseler, M.; Shen, H.; et al. Integrated strategy toward self-powering and selectivity tuning of semiconductor gas sensors. ACS Sens. 2016, 1, 1256–1264. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Huang, J.; Lim, L.Y.; Li, W.; Deng, J.; Gong, D.; Tang, Y.; Lai, Y.; Chen, Z. Titanate and titania nanostructured materials for environmental and energy applications: A review. RSC Adv. 2015, 5, 79479–79510. [Google Scholar] [CrossRef]
- Fedorov, F.S.; Varezhnikov, A.S.; Kiselev, I.; Kolesnichenko, V.V.; Burmistrov, I.N.; Sommer, M.; Fuchs, D.; Kuebel, C.; Gorokhovsky, A.V.; Sysoev, V.V. Potassium polytitanate gas-sensor study by impedance spectroscopy. Anal. Chim. Acta 2015, 897, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shi, Y.; Xue, D. Large aspect ratio titanate nanowire prepared by monodispersed titania submicron sphere via simple wet-chemical reactions. J. Solid State Chem. 2007, 180, 1028–1037. [Google Scholar] [CrossRef]
- Gorokhovsky, A.; Escalante-Garcia, J.; Sanches-Monjares, T.; Vargas-Gutierrez, G. Synthesis of barium titanate powders and coatings by treatment of TiO2 with molten mixtures of Ba(NO3)2, KNO3 and KOH. Mater. Lett. 2004, 58, 2227–2230. [Google Scholar] [CrossRef]
- Liu, C.; Lu, X.; Yu, G.; Feng, X.; Zhang, Q.; Xu, Z. Role of an intermediate phase in solid state reaction of hydrous titanium oxide with potassium carbonate. Mater. Chem. Phys. 2005, 94, 401–407. [Google Scholar] [CrossRef]
- Kang, S.-O.; Jang, H.-S.; Kim, Y.-I.; Kim, K.-B.; Jung, M.-J. Study on the growth of potassium titanate nanostructures prepared by sol-gel-calcination process. Mater. Lett. 2007, 61, 473–477. [Google Scholar] [CrossRef]
- Sanchez-Monjaras, T.; Gorokhovsky, A.; Escalante-Garcia, J.I. Molten salt synthesis and characterization of potassium polytitanate ceramic precursors with varied TiO2/K2O molar ratios. J. Am. Ceram. Soc. 2008, 91, 3058–3065. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, S.; Zhai, L.; Yu, J.; Shi, Y.; Du, Y. A simple molten salt method to synthesize single-crystalline potassium titanate nanobelts. Mater. Lett. 2009, 63, 887–889. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, T.; Zhang, Y. Synthesis of hexatitanate and titanium dioxide fibers by ion-exchange approach. Mater. Res. Bull. 2007, 42, 40–45. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Schneider, T.; Goschnick, J.; Kiselev, I.; Habicht, W.; Hahn, H.; Strelcov, E.; Kolmakov, A. Percolating SnO2 nanowire network as a stable gas sensor: Direct comparison of long-term performance versus SnO2 nanoparticle films. Sens. Actuators B 2009, 139, 699–703. [Google Scholar] [CrossRef]
- Burmistrov, I.N.; Kuznetsov, D.V.; Yudin, A.G.; Muratov, D.S.; Milyaeva, S.I.; Kostitsyn, M.A.; Gorshenkov, M.V. Analysis of the effect of preparation conditions for potassium polytitanates on their morphological properties. Refract. Ind. Ceram. 2012, 52, 393–397. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Z.; Chung, J.S. Formation and structural characterization of potassium titanates and the potassium ion exchange property. Mater. Res. Bull. 2009, 44, 1973–1977. [Google Scholar] [CrossRef]
- Lee, C.-T.; Um, M.-H.; Kumazawa, H. Synthesis of titanate derivatives using ion-exchange reaction. J. Am. Ceram. Soc. 2000, 83, 1098–1102. [Google Scholar] [CrossRef]
- Papp, S.; Korosi, L.; Meynen, V.; Cool, P.; Vansant, E.F.; Dekany, I. The influence of temperature on the structural behaviour of sodium tri- and hexa-titanates and their protonated forms. J. Solid State Chem. 2005, 178, 1614–1619. [Google Scholar] [CrossRef]
- Dumont, O.S.; Reckhard, H. Phase diagrams for ceramists. In Applications of Phase Diagrams in Metallurgy and Ceramics; Levin, E.M., Robbins, C., Mcmurdie, H.F., Eds.; American Ceramic Society: Columbus, OH, US, 1964; Volume 2, p. 87. [Google Scholar]
- Wen, P.; Itoh, H.; Tang, W.; Feng, Q. Transformation of layered titanate nanosheets into nanostructured porous titanium dioxide in polycation solution. Microporous Mesoporous Mater. 2008, 116, 147–156. [Google Scholar] [CrossRef]
- Marchand, R.; Brohan, L.; M’Bedi, R.; Tounoux, M. Hydrolysis—Pyrolysis process applied to K2Ti4O9. Rev. Chim. Miner. 1984, 21, 476–486. [Google Scholar]
- Du, G.H.; Chen, Q.; Han, P.D.; Yu, Y.; Peng, L.M. Potassium titanate nanowires: Structure, growth, and optical properties. Phys. Rev. B 2003, 67, 353231–353237. [Google Scholar] [CrossRef]
- Xu, C.-Y.; Liu, Y.-Z.; Zhen, L.; Wang, Z.L. Disket-nanorings of K2Ti6O13 formed by self-spiraling of a nanobelt. J. Phys. Chem. C 2008, 112, 7547–7551. [Google Scholar] [CrossRef]
- Bao, N.; Feng, X.; Shen, L.; Lu, X. Calcination syntheses of a series of potassium titanates and their morphologic evolution. Cryst. Growth Des. 2002, 2, 437–442. [Google Scholar] [CrossRef]
- Kukovecz, A.; Kordas, K.; Kiss, J.; Konya, Z. Atomic scale characterization and surface chemistry of metal modified titanate nanotubes and nanowires. Surf. Sci. Rep. 2016, 71, 473–546. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Goschnick, J.; Schneider, T.; Strelcov, E.; Kolmakov, A. A gradient microarray electronic nose based on percolating SnO2 nanowire sensing elements. Nano Lett. 2007, 7, 3182–3188. [Google Scholar] [CrossRef] [PubMed]
- Go, J.; Sysoev, V.V.; Kolmakov, A.; Pimparkar, N.; Alam, M.A. A novel model for (percolating) nanonet chemical sensors for microarray-based E-nose application. In Proceedings of the 2009 IEEE International Electron Devices Meeting (IEDM), Baltimore, MD, USA, 7–9 December 2009. [Google Scholar]
- Sysoev, V.V.; Kiselev, I.; Trouillet, V.; Bruns, M. Enhancing the gas selectivity of single-crystal SnO2: Pt thin-film chemiresistor microarray by SiO2 membrane coating. Sens. Actuators B 2013, 185, 59–69. [Google Scholar] [CrossRef]
- Kiselev, I.; Sommer, M.; Mann, J.K.; Sysoev, V.V. Employment of electric potential to build a gas-selective response of metal oxide gas sensor array. IEEE Sens. J. 2010, 10, 849–855. [Google Scholar] [CrossRef]
- Goschnick, J. An electronic nose for intelligent consumer products based on a gas analytical gradient microarray. Microelectron. Eng. 2001, 57–58, 693–704. [Google Scholar] [CrossRef]
- Burmistrov, I.N.; Varezhnikov, A.S.; Musatov, V.Y.; Lashkov, A.V.; Gorokhovsky, A.V.; Yudinceva, T.I.; Sysoev, V.V. Room temperature gas sensing with potassium titanate nanowires. In Proceedings of the 2015 IEEE Sensors, Busan, Korea, 1–4 November 2015; pp. 1593–1596. [Google Scholar]
- Fedorov, F.; Podgainov, D.; Varezhnikov, A.; Lashkov, A.; Gorshenkov, M.; Burmistrov, I.; Sommer, M.; Sysoev, V. The potentiodynamic bottom-up growth of the tin oxide nanostructured layer for gas-analytical multisensor array chips. Sensors 2017, 17, 1908. [Google Scholar] [CrossRef] [PubMed]
- Sysoev, V.V.; Strelcov, E.; Sommer, M.; Bruns, M.; Kiselev, I.; Habicht, W.; Kar, S.; Gregoratti, L.; Kiskinova, M.; Kolmakov, A. Single-nanobelt electronic nose: Engineering and tests of the simplest analytical element. ACS Nano 2017, 4, 4487–4494. [Google Scholar] [CrossRef] [PubMed]
- Hierlemann, A.; Gutierrez-Osuna, R. Higher-order chemical sensing. Chem. Rev. 2008, 108, 563–613. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varezhnikov, A.S.; Fedorov, F.S.; Burmistrov, I.N.; Plugin, I.A.; Sommer, M.; Lashkov, A.V.; Gorokhovsky, A.V.; Nasibulin, A.G.; Kuznetsov, D.V.; Gorshenkov, M.V.; et al. The Room-Temperature Chemiresistive Properties of Potassium Titanate Whiskers versus Organic Vapors. Nanomaterials 2017, 7, 455. https://doi.org/10.3390/nano7120455
Varezhnikov AS, Fedorov FS, Burmistrov IN, Plugin IA, Sommer M, Lashkov AV, Gorokhovsky AV, Nasibulin AG, Kuznetsov DV, Gorshenkov MV, et al. The Room-Temperature Chemiresistive Properties of Potassium Titanate Whiskers versus Organic Vapors. Nanomaterials. 2017; 7(12):455. https://doi.org/10.3390/nano7120455
Chicago/Turabian StyleVarezhnikov, Alexey S., Fedor S. Fedorov, Igor N. Burmistrov, Ilya A. Plugin, Martin Sommer, Andrey V. Lashkov, Alexander V. Gorokhovsky, Albert G. Nasibulin, Denis V. Kuznetsov, Michail V. Gorshenkov, and et al. 2017. "The Room-Temperature Chemiresistive Properties of Potassium Titanate Whiskers versus Organic Vapors" Nanomaterials 7, no. 12: 455. https://doi.org/10.3390/nano7120455