Pure and Highly Nb-Doped Titanium Dioxide Nanotubular Arrays: Characterization of Local Surface Properties
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiu, J.; Isoda, S.; Wang, F.; Adachi, M. Dye-sensitized solar cells based on a single-crystalline TiO2 nanorod. J. Phys. Chem. B 2006, 110, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Varghese, K.; Paulose, M.; Grimes, C.A. Long vertically aligned titania nanotubes on transparent conducting oxide for highly efficient solar cells. Nat. Nanotechnol. 2009, 4, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Vomiero, A.; Concina, I.; Braga, A.; Brisotto, M.; Bontempi, E.; Faglia, G.; Sberveglieri, G. Vertically aligned TiO2 nanotubes on plastic substrates for flexible solar cells. Small 2011, 7, 2437–2442. [Google Scholar] [CrossRef] [PubMed]
- Yuxiang, Y.; Dongsheng, X. Single-crystalline TiO2 nanorods: Highly active and easily recycled photocatalysts. Appl. Catal. B Environ. 2007, 73, 166–171. [Google Scholar]
- Kang, A.-Z.; Xu, Z.; Song, Y.; Mu, J. Photocatalytic activity of high aspect ratio TiO2 nanorods. J. Dispers. Sci. Technol. 2006, 27, 857–859. [Google Scholar] [CrossRef]
- Akbar, S.A.; Younkman, L.B. Sensing mechanism of a carbon monoxide sensor based on anatase titania. J. Electrochem. Soc. 1997, 144, 1750–1753. [Google Scholar] [CrossRef]
- Harris, L.A. A titanium-dioxide hydrogen detector. J. Electrochem. Soc. 1980, 127, 2657–2662. [Google Scholar] [CrossRef]
- Satake, K.; Katayama, A.; Ohkoshi, H.; Nakahara, T.; Takeuchi, T. Titania NOx sensors for exhaust monitoring. Sens. Actuators B Chem. 1994, 20, 111–117. [Google Scholar] [CrossRef]
- Comini, E.; Sberveglieri, G.; Ferroni, M.; Guidi, V.; Martinelli, G. Response to ethanol of thin films based on Mo and Ti oxides deposited by sputtering. Sens. Actuators B Chem. 2003, 93, 409–415. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Baratto, C.; Ponzoni, A.; Ferroni, M.; Poli, N.; Faglia, G.; Bontempi, E.; Brisotto, M.; Sberveglieri, G. Large surface area biphase titania for chemical sensing. Sens. Actuators B Chem. 2015, 209, 1091–1096. [Google Scholar] [CrossRef]
- Du, G.H.; Chen, Q.; Che, R.C.; Yuan, Z.Y.; Peng, L.M. Preparation, structure analysis of titanium oxide nanotubes. Appl. Phys. Lett. 2001, 79, 3702–3704. [Google Scholar] [CrossRef]
- Alessandri, I.; Comini, E.; Bontempi, E.; Faglia, G.; Depero, L.E.; Sberveglieri, G. Cr-inserted TiO2 thin films for chemical gas sensors. Sens. Actuators B Chem. 2007, 128, 312–319. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, J.B.; Jia, Y.C.; Xiao, P.; Tang, J. TiO2 nanotube array sensor for detecting the SF6 decomposition product SO2. Sensors 2012, 12, 3302–3313. [Google Scholar] [CrossRef] [PubMed]
- Körner, W.; Elsässer, C. Density functional theory study of dopants in polycrystalline TiO2. Phys. Rev. B 2011, 83, 205315. [Google Scholar] [CrossRef]
- Sotter, E.; Vilanova, X.; Llobet, E.; Stankova, M.; Correig, X. Niobium-doped titania nanopowders for gas sensor applications. J. Optoel. Adv. Mater. 2005, 7, 1395–1398. [Google Scholar]
- Edelman, F.; Hahn, H.; Seifried, S.; Alof, C.; Hoche, H.; Balogh, A.; Werner, P.; Zakrzewska, K.; Radecka, M.; Pasierb, P.; et al. Structural evolution of SnO2-TiO2 nanocrystalline films for gas sensors. Mater. Sci. Eng. B 2000, 69–70, 386–391. [Google Scholar] [CrossRef]
- Lee, H.; Leu, I.C.; Hsu, M.C.; Chung, Y.W.; Hon, M.H. Fabrication of aligned TiO2 one-dimensional nanostructured arrays using a one-step templating solution approach. J. Phys. Chem. B 2005, 109, 13056–13059. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, P. Formation of titanium dioxide nanotube array. Langmuir 1996, 12, 1411–1413. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 Nanotubes: Recent advances in synthesis and gas sensing properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Comini, E.; Vomiero, A.; Ponzoni, A.; Concina, I.; Brisotto, M.; Bontempi, E.; Faglia, G.; Sberveglieri, G. Fabrication of pure and Nb-TiO2 nanotubes and their functional properties. J. Alloys Comp. 2012, 536, S488–S490. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Vomiero, A.; Borgese, L.; Bontempi, E.; Sberveglieri, G. Fabrication and investigation of gas sensing properties of Nb-doped TiO2 nanotubular arrays. Nanotechnology 2012, 23, 235706. [Google Scholar] [CrossRef] [PubMed]
- Comini, E.; Galstyan, V.; Faglia, G.; Bontempi, E.; Sberveglieri, G. Highly conductive titanium oxide nanotubes chemical sensors. Microporous Mesoporous Mater. 2015, 208, 165–170. [Google Scholar] [CrossRef]
- Kwoka, M.; Ottaviano, L.; Szuber, J. Photoemission studies of the surface electronic properties of L-CVD SnO2 ultra thin films. Appl. Surf. Sci. 2012, 258, 8425–8429. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Q.; Gao, S.; Shang, J. Synthesis and characterization of niobium-doped TiO2 nanotube arrays by anodization of Ti-20Nb alloys. J. Mater. Sci. Technol. 2012, 28, 865–870. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sool, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer: Eden Prairie, MN, USA, 1992; ISBN 0962702625. [Google Scholar]
- Watts, J.F.; Wolstenholme, J. An Introduction to Surface Analysis by XPS and AES; John Wiley & Sons: Chichester, UK, 2003; ISBN 978-0-470-84713-8. [Google Scholar]
- Surface Analysis—The Principal Techniques, 2nd ed; Vickerman, J.C.; Gilmore, I.S. (Eds.) John Wiley & Sons: Chichester, UK, 2009; ISBN 978-0-470-01763-0. [Google Scholar]
- Mahajan, V.K.; Misra, M.; Raja, K.S.; Mohapatra, S.K. Self-organized TiO2 nanotubular arrays for photoelectrochemical hydrogen generation: Effect of crystallization and defect structures. J. Phys. D Appl. Phys. 2008, 41, 125307–125315. [Google Scholar] [CrossRef]
- Mohanta, R.R.; Medicherla, V.R.R.; Mohanta, K.L.; Nayak, N.C.; Majumder, S.; Solanki, V.; Varma, S.; Bapna, K.; Phase, D.M.; Sathe, V. Ion beam induced chemical and morphological changes in TiO2 films deposited on Si(111) surface by pulsed laser deposition. Appl. Surf. Sci. 2015, 325, 185–191. [Google Scholar] [CrossRef]
- Kondalkar, V.V.; Mali, S.S.; Pawar, N.B.; Mane, R.M.; Choudhury, S.; Hong, C.K.; Patil, P.S.; Patil, S.R.; Bhosalea, P.N.; Kim, J.H. Microwave-assisted rapid synthesis of highly porous TiO2 thin films with nanocrystalline framework for efficient photoelectrochemical conversion. Electrochim. Acta 2014, 143, 89–97. [Google Scholar] [CrossRef]
- Gao, Y.-F.; Masuda, Y.; Koumoto, K. Light-Excited superhydrophilicity of amorphous TiO2 thin films deposited in an aqueous peroxotitanate solution. Langmuir 2004, 20, 3188–3194. [Google Scholar] [CrossRef] [PubMed]
- Chennakesavulu, K.; Reddy, G.R.; Prasath, S.S.; Supriya, S.; Sivanesan, S. Visible light driven photocatalytic degradation of the reactive red-198, methylene blue and 3-chlorophenol by Nb2O5@ZnO: Synthesis and characterization. Adv. Mater. Lett. 2015, 6, 518–526. [Google Scholar] [CrossRef]
- Martinez-Mendez, S.; Henriquez, Y.; Dominguez, O.; D’Ornelas, L.; Krentzien, H. Catalytic properties of silica supported titanium, vanadium and niobium oxide nanoparticles towards the oxidation of saturated and unsaturated hydrocarbons. J. Mol. Catal. A Chem. 2006, 252, 226–234. [Google Scholar] [CrossRef]
- Tucker, R.T.; Beckers, N.A.; Fleischauer, M.D.; Brett, M.J. Electron beam deposited Nb-doped TiO2 toward nanostructured transparent conductive thin films. Thin Solid Films 2012, 525, 28–34. [Google Scholar] [CrossRef]
- Antony, R.P.; Mathews, T.; Dash, S.; Tyagi, A.K.; Raj, B. X-ray photoelectron spectroscopic studies of anodically synthesized self aligned TiO2 nanotube arrays and the effect of electrochemical parameters on tube morphology. Mater. Chem. Phys. 2012, 132, 957–966. [Google Scholar] [CrossRef]
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R.J. NIST X-ray Photoelectron Spectroscopy Database; NIST NSRDS: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Cottineau, T.; Bealu, N.; Gross, P.-A.; Pronkin, S.N.; Keller, N.; Savinova, E.R.; Keller, V. One step synthesis of niobium doped titania nanotube arrays to form (N, Nb) co-doped TiO2 with high visible light hotoelectrochemical activity. J. Mater. Chem. A 2013, 1, 2151–2160. [Google Scholar] [CrossRef]
- Ohtsu, N.; Masahashi, N.; Mizukoshi, Y. Angle resolved XPS studies on an anodic oxide formed on Ti–Nb–Sn alloy and the photo-induced change in carbon contaminants adsorbed on its surface. Appl. Surf. Sci. 2012, 258, 6052–6055. [Google Scholar] [CrossRef]
- Atashbar, M.Z.; Sun, H.T.; Gong, B.; Wlodarski, W.; Lamb, R. XPS study of Nb-doped oxygen sensing TiO2 thin films prepared by sol-gel method. Thin Solid Films 1998, 326, 238–244. [Google Scholar] [CrossRef]
- Karthick, S.N.; Prabakar, K.; Subramania, A.; Hong, J.-T.; Jang, J.-J.; Kim, H.-J. Formation of anatase TiO2 nanoparticles by simple polymer gel technique and their properties. Powder Technol. 2011, 205, 36–41. [Google Scholar] [CrossRef]
- Kwoka, M.; Ottaviano, L.; Passacantando, M.; Santucci, S.; Szuber, J. XPS depth profiling studies of L-CVD SnO2 thin films. Appl. Surf. Sci. 2006, 252, 7730–7733. [Google Scholar] [CrossRef]
- Alam, A.U.; Howlader, M.M.R.; Deen, M.J. Oxygen plasma and humidity dependent surface analysis of silicon, silicon dioxide and glass for direct wafer bonding. ECS J. Solid State Sci. Technol. 2013, 2, P515–P523. [Google Scholar] [CrossRef]
- Sitarz, M.; Kwoka, M.; Comini, E.; Zappa, D.; Szuber, J. Surface chemistry of the SnO2 nanowires on Ag catalyst-covered Si substrate studied by XPS and TDS methods. Nanoscale Res. Lett. 2014, 9, 43. [Google Scholar] [CrossRef] [PubMed]
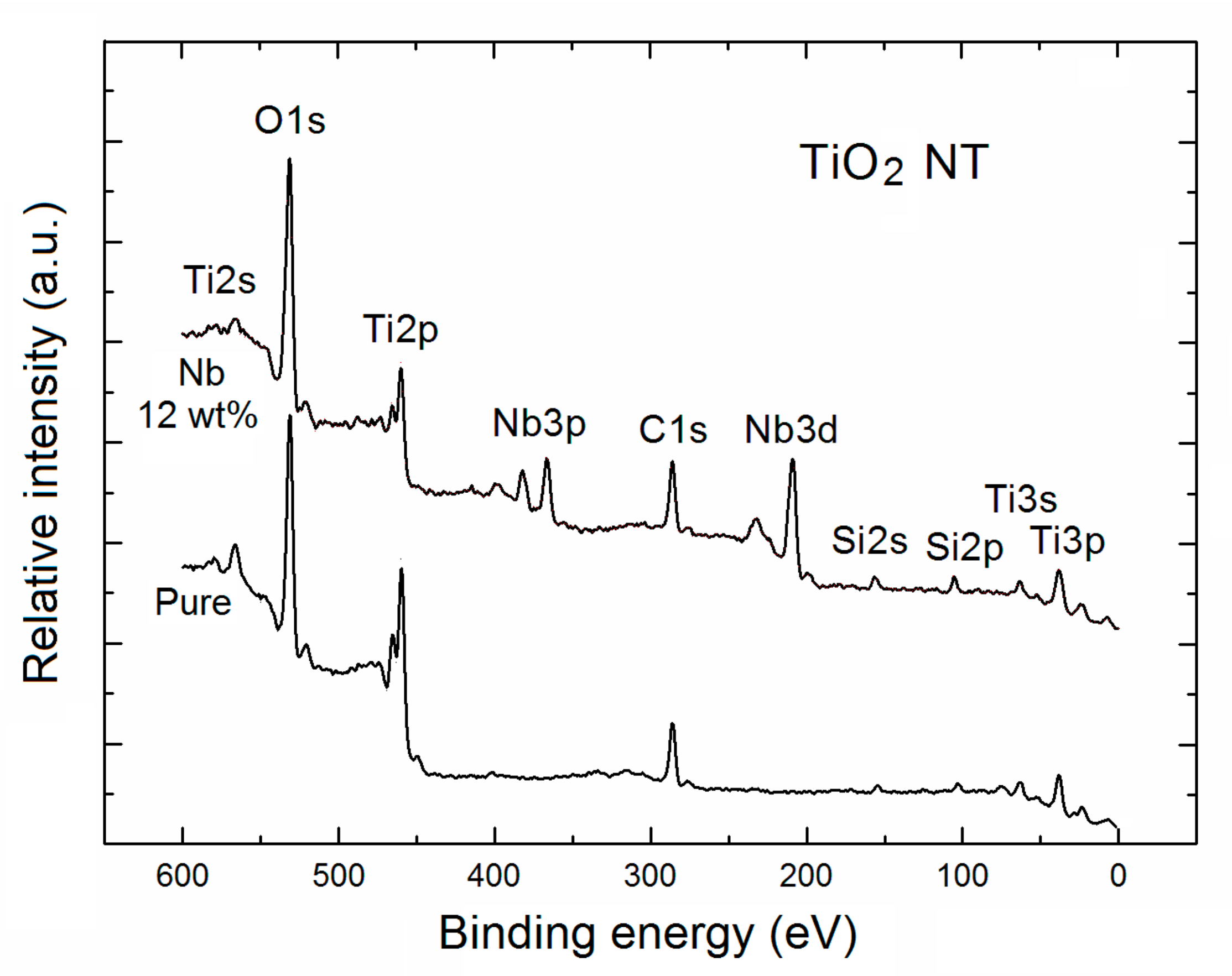
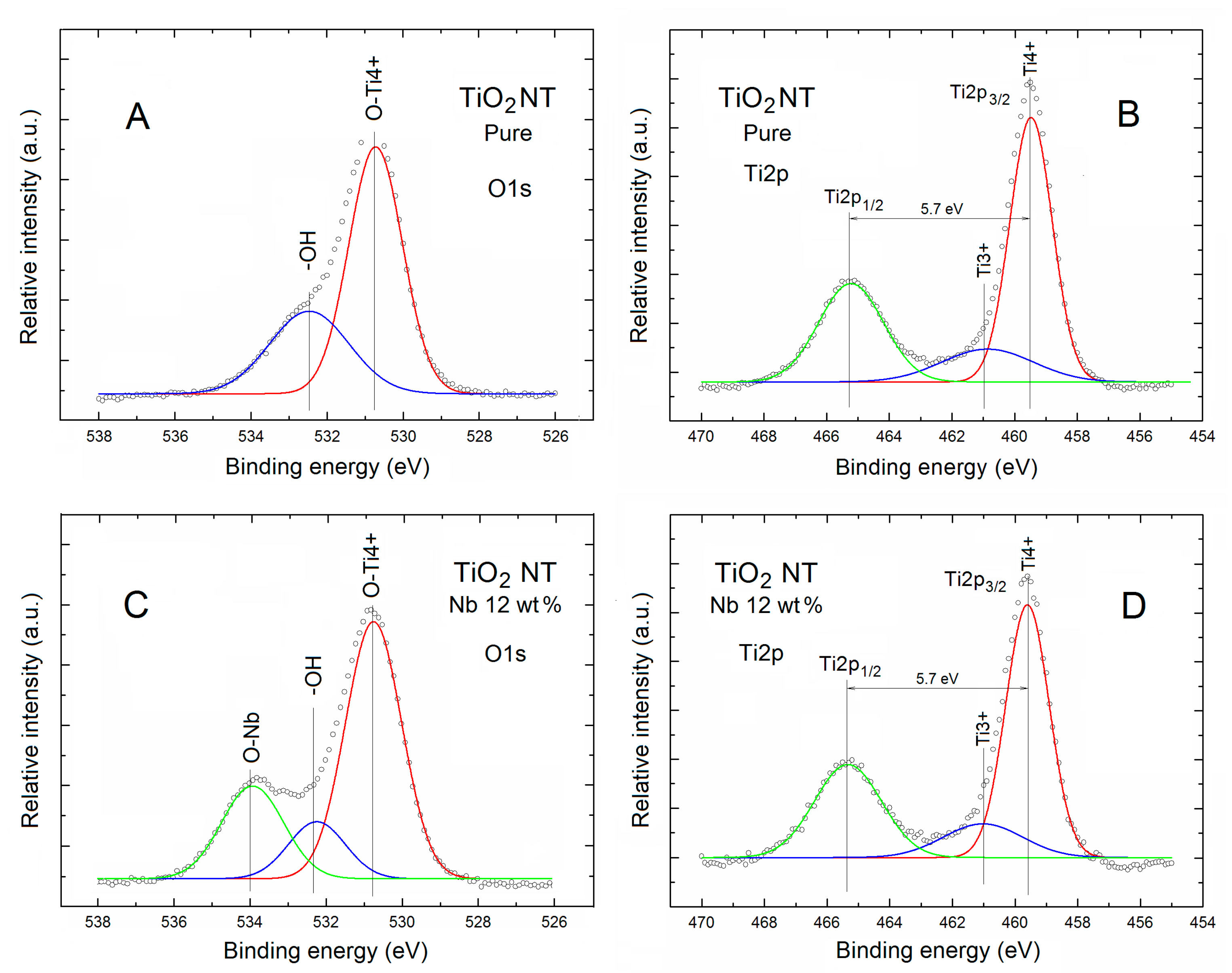
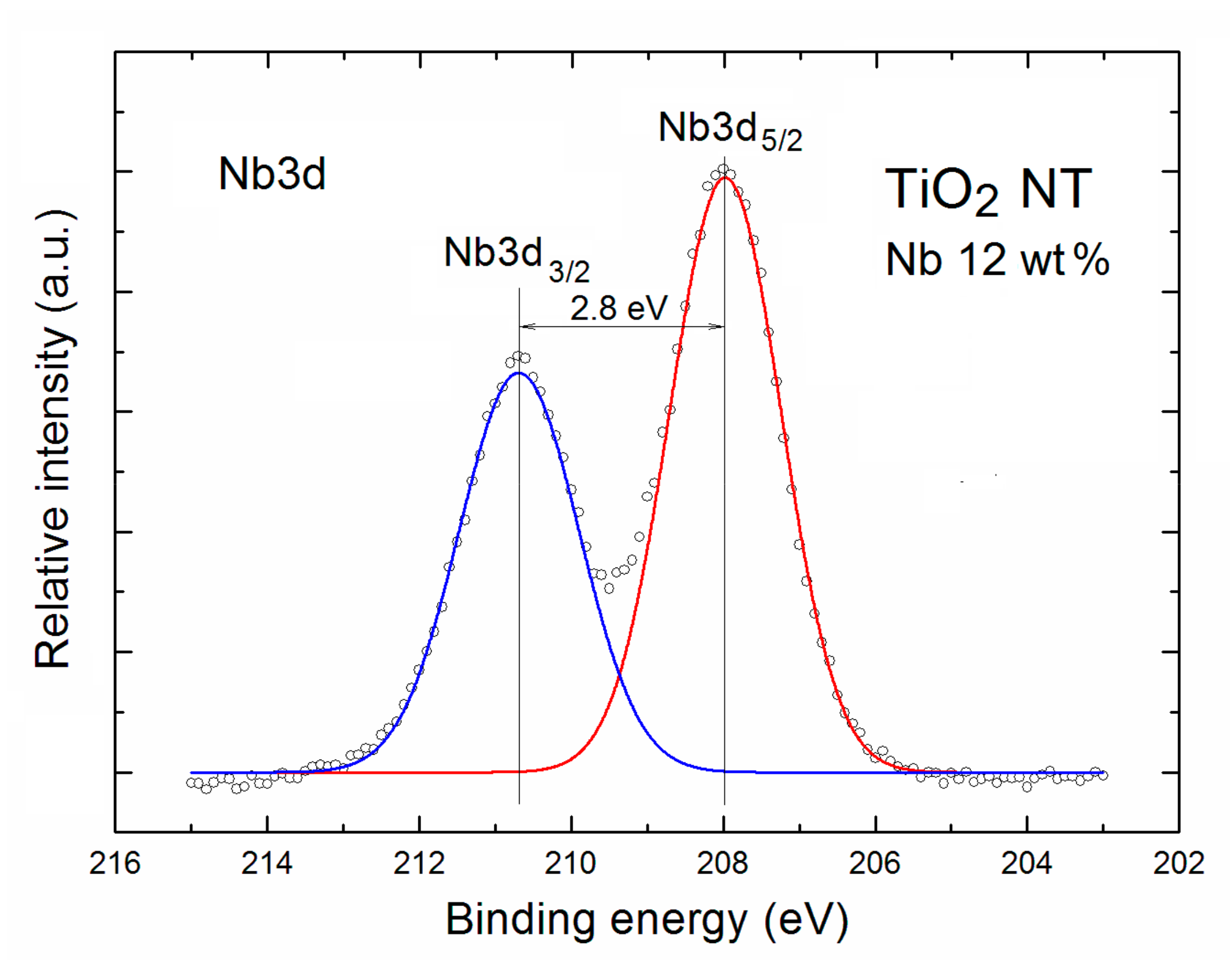
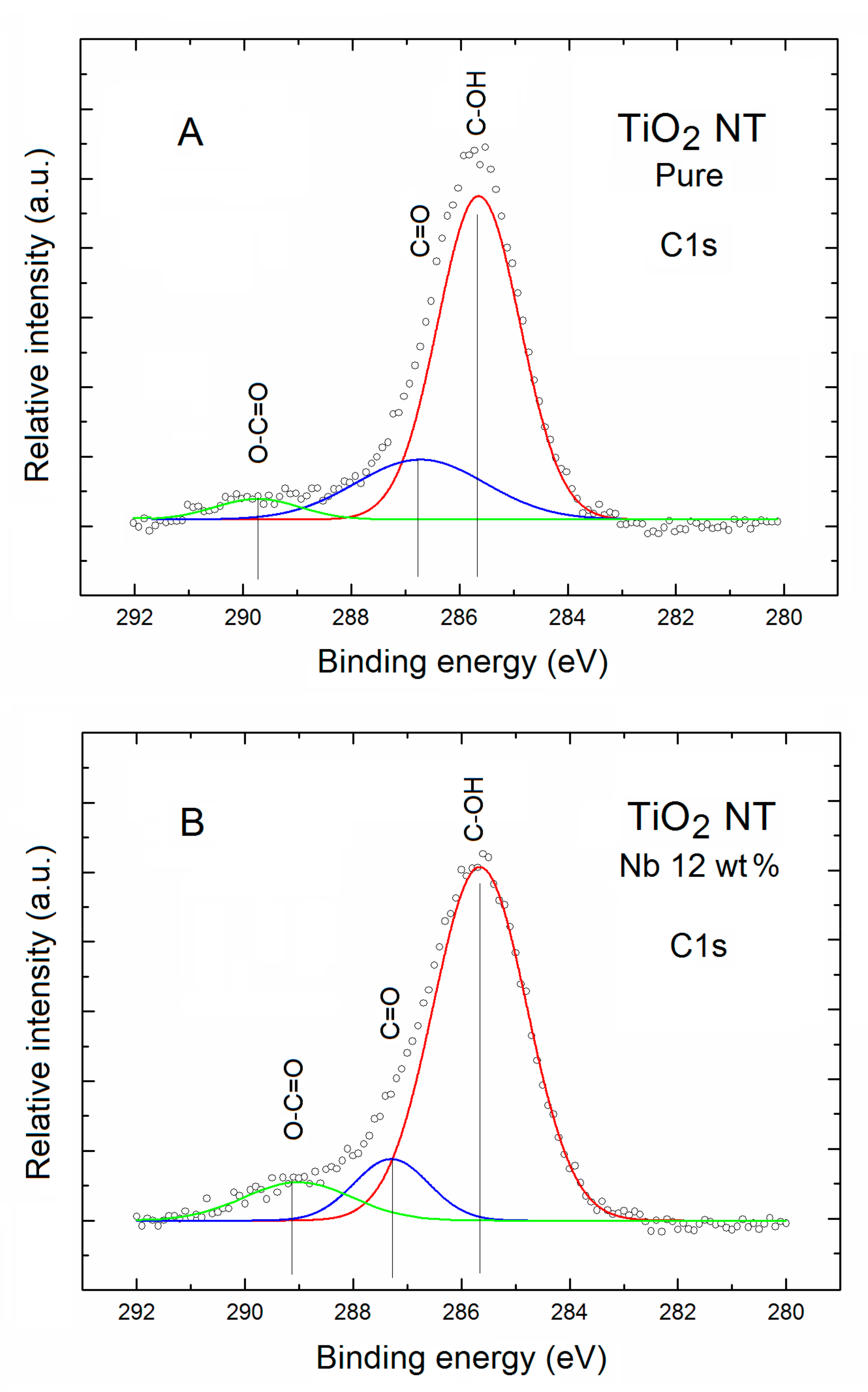


| TNT | Relative Concentration of Specific Elements with Respect to All the Surface Atoms | Partial Surface Concentration of Specific Elements (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| O | Ti | Nb | C | O | Ti | Nb | C | |
| Pure | 0.46 | 0.18 | 0 | 0.36 | 46 | 18 | 0 | 36 |
| Nb-doped | 0.42 | 0.12 | 0.09 | 0.36 | 42 | 12 | 9 | 36 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwoka, M.; Galstyan, V.; Comini, E.; Szuber, J. Pure and Highly Nb-Doped Titanium Dioxide Nanotubular Arrays: Characterization of Local Surface Properties. Nanomaterials 2017, 7, 456. https://doi.org/10.3390/nano7120456
Kwoka M, Galstyan V, Comini E, Szuber J. Pure and Highly Nb-Doped Titanium Dioxide Nanotubular Arrays: Characterization of Local Surface Properties. Nanomaterials. 2017; 7(12):456. https://doi.org/10.3390/nano7120456
Chicago/Turabian StyleKwoka, Monika, Vardan Galstyan, Elisabetta Comini, and Jacek Szuber. 2017. "Pure and Highly Nb-Doped Titanium Dioxide Nanotubular Arrays: Characterization of Local Surface Properties" Nanomaterials 7, no. 12: 456. https://doi.org/10.3390/nano7120456







