Fabrication of GO/Cement Composites by Incorporation of Few-Layered GO Nanosheets and Characterization of Their Crystal/Chemical Structure and Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Preparation of GO Nanosheets Suspension
2.3. Planning and Preparation of GO/Cement Composites
2.4. Test Methods
3. Results and Discussion
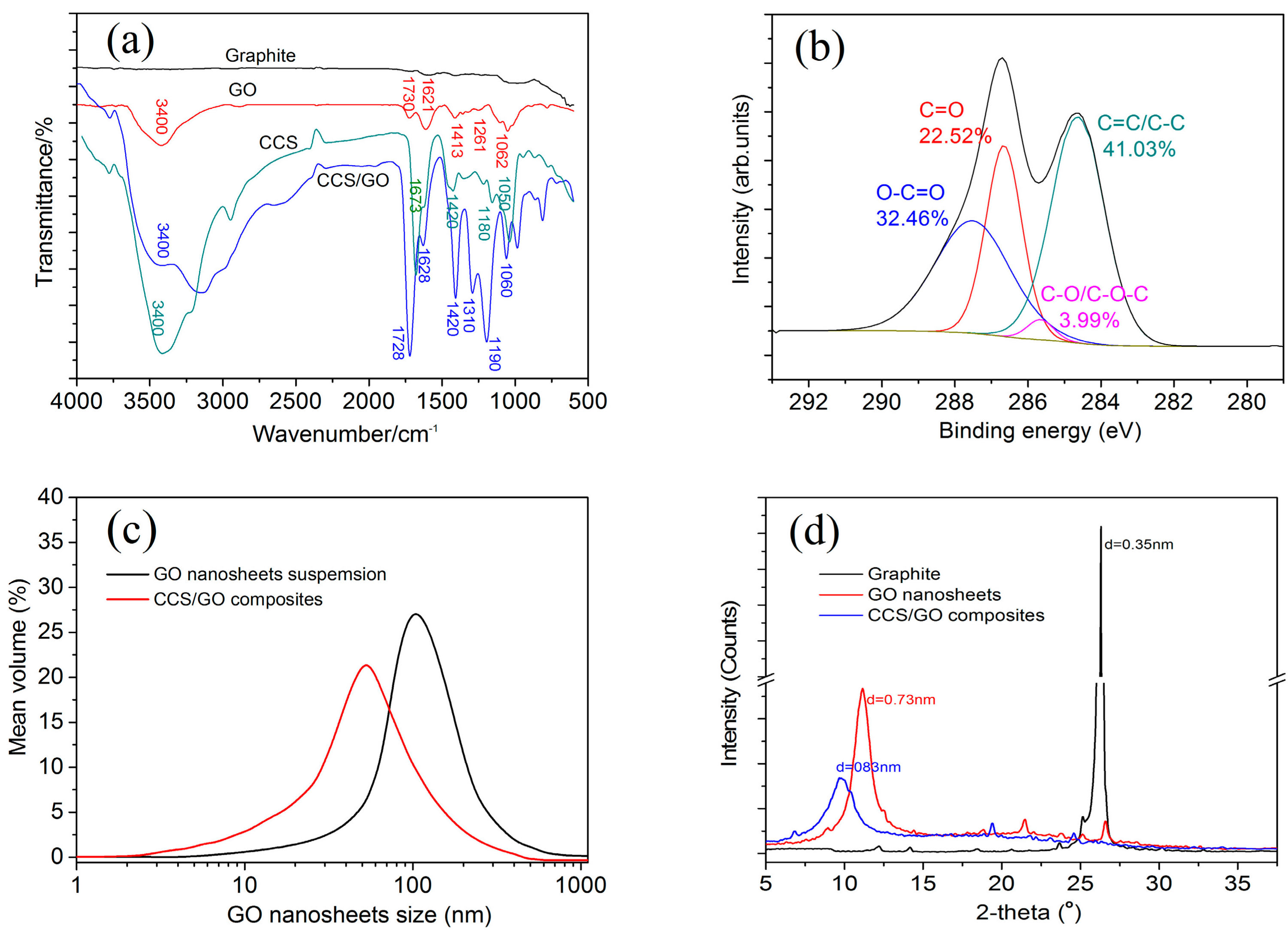
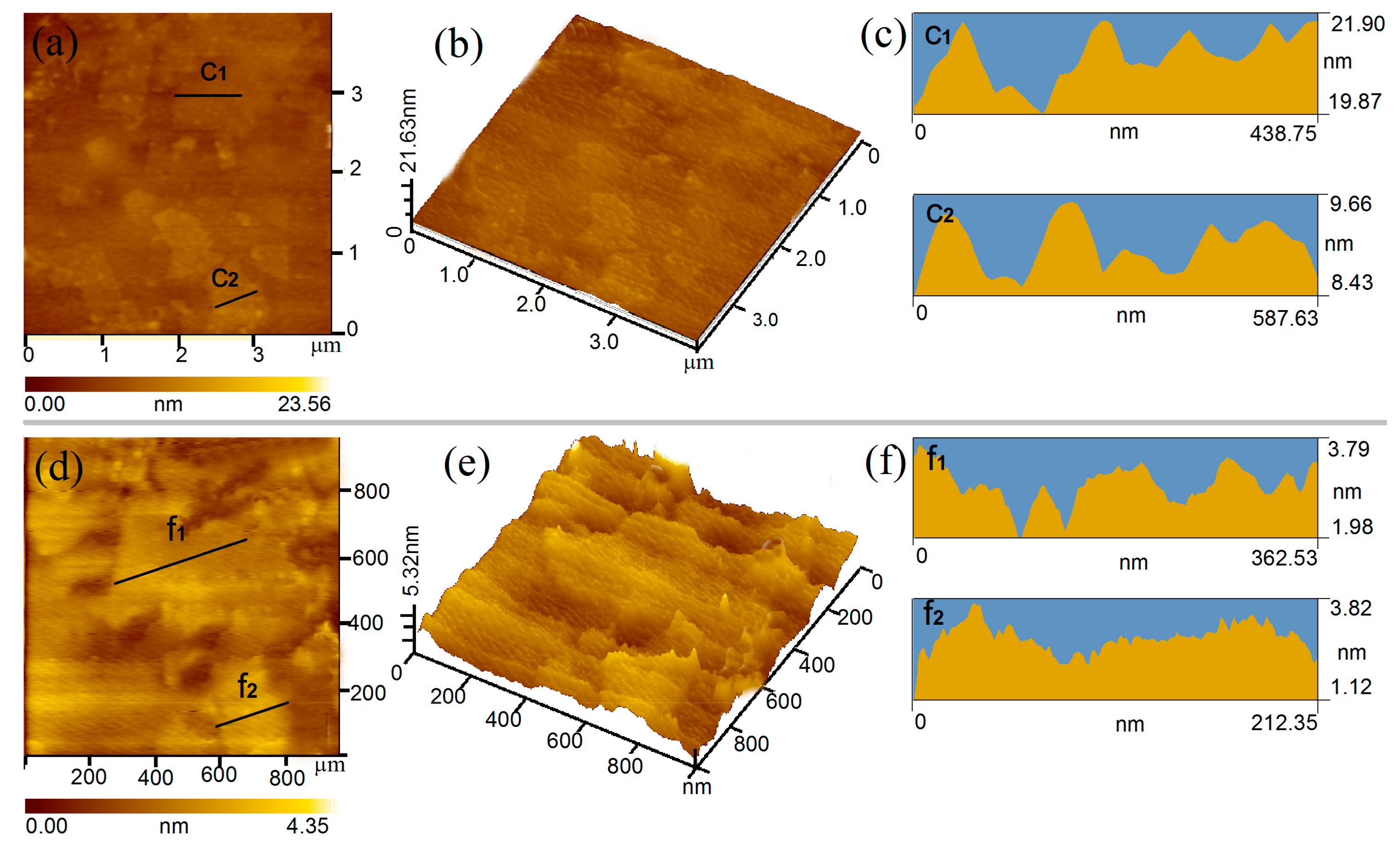
3.1. Chemical Structure and Microstructure of GO Nanosheets
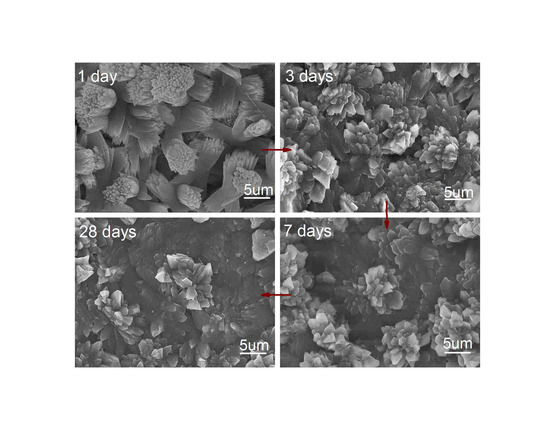
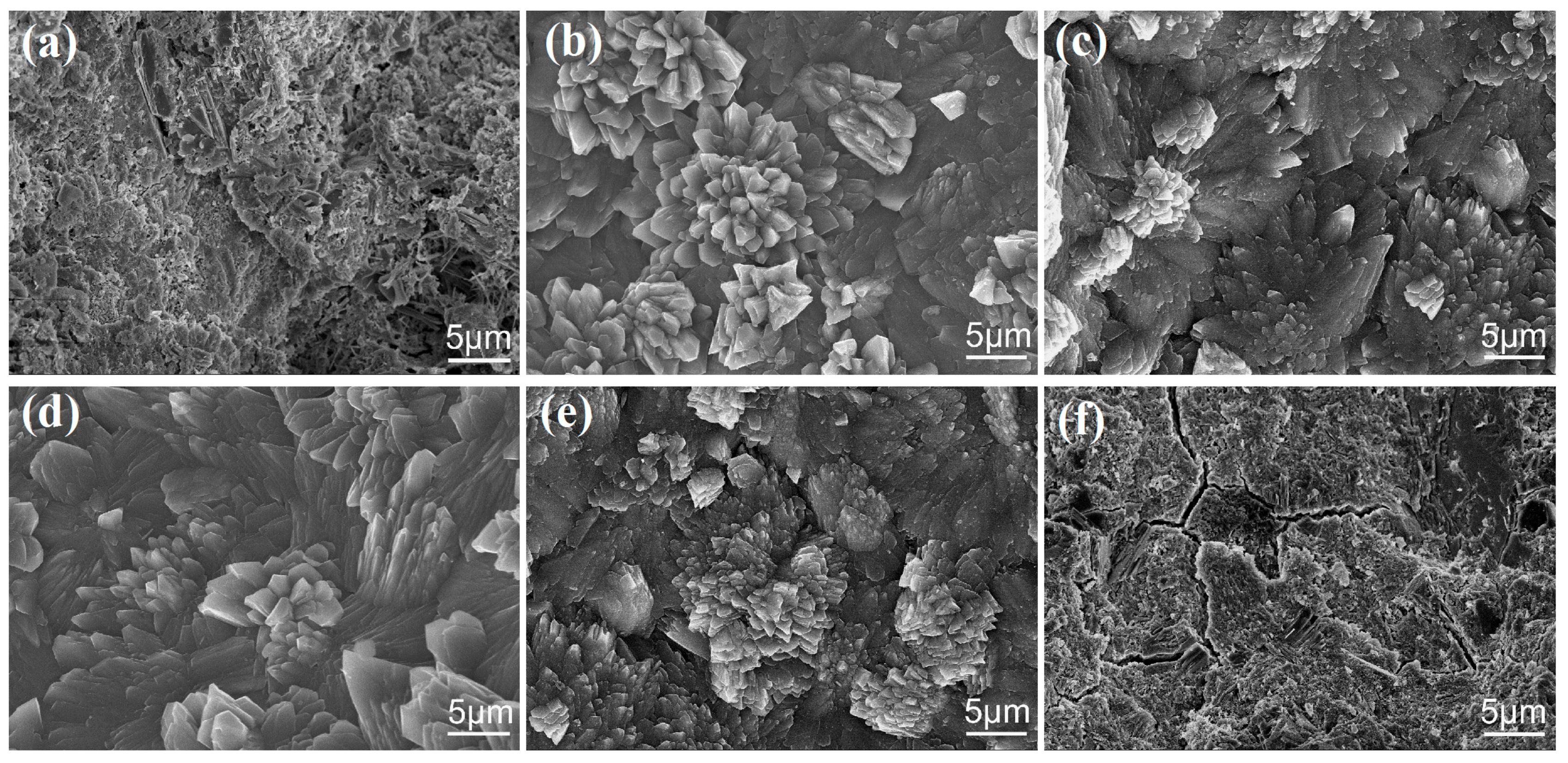
3.2. Microstructure of Cement Composites
3.3. Crystal Structure of Cement Hydration Crystals
3.4. Formation Mechanism of Cement Hydration Crystals and Ordered Microstructure
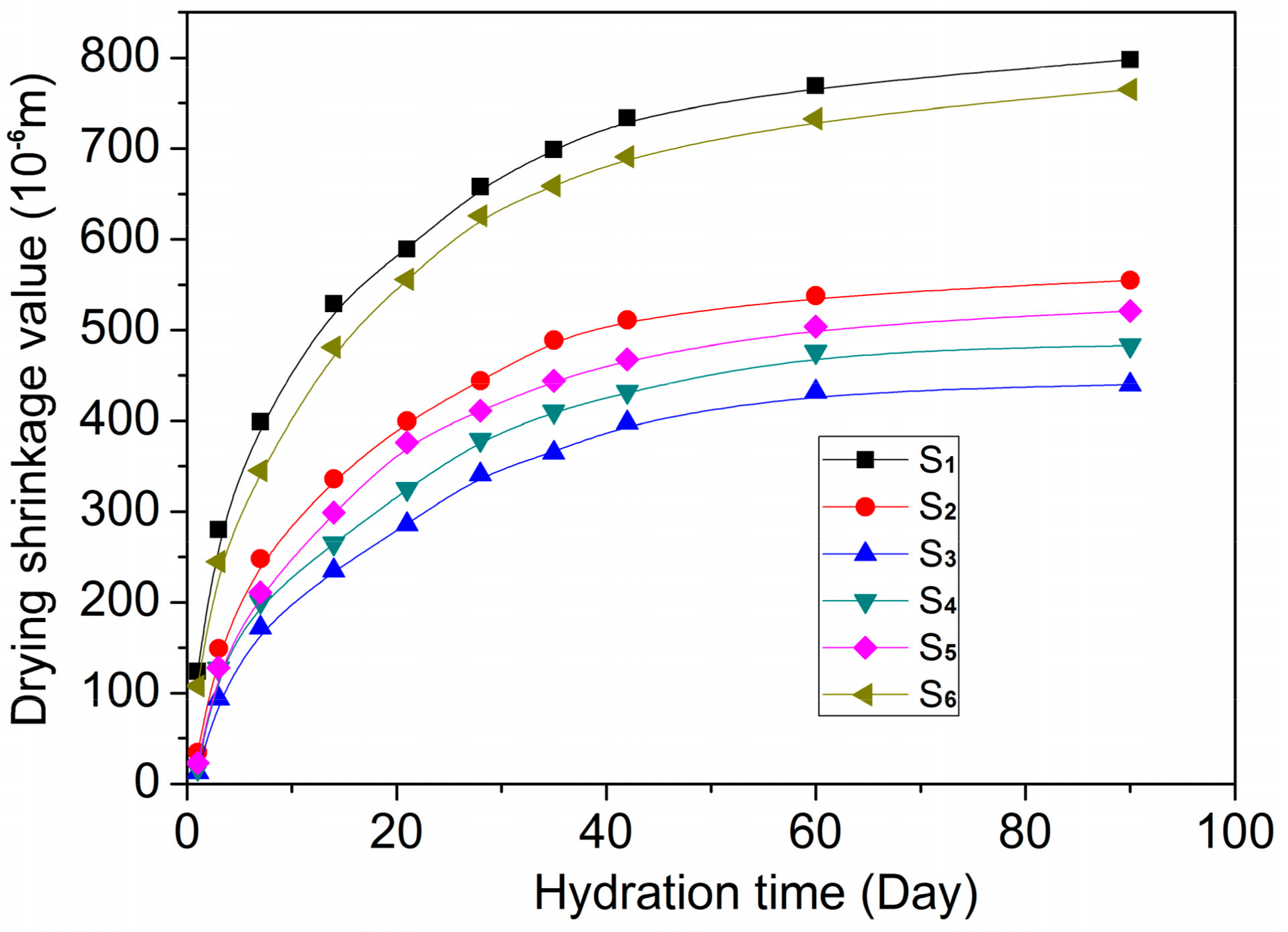
3.5. Mechanical Properties and Durability Parameters of GO/Cement Composites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bishop, M.; Bott, S.G.; Barron, A.R. A new mechanism for cement hydration Inhibition: Solid-state chemistry of calcium nitrilotris (methylene) triphosphonate. Chem. Mater. 2003, 15, 3074–3088. [Google Scholar] [CrossRef]
- Lu, X.L.; Ye, Z.M.; Zhang, L.; Hou, P.; Cheng, X. The influence of ethanol-diisopropanolamine on the hydration and mechanical properties of Portland cement. Constr. Build. Mater. 2017, 135, 484–489. [Google Scholar] [CrossRef]
- Kadam, M.P.; Patil, Y.D. Strength, Durability and Micro Structural Properties of Concrete Incorporating MS and GCBA as Sand Substitute. J. Sci. Ind. Res. India 2017, 76, 644–653. [Google Scholar]
- Yoo, D.Y.; Kim, S.; Park, G.J.; Park, J.J.; Kim, S.W. Effects of fiber shape, aspect ratio and volume fraction on flexural behavior of ultra-high-performance fiber-reinforced cement composites. Compos. Struct. 2017, 174, 375–388. [Google Scholar] [CrossRef]
- Zegardlo, B.; Szelag, M.; Ogrodnik, P. Ultra-high strength concrete made with recycled aggregate from sanitary ceramic wastes. The method of production and the interfacial transition zone. Constr. Build. Mater. 2016, 122, 736–742. [Google Scholar] [CrossRef]
- Ganesh, P.; Murthy, A.R.; Kumar, S.S. Effect of nanosilica on durability and mechanical properties of high-strength concrete. Mag. Concr. Res. 2016, 68, 229–236. [Google Scholar] [CrossRef]
- Chithra, S.; Kumar, S.R.S.; Chinnaraju, K. The effect of colloidal nano-silica on workability, mechanical and durability properties of high performance concrete with copper slag as partial fine aggregate. Constr. Build. Mater. 2016, 113, 794–804. [Google Scholar] [CrossRef]
- Atmaca, N.; Abbas, M.L.; Atmaca, A. Effects of nano-silica on the gas permeability, durability and mechanical properties of high-strength lightweight concrete. Constr. Build. Mater. 2017, 147, 17–26. [Google Scholar] [CrossRef]
- Asgari, H.; Ramezanianpour, A.; Butt, H.J. Effect of water and nano-silica solution on the early stages cement hydration. Constr. Build. Mater. 2016, 129, 11–24. [Google Scholar] [CrossRef]
- Papatzani, S. Effect of nanosilica and montmorillonite nanoclay particles on cement hydration and microstructure. Mater. Sci. Technol. 2016, 32, 138–153. [Google Scholar] [CrossRef]
- Ronquillo, G.J.B.; Madlangbayan, M.S.; Ignacio, M.C.C.D. Morphological and characterization-based verification of the properties of concrete with amorphous nanosilica synthesized from rice hull ash. Asia Life Sci. 2016, 25, 311–324. [Google Scholar]
- Sutan, N.M.; Yakub, I.; Jaafar, M.S. Sustainable nanopozzolan modified cement: Characterizations and morphology of calcium silicate hydrate during hydration. J. Nanomater 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Shaikh, F.U.A.; Supit, S.W.M. Mechanical and durability properties of high volume fly ash (HVFA) concrete containing calcium carbonate (CaCO3) nanoparticles. Constr. Build. Mater. 2014, 70, 309–321. [Google Scholar] [CrossRef]
- Mang, C.; Jason, L.; Davenne, L. Crack opening estimate in reinforced concrete walls using a steel–concrete bond model. Arch. Civ. Mech. Eng. 2016, 16, 422–436. [Google Scholar] [CrossRef]
- Han, J.D.; Sun, W.; Pan, G.H. Monitoring the evolution of accelerated carbonation of hardened cement pastes by X-ray computed tomography. J. Mater. Civ. Eng. 2013, 25, 347–354. [Google Scholar] [CrossRef]
- Benabed, B.; Kadri, E.H.; Azzouz, L.; Kenai, S. Properties of self-compacting mortar made with various types of sand. Cem. Concr. Comp. 2012, 10, 1167–1173. [Google Scholar] [CrossRef]
- Alyhya, W.S.; Kulasegaram, S.; Karihaloo, B.L. Simulation of the flow of self-compacting concrete in the V-funnel by SPH. Cem. Concr. Res. 2017, 100, 47–59. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Jiao, Y. Quantitative analysis of concrete property under effects of crack, freeze-thaw and carbonation. Constr. Build. Mater. 2016, 129, 106–115. [Google Scholar] [CrossRef]
- Jalal, M.; Mansouri, E.; Sharifipour, M. Mechanical, rheological, durability and microstructural properties of high performance self-compacting concrete containing SiO2 micro and nanoparticles. Mater. Des. 2012, 34, 389–400. [Google Scholar] [CrossRef]
- Zhang, M.H.; Li, H. Pore structure and chloride permeability of concrete containing nano-particles for pavement. Constr. Build. Mater. 2011, 25, 608–616. [Google Scholar] [CrossRef]
- Ghafari, E.; Ghahari, S.A.; Costa, H.; Julio, E.; Portugal, A.; Duraes, L. Effect of supplementary cementitious materials on autogenous shrinkage of ultra-high performance concrete. Constr. Build. Mater. 2016, 127, 43–48. [Google Scholar] [CrossRef]
- Sasi, E.A.; Peled, A. Three dimensional (3D) fabrics as reinforcements for cement-based composites. Compos. Part A Appl. Sci. Manuf. 2015, 74, 153–165. [Google Scholar] [CrossRef]
- Afroughsabet, V.; Biolzi, L.; Ozbakkaloglu, T. Influence of double hooked-end steel fibers and slag on mechanical and durability properties of high performance recycled aggregate concrete. Compos. Struct. 2017, 181, 273–284. [Google Scholar] [CrossRef]
- Kurad, R.; Silvestre, J.D.; Brito, J.D.; Ahmed, H. Effect of incorporation of high volume of recycled concrete aggregates and fly ash on the strength and global warming potential of concrete. J. Clean. Prod. 2017, 166, 485–502. [Google Scholar] [CrossRef]
- Soltan, D.G.; das Neves, P.; Olvera, A.; Savastano, H.; Li, V.C. Introducing a curaua fiber reinforced cement-based composite with strain-hardening behavior. Ind. Crops Prod. 2017, 103, 1–12. [Google Scholar] [CrossRef]
- Lago, B.D.; Taylor, S.E.; Deegan, P.; Ferrara, L.; Sonebi, M.; Crosset, P.; Pattarini, A. Full-scale testing and numerical analysis of a precast fibre reinforced self-compacting concrete slab pre-stressed with basalt fibre reinforced polymer bars. Compos. Part B Eng. 2017, 128, 120–133. [Google Scholar] [CrossRef]
- Lv, S.H.; Ma, Y.; Qiu, C.; Sun, T.; Liu, J.; Zhou, Q. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Lv, S.H.; Sun, T.; Liu, J.J.; Zhou, Q.F. Use of graphene oxide nanosheets to regulate the microstructure of hardened cement paste to increase its strength and toughness. CrystEngComm 2014, 16, 8508–8516. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Li, X.; Lu, Z.; Chuah, S.; Li, W.; Li, Z. Effects of graphene oxide aggregates on hydration degree, sorptivity and tensile splitting strength of cement paste. Compos. Part A Appl. Sci. Manuf. 2017, 100, 1–8. [Google Scholar] [CrossRef]
- Chu, H.; Jiang, J.; Sun, W.; Zhang, M. Effects of graphene sulfonate nanosheets on mechanical and thermal properties of sacrificial concrete during high temperature exposure. Cem. Concr. Comp. 2017, 82, 252–264. [Google Scholar] [CrossRef]
- Dubey, N.; Rajan, S.S.; Bello, Y.D.; Min, K.S.; Rosa, V. Graphene Nanosheets to Improve Physico-Mechanical Properties of Bioactive Calcium Silicate Cements. Materials 2017, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, S.P.; Duffour, N.T.; Skipper, M.; Billing, Y.B. An investigation into the colloidal stability of graphene oxide nano-layers in alite paste. Cem. Concr. Res. 2017, 99, 116–128. [Google Scholar] [CrossRef]
- Cui, H.; Yan, X.; Tang, L.; Xing, F. Possible pitfall in sample preparation for SEM analysis-A discussion of the paper “Fabrication of polycarboxylate/graphene oxide nanosheet composites by copolymerization for reinforcing and toughening cement composites” by Lv et al. Cem. Concre. Comp. 2017, 77, 81–85. [Google Scholar] [CrossRef]
- Mokhtar, M.M.; Abo-El-Enein, S.A.; Hassaan, M.Y.; Morsy, M.S.; Khalil, M.H. Mechanical performance, pore structure and micro-structural characteristics of graphene oxide nano platelets reinforced cement. Constr. Build. Mater. 2017, 138, 333–339. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, R.; Wu, Z. Investigation of the mechanical properties and microstructure of graphene nanoplatelet-cement composite. Nanomaterials 2016, 6, 200. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.; Duarte, P.; Barbosa, D.; Ferreira, I. New composite of natural hydraulic lime mortar with graphene oxide. Constr. Build. Mater. 2017, 156, 1150–1157. [Google Scholar] [CrossRef]
- Bastos, G.; Patiño-Barbeito, F.; Armesto, J. Nano-Inclusions Applied in Cement-Matrix Composites: A Review. Materials 2016, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Dong, O. Properties of Cement mortar and ultra-high strength concrete incorporating graphene oxide nanosheets. Nanomaterials 2017, 7, 187. [Google Scholar]
- Lv, S.H.; Zhang, J.; Zhu, L.L.; Jia, C.M. Preparation of cement composites with ordered microstructures via doping with graphene oxide nanosheets and an investigation of their strength and durability. Materials 2016, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.H.; Yang, W.Q.; Zhao, H.R.; Zhou, Q.F.; Cui, Y.Y. Effect of polyacrylate/GO nanocomposites on properties of cement paste. Mag. Concr. Res. 2016, 68, 187–196. [Google Scholar] [CrossRef]
- Lv, S.H.; Deng, L.J.; Yang, W.Q.; Zhou, Q.F.; Cui, Y.Y. Fabrication of polycarboxylate/graphene oxide nanosheet composites by copolymerization for reinforcing and toughening cement composites. Cem. Concr. Comp. 2016, 66, 1–9. [Google Scholar] [CrossRef]
- Appelo, C.A.J. Solute transport solved with the Nernst-Planck equation for concrete pores with ‘free’ water and a double layer. Cem. Concr. Res. 2017, 101, 102–113. [Google Scholar] [CrossRef]
- Gorzelańczyk, T.; Hoła, J. Pore structure of self-compacting concretes made using different superplasticizers. Arch. Civ. Mech. Eng. 2011, 11, 611–621. [Google Scholar] [CrossRef]


| Chemical Components | Content (%) | Mineral Compositions | Content (%) | |
|---|---|---|---|---|
| Calcium oxide (CaO) | 63.15 | Tricalcium silicate (C3S, 3CaO·SiO2) | 50.65 | |
| Silicon dioxide (SiO2) | 21.21 | Dicalcium silicate (C2S, 2CaO·SiO2) | 20.32 | |
| luminum oxide (Al2O3) | 6.35 | Dicalcium aluminate (C3A, 3CaO·Al2O3) | 15.63 | |
| Ferric oxide (Fe2O3) | 3.34 | Tetracalciumaluminoferrite (C4AF, 4CaO Al2O3·Fe2O3) | 8.35 | |
| Alkalis (Na2O equivalent) | 1.45 | Gypsum (SCH2, CaSO4·2H2O) | 4.27 | |
| Magnesium oxide (MgO) | 2.75 | f-CaO | 0.78 | |
| Potassium oxide (K2O) | 1.22 | |||
| Sulfur trioxide (SO3) | 0.53 | |||
| Samples | Component and Composition (Weight) | Density (g/cm3) | Strength (MPa) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cement | Sand | Water | PCs | CCS | GO | Compressive | Flexural | ||
| S1 | 100 | 0 | 20 | 0.6 | 1.0 | 0 | 2.26 | 116.53 | 10.68 |
| S2 | 100 | 0 | 20 | 0.6 | 1.0 | 0.03 | 2.28 | 149.36 | 22.82 |
| S3 | 100 | 0 | 20 | 0.6 | 1.0 | 0.05 | 2.29 | 175.64 | 31.67 |
| S4 | 100 | 0 | 20 | 0.6 | 1.0 | 0.07 | 2.31 | 166.23 | 26.38 |
| S5 | 100 | 80 | 20 | 0.6 | 1.0 | 0.05 | 2.35 | 155.46 | 29.65 |
| S6 | 100 | 80 | 20 | 0.6 | 1.0 | 0 | 2.33 | 128.63 | 21.26 |
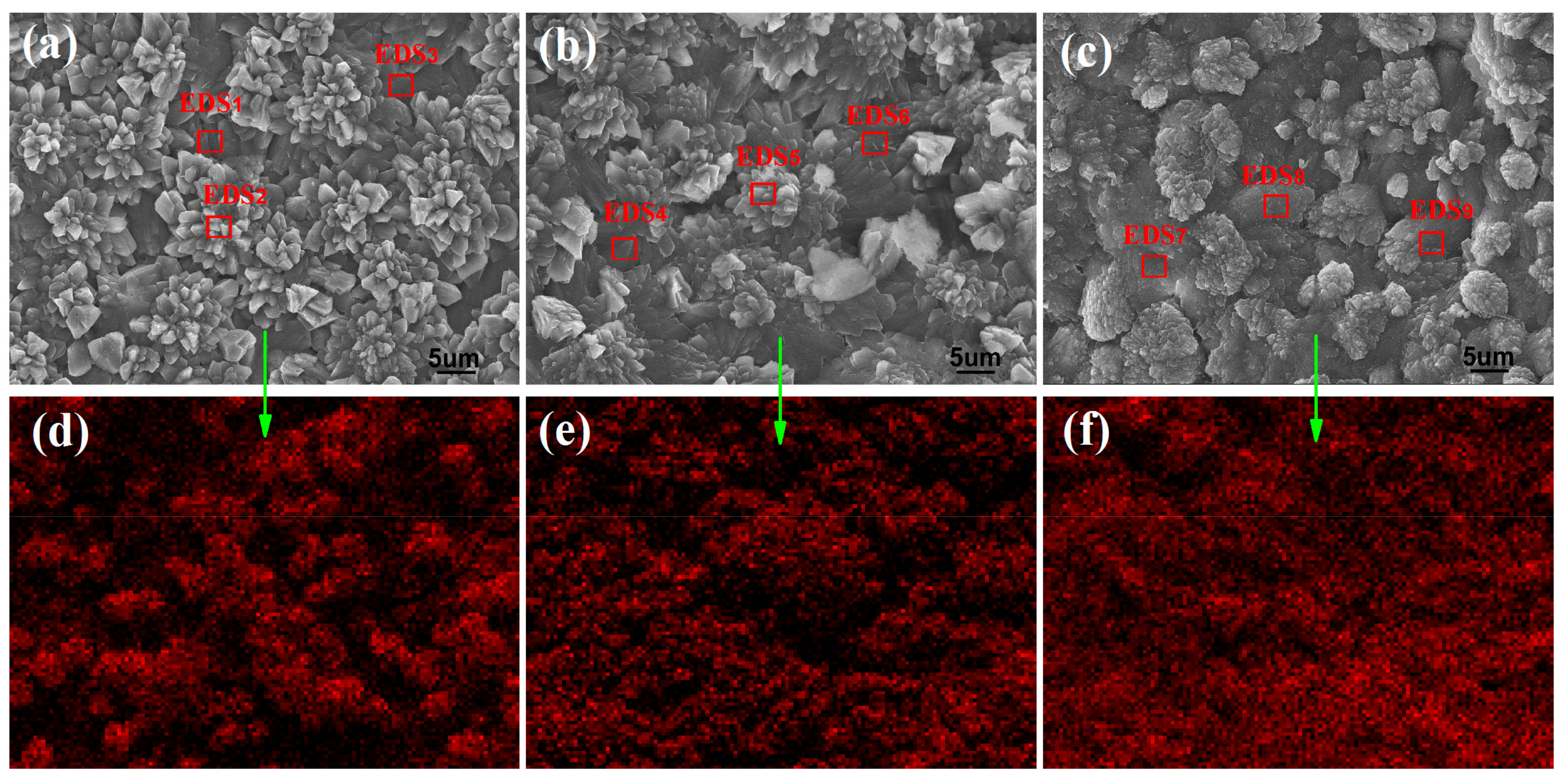
| Samples | Elemental Content (wt %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Si | Ca | Al | Mg | Na | K | Fe | S | |
| Cement | 1.34 | 34.83 | 9.63 | 44.31 | 3.52 | 1.68 | 1.13 | 0.95 | 2.38 | 0.23 |
| DES1 | 4.28 | 39.56 | 2.26 | 46.08 | 2.94 | 1.69 | 1.02 | 0.94 | 0.97 | 0.26 |
| EDS2 | 5.63 | 38.54 | 2.51 | 44.91 | 3.41 | 1.65 | 1.15 | 0.65 | 1.32 | 0.23 |
| EDS3 | 4.56 | 37.56 | 2.89 | 45.62 | 3.52 | 1.68 | 1.24 | 1.34 | 1.32 | 0.27 |
| EDS4 | 6.45 | 41.32 | 2.81 | 42.45 | 2.61 | 1.56 | 1.38 | 0.81 | 0.44 | 0.17 |
| EDS5 | 5.52 | 42.39 | 3.21 | 43.42 | 1.42 | 1.62 | 1.25 | 0.64 | 0.34 | 0.19 |
| EDS6 | 6.65 | 40.49 | 3.21 | 42.39 | 2.56 | 1.78 | 1.12 | 0.78 | 0.81 | 0.21 |
| DES7 | 9.85 | 40.42 | 2.91 | 40.71 | 1.95 | 1.62 | 1.13 | 0.35 | 0.85 | 0.21 |
| EDS8 | 10.23 | 40.56 | 2.43 | 40.15 | 2.86 | 1.45 | 1.15 | 0.27 | 0.63 | 0.27 |
| EDS9 | 9.94 | 40.55 | 2.35 | 40.81 | 1.98 | 1.68 | 1.35 | 0.35 | 0.76 | 0.23 |
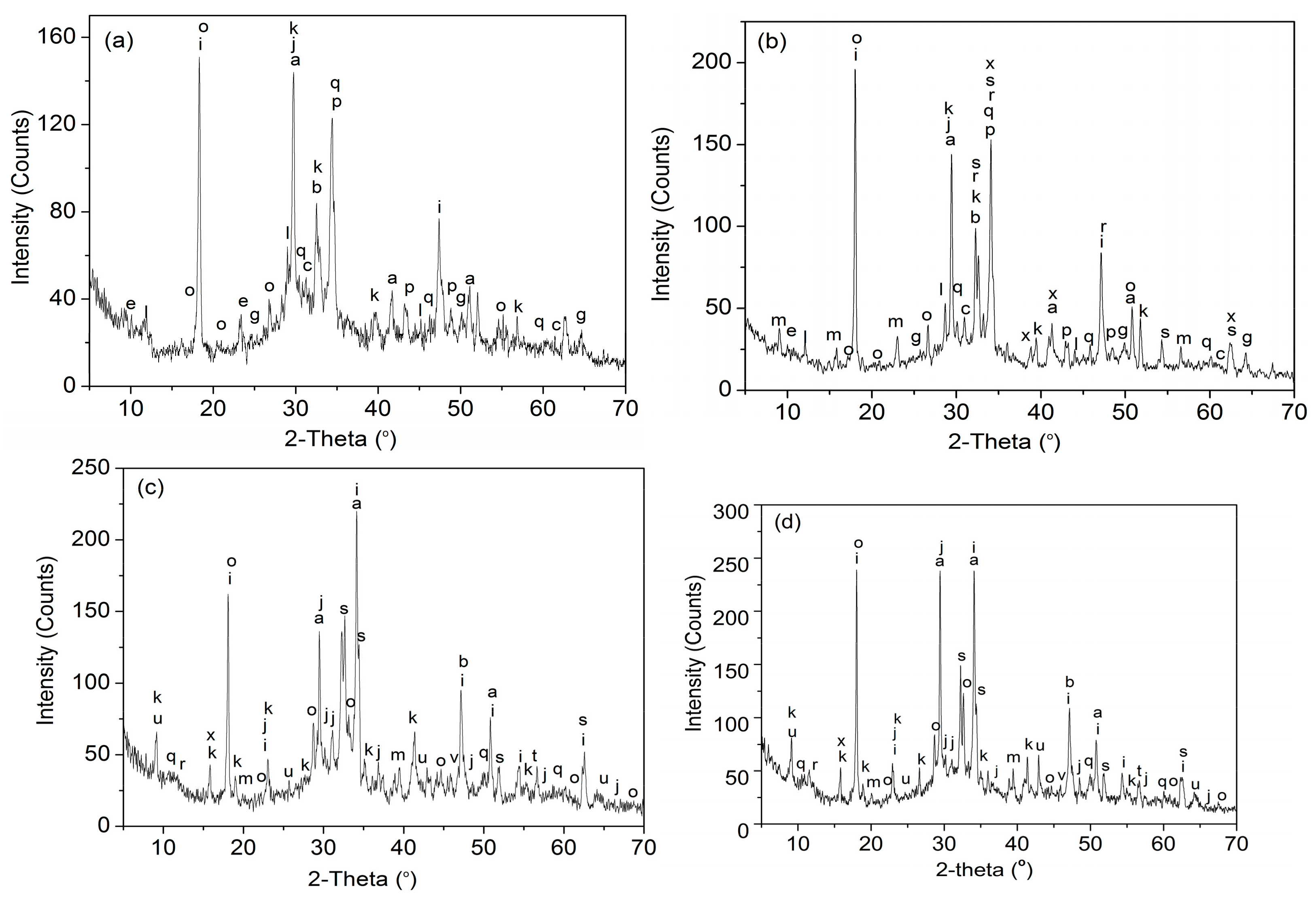
| Hydration Products | Crystal System | Cement Composites * | |||
|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||
| C3S, Ca2SiO5 | Monoclinic | + | + | + | + |
| C2S, Ca2SiO4 | Monoclinic | + | + | + | + |
| C3A, Ca3Al2O6 | Orthorhombic | + | + | ||
| C4AF,Ca4Al2Fe2O10 | Tetrahedral | + | |||
| CaSO4·2H2O | Monoclinic | + | |||
| Al2O3 | Hexagonal | ||||
| SiO2 | Tetragonal | + | + | + | + |
| CaO | Cubic | ||||
| Ca(OH)2 | Hexagonal | + | + | + | + |
| CaCO3 | Hexagonal | + | + | + | + |
| AFt,Ca6Al2(SO4)3(OH)12·26H2O | Hexagonal | + | + | + | + |
| AFm,Ca4Al2O6(SO4)·14H2O) | Hexagonal | + | + | + | + |
| C–S–H,Ca3Si2O7·xH2O) | Amorphous | + | + | + | + |
| C–S–H(Ca3Si2O7·xH2O) | Monoclinic | + | + | + | |
| CaAl2Si6O16·6H2O | Tetragonal | + | + | + | + |
| Ca2H2Si2O7 | Orthorhombic | + | + | + | |
| Ca6(AlSiO4)12·30H2O | Cubic | + | + | + | + |
| Ca4Si4O4(OH)24·3H2O | Monoclinic | + | + | + | |
| Ca3Si(OH)6(CO3)(SO4) 12H2O | Hexagonal | + | + | + | |
| K2Ca5(SO4)6·H2O | Monoclinic | + | + | ||
| CaFe5AlO10 | Tetragonal | + | + | + | |
| Ca2Al2Fe2O8 | Orthorhombic | + | + | ||
| Samples | Compressive Strength (MPa) | Flexural Strength (MPa) | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 Days | 7 Days | 28 Days | 60 Days | 3 Days | 7 Days | 28 Days | 60 Days | |
| S1 | 40.67 | 75.25 | 106.53 | 117.73 | 3.42 | 8.52 | 12.68 | 13.54 |
| S2 | 32.65 | 91.56 | 151.36 | 154.62 | 7.46 | 13.54 | 22.83 | 23.47 |
| S3 | 35.41 | 95.75 | 175.64 | 177.36 | 7.85 | 17.28 | 31.67 | 32.46 |
| S4 | 36.23 | 98.23 | 166.23 | 168.34 | 7.31 | 16.62 | 29.38 | 29.43 |
| S5 | 31.63 | 87.43 | 155.46 | 158.42 | 6.87 | 14.32 | 28.65 | 27.36 |
| S6 | 22.15 | 91.56 | 108.63 | 129.63 | 5.38 | 9.98 | 11.26 | 12.42 |
| Samples | Penetration Resistance | Freeze–Thaw Cycles * (×100) | Carbonation Depth (mm) | ||||
|---|---|---|---|---|---|---|---|
| Osmotic Pressure (MPa) | Seepage Height (mm) | m0 (g) | mloss (g) | P (%) | 7 Days | 28 Days | |
| S1 | 3.5 | 15.4 | 9837 | 0.55 | 71.52 | 3.73 | 4.94 |
| S2 | 3.5 | 4.7 | 9833 | 0 | 89.5 | 2.73 | 3.23 |
| S3 | 3.5 | 3.6 | 9845 | 0 | 96.53 | 0.84 | 1.84 |
| S4 | 3.5 | 3.7 | 9836 | 0 | 98.76 | 0.65 | 1.35 |
| S5 | 3.5 | 4.1 | 9841 | 0 | 97.65 | 0.52 | 1.62 |
| S6 | 3.5 | 11.3 | 9851 | 0.45 | 73.34 | 3.53 | 4.34 |
| Samples | Pore Structure of Cement Composites | ||||
|---|---|---|---|---|---|
| Total Pore Area (m2/g) | Median Porediameter (nm) | Average Pore Diameter (nm) | Apparent Density (g/cm3) | Porosity (%) | |
| S1 | 24.86 | 39.42 | 55.13 | 2.21 | 23.74 |
| S2 | 16.59 | 22.34 | 21.94 | 2.31 | 17.36 |
| S3 | 13.68 | 15.25 | 14.67 | 2.35 | 11.25 |
| S4 | 12.32 | 14.32 | 13.45 | 2.33 | 10.25 |
| S5 | 15.14 | 17.67 | 19.32 | 2.35 | 15.43 |
| S6 | 27.43 | 45.72 | 45.65 | 2.34 | 21.62 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Hu, H.; Zhang, J.; Luo, X.; Lei, Y.; Sun, L. Fabrication of GO/Cement Composites by Incorporation of Few-Layered GO Nanosheets and Characterization of Their Crystal/Chemical Structure and Properties. Nanomaterials 2017, 7, 457. https://doi.org/10.3390/nano7120457
Lv S, Hu H, Zhang J, Luo X, Lei Y, Sun L. Fabrication of GO/Cement Composites by Incorporation of Few-Layered GO Nanosheets and Characterization of Their Crystal/Chemical Structure and Properties. Nanomaterials. 2017; 7(12):457. https://doi.org/10.3390/nano7120457
Chicago/Turabian StyleLv, Shenghua, Haoyan Hu, Jia Zhang, Xiaoqian Luo, Ying Lei, and Li Sun. 2017. "Fabrication of GO/Cement Composites by Incorporation of Few-Layered GO Nanosheets and Characterization of Their Crystal/Chemical Structure and Properties" Nanomaterials 7, no. 12: 457. https://doi.org/10.3390/nano7120457