Formation of Platinum Catalyst on Carbon Black Using an In‐Liquid Plasma Method for Fuel Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Formation of Platinum Catalyst on Carbon Black
2.2. Fabrication and Characterization of Fuel Cells Using Platinum Catalyst Formed by In-Liquid Plasma
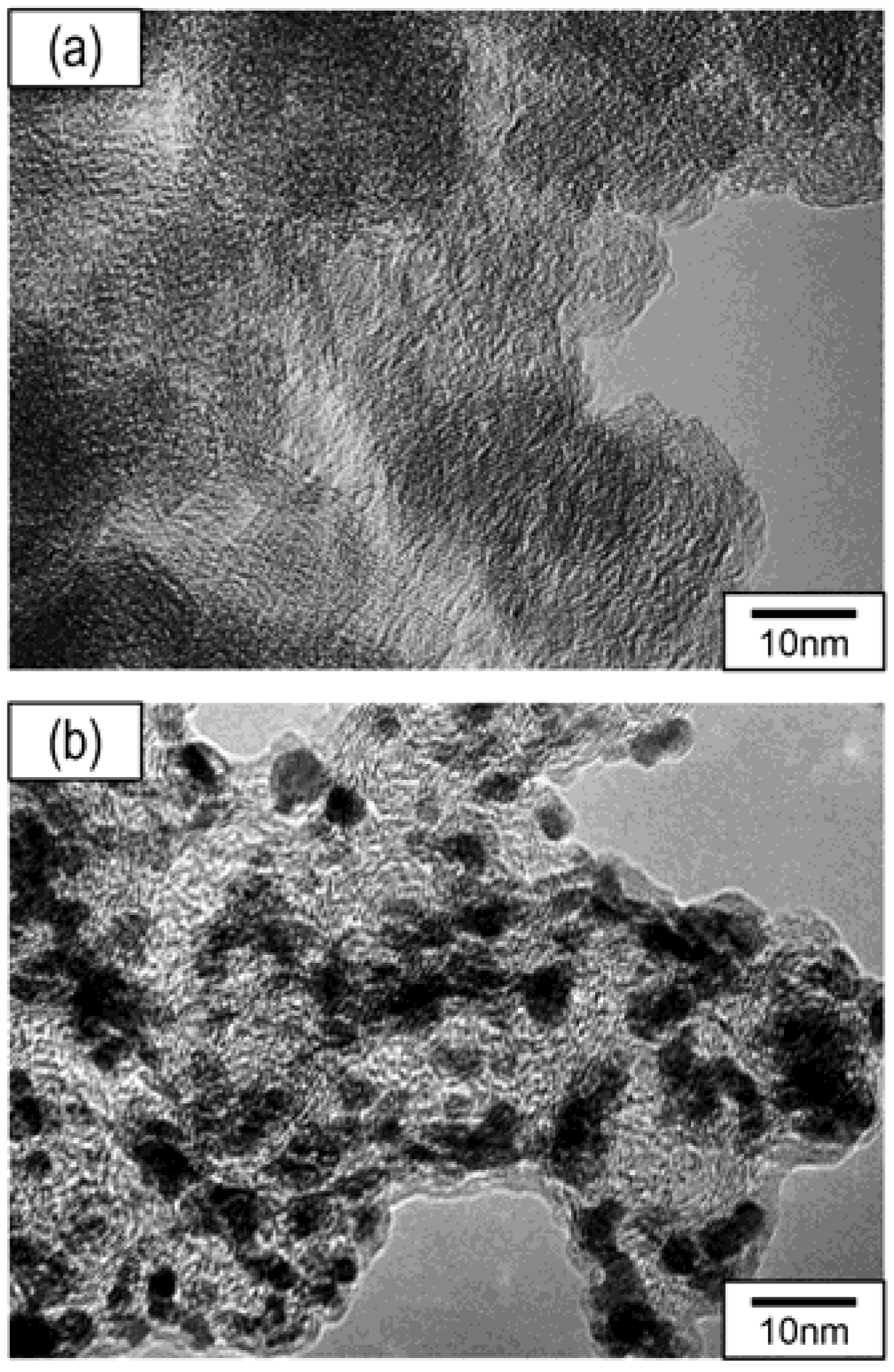
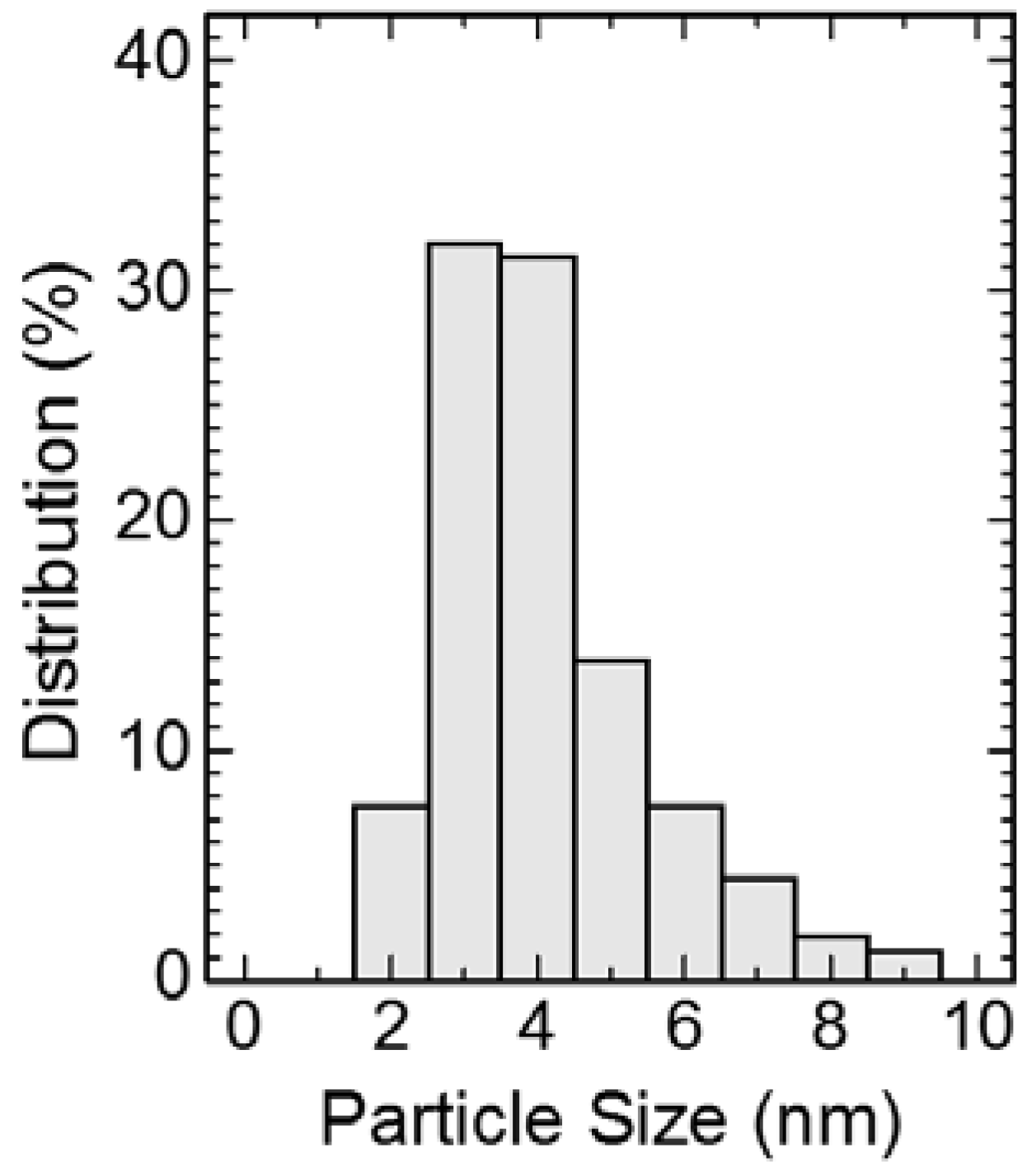
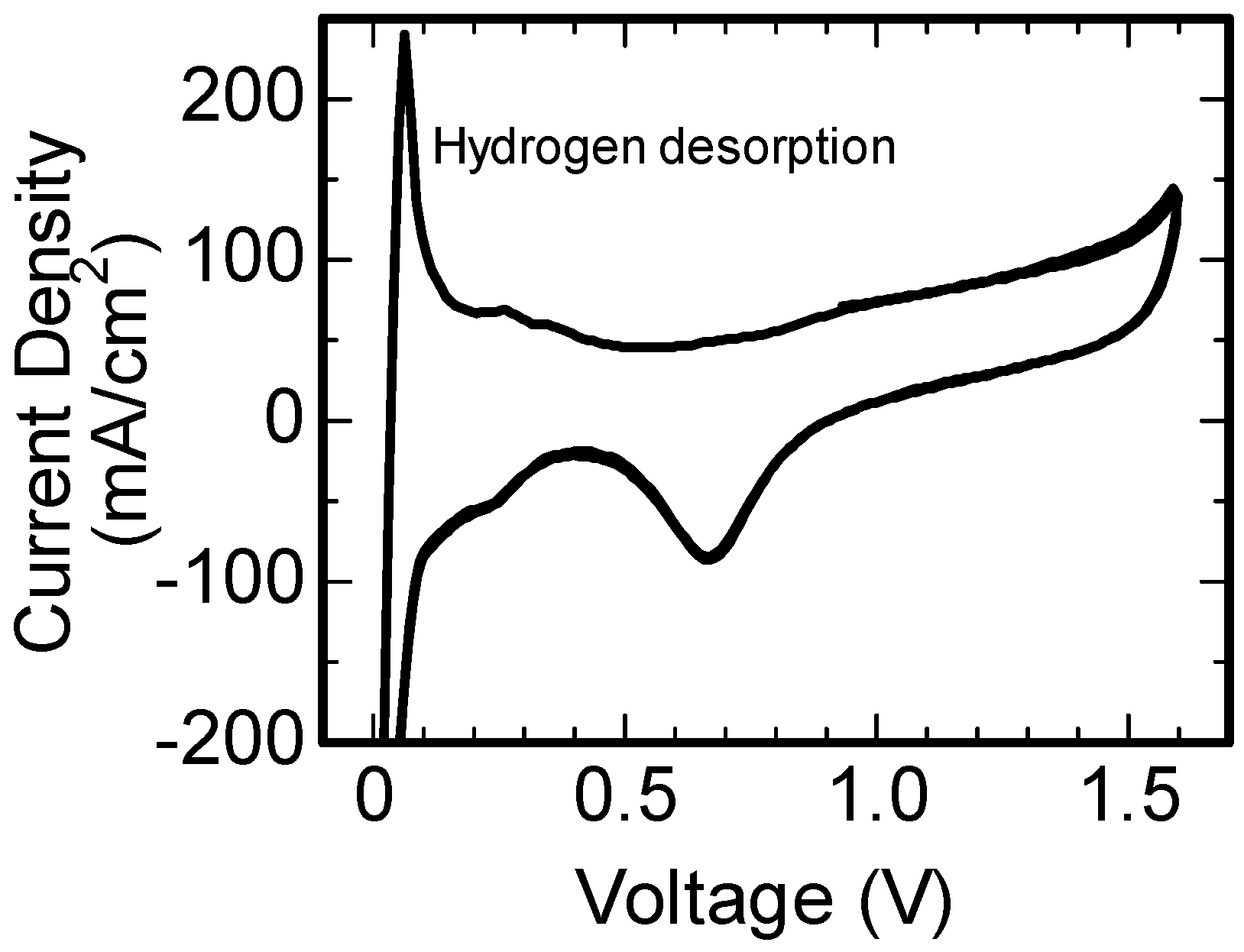
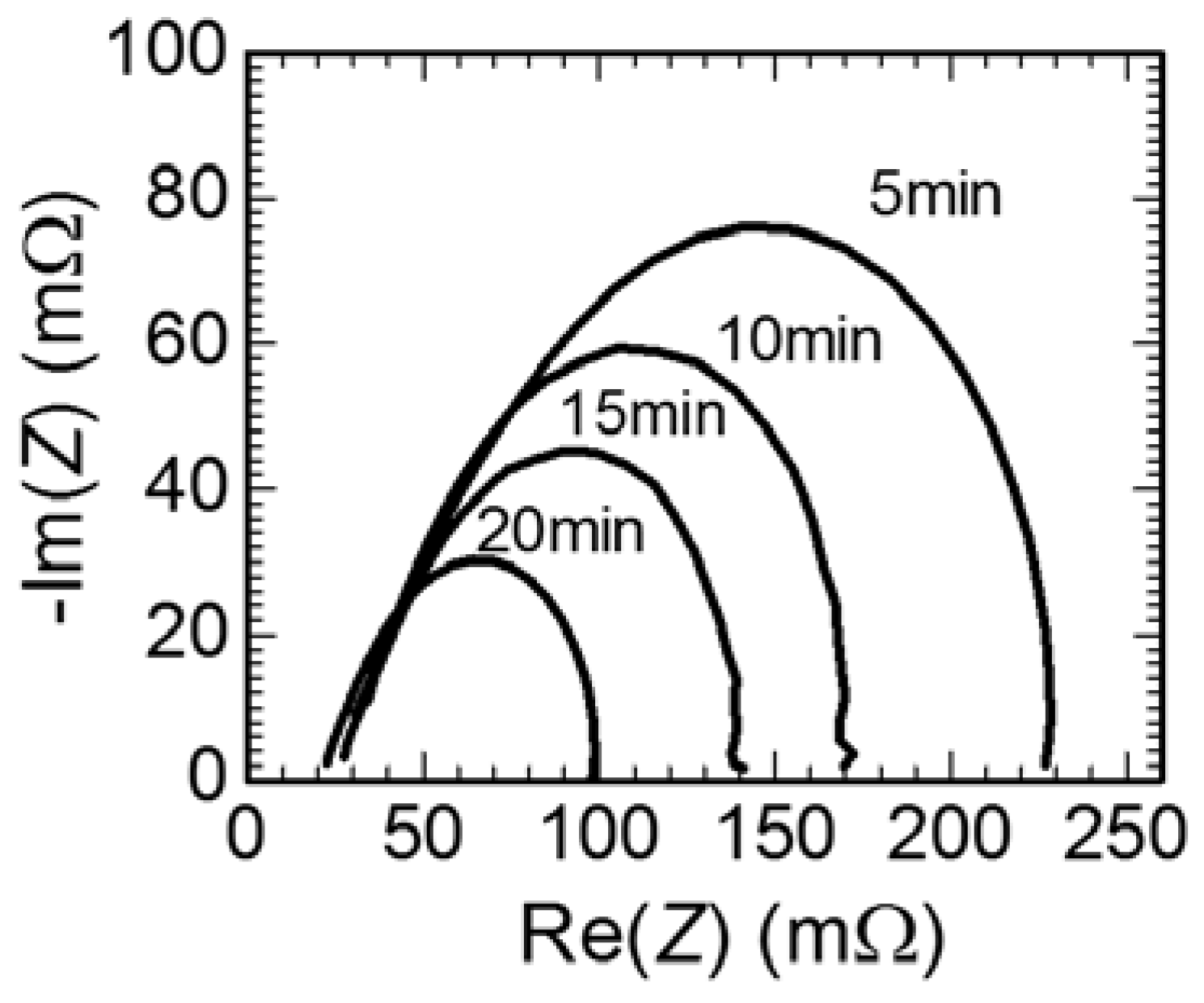
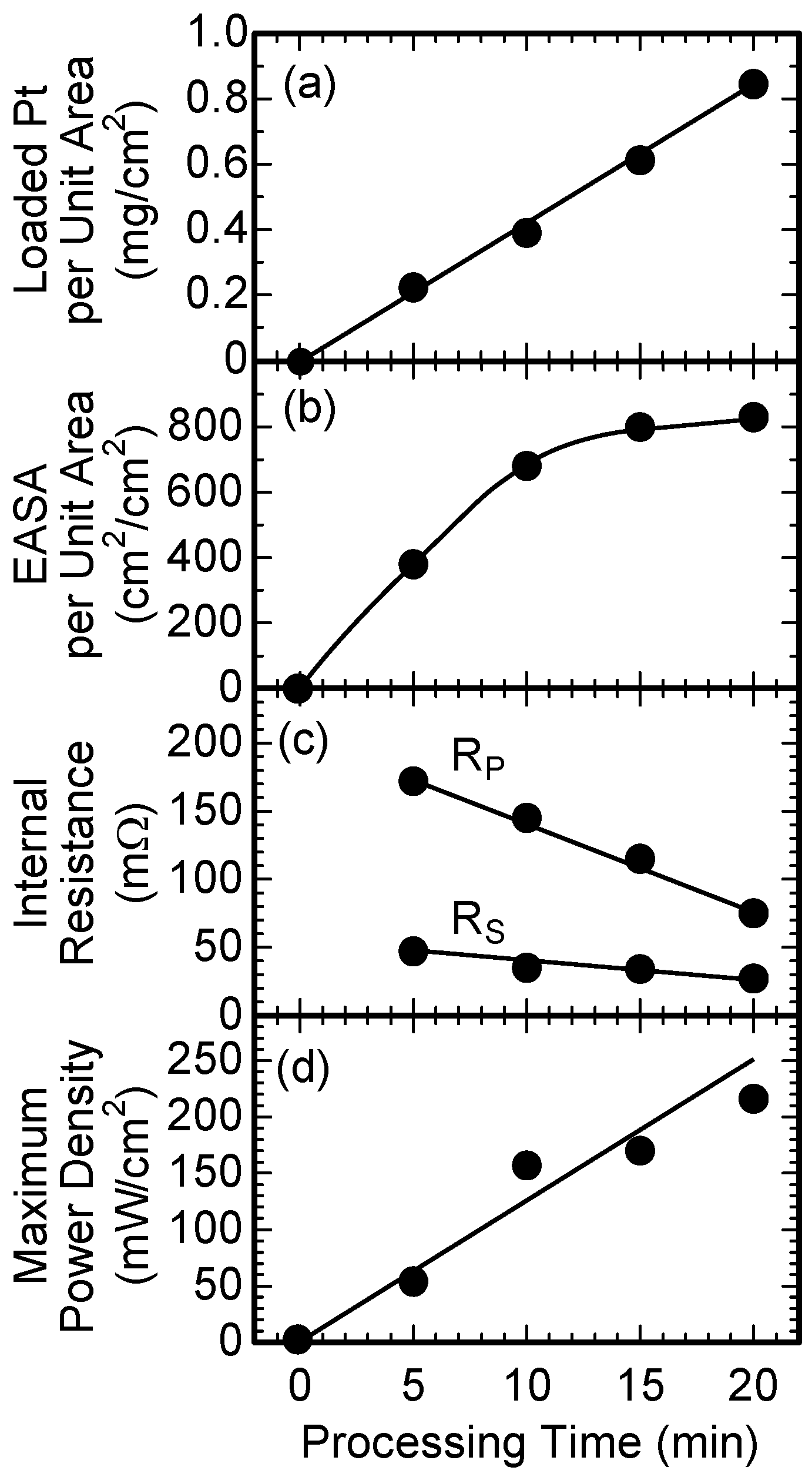
3. Results and Discussion
4. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- EG&G Technical Services, Inc. Fuel Cell Handbook, 7th ed.U.S. Department of Energy: Morgantown, WV, USA, 2004.
- Slade, S.; Campbell, S.A.; Ralph, T.R.; Walshc, F.C. Ionic Conductivity of an Extruded Nafion 1100 EW Series of Membranes. J. Electrochem. Soc. 2002, 149, A1556–A1564. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Yoshida, T.; Kojima, K. Toyota MIRAI fuel cell vehicle and progress toward a future hydrogen society. Electrochem. Soc. Interface Summer 2015, 24, 45–49. [Google Scholar] [CrossRef]
- Alaswad, A.; Baroutaji, A.; Achour, H.; Carton, J.; Al Makky, A.; Olabi, A.G. Developments in fuel cell technologies in the transport sector. Int. J. Hydrog. Energy 2016, 41, 16499–16508. [Google Scholar] [CrossRef]
- Pozio, A.; de Francesco, M.; Cemmi, A.; Cardellini, F.; Giorgi, L. Comparison of high surface Pt/C catalysts by cyclic voltammetry. J. Power Source 2002, 105, 13–19. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: Particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E. Structural parameters of supported fuel cell catalysts: The effect of particle size, inter-particle distance and metal loading on catalytic activity and fuel cell performance. Appl. Catal. B 2016, 181, 298–313. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Nguyen, T.T.L.; Nguyen, K.M.; Ha, T.H.; Nguyen, Q.H. Preparation of the vulcan XC-72R-supported Pt nanoparticles for the hydrogen evolution reaction in PEM water electrolysers. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 025012. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, W.H.; Kim, H. A novel synthetic route for the preparation of core shell like carbon-supported nanoparticles with a Pt-rich shell. J. Mater. Chem. A 2014, 2, 11635–11641. [Google Scholar] [CrossRef]
- Sonstrom, P.; Baumer, M. Supported colloidal nanoparticles in heterogeneous gas phase catalysis: On the way to tailored catalysts. Phys. Chem. Chem. Phys. 2011, 13, 19270–19284. [Google Scholar] [CrossRef] [PubMed]
- O’Hayre, R.; Lee, S.; Cha, S.; Prinz, F.B. A sharp peak in the performance of sputtered platinum fuel cells at ultra-low platinum loading. J. Power Source 2002, 109, 483–493. [Google Scholar] [CrossRef]
- Gruber, D.; Ponath, N.; Müller, J.; Lindstaedt, F. Sputter-deposited ultra-low catalyst loadings for PEM fuel cells. J. Power Source 2005, 150, 67–72. [Google Scholar] [CrossRef]
- Alvisia, M.; Galtieria, G.; Giorgib, L.; Giorgib, R.; Serrab, E.; Signorea, M.A. Sputter deposition of Pt nanoclusters and thin films on PEM fuel cell electrodes. Surface Coat. Technol. 2005, 200, 1325–1329. [Google Scholar] [CrossRef]
- Ichin, Y.; Mitamura, K.; Saito, N.; Takai, O. Characterization of platinum catalyst supported on carbon nanoballs prepared by solution plasma processing. J. Vac. Sci. Technol. A 2009, 27, 826–830. [Google Scholar] [CrossRef]
- Hua, X.; Shena, X.; Takaic, O.; Saito, N. Facile fabrication of PtAu alloy clusters using solution plasma sputtering and their electrocatalytic activity. J. Alloys Compd. 2013, 552, 351–355. [Google Scholar] [CrossRef]
- Hu, X.; Takai, O.; Saito, N. Simple Synthesis of Platinum Nanoparticles by Plasma Sputtering in Water. Jpn. J. Appl. Phys. 2013, 52, 01AN05. [Google Scholar] [CrossRef]
- Show, Y.; Hirai, A.; Almowarai, A.; Ueno, Y. Platinum catalyst formed on carbon nanotube by the in-liquid plasma method for fuel cell. Thin Solid Films 2015, 596, 198–200. [Google Scholar] [CrossRef]
- Antolini, E.; Giorgi, L.; Pozio, A.; Passalacqua, E. Influence of Nafion loading in the catalyst layer of gas-diffusion electrodes for PEFC. J. Power Source 1999, 77, 136–142. [Google Scholar] [CrossRef]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Witte, J.; Bongard, H.J.; Topalov, A.A.; Baldizzone, C.; Mezzavilla, S.; Schüth, F.; Mayrhofer, K.J.J. Design criteria for stable Pt/C fuel cell catalysts. Beilstein J. Nanotechnol. 2014, 5, 44–67. [Google Scholar] [CrossRef] [PubMed]
- Colon-Mercado, H.R.; Popov, B.N. Stability of platinum based alloy cathode catalysts in PEM fuel cells. J. Power Source 2006, 155, 253–263. [Google Scholar] [CrossRef]
- Watt-Smith, M.J.; Friedrich, J.M.; Rigby, S.P.; Ralph, T.R.; Walsh, F.C. Determination of the electrochemically active surface area of Pt/C PEM fuel cell electrodes using different adsorbates. J. Phys. D 2008, 41, 174004. [Google Scholar] [CrossRef]
- Park, D.; Jeon, Y.; Ok, J.; Park, J.; Yoon, S.; Choy, J.; Shul, Y. Pt Nanoparticle-Reduced Graphene Oxide Nanohybrid for Proton Exchange Membrane Fuel Cells. J. Nanosci. Nanotechnol. 2012, 12, 5669–5672. [Google Scholar] [CrossRef] [PubMed]
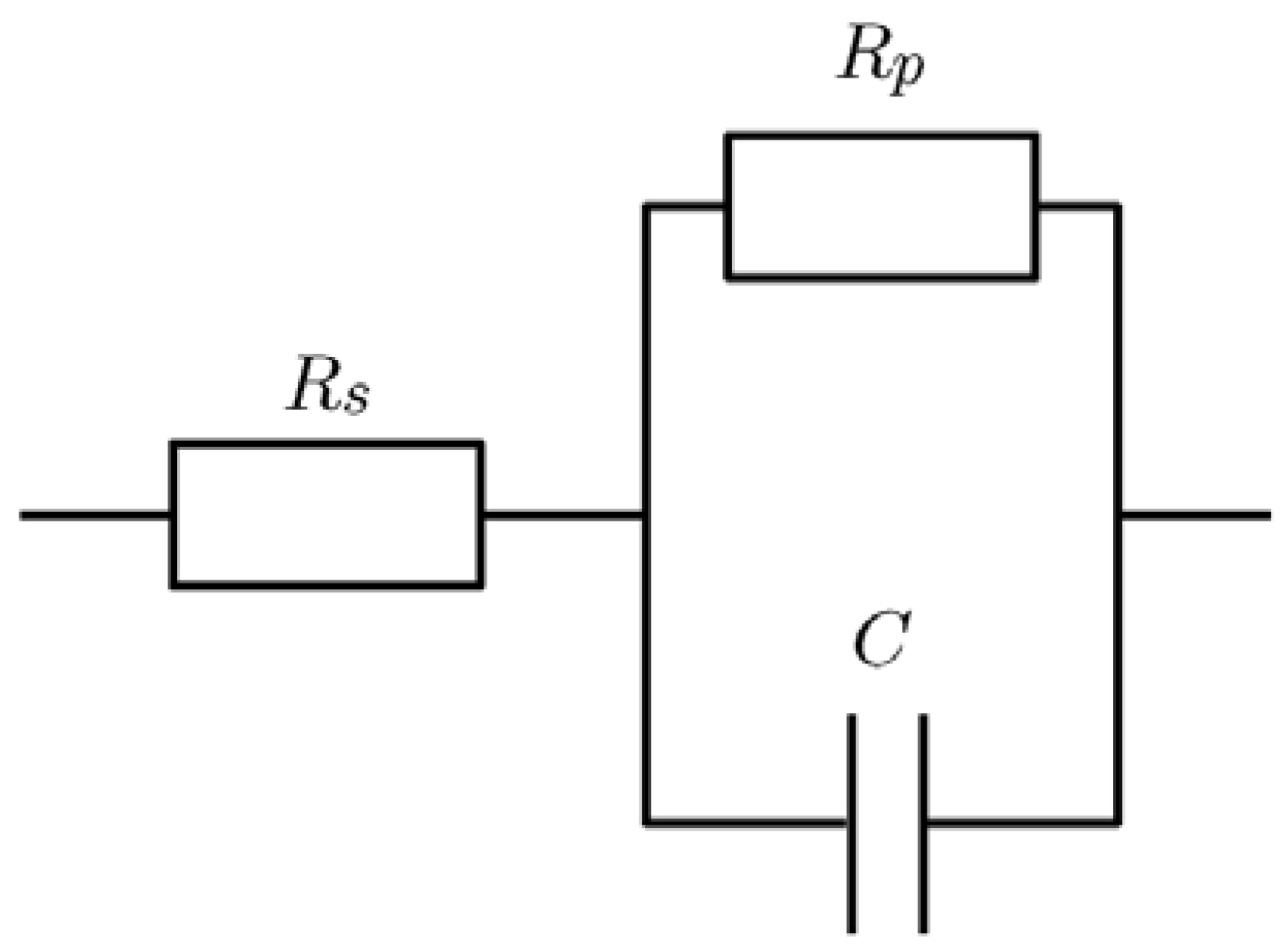
- Yuan, X.; Wang, H.; Sun, J.; Zhang, J. AC impedance technique in PEM fuel cell diagnosis—A review. Int. J. Hydrog. Energy 2007, 32, 4365–4380. [Google Scholar] [CrossRef]
- Niya, S.M.R.; Hoorfar, M. Study of proton exchange membrane fuel cells using electrochemical impedance spectroscopy technique—A review. J. Power Source 2013, 240, 281–293. [Google Scholar] [CrossRef]
- Fouquet, N.; Doulet, C.; Nouillant, C.; Dauphin-Tanguy, G.; Ould-Bouamamab, B. Model based PEM fuel cell state-of-health monitoring via ac impedance measurements. J. Power Source 2006, 159, 905–913. [Google Scholar] [CrossRef]
- Yang, M.; Hsueh, C. Impedance Analysis of Working PEMFCs in the Presence of Carbon Monoxide. J. Electrochem. Soc. 2006, 153, A1043–A1048. [Google Scholar] [CrossRef]
- Oono, Y.; Fukuda, T.; Sounaib, A.; Horia, M. Influence of operating temperature on cell performance and endurance of high temperature proton exchange membrane fuel cells. J. Power Source 2010, 195, 1007–1014. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Show, Y.; Ueno, Y. Formation of Platinum Catalyst on Carbon Black Using an In‐Liquid Plasma Method for Fuel Cells. Nanomaterials 2017, 7, 31. https://doi.org/10.3390/nano7020031
Show Y, Ueno Y. Formation of Platinum Catalyst on Carbon Black Using an In‐Liquid Plasma Method for Fuel Cells. Nanomaterials. 2017; 7(2):31. https://doi.org/10.3390/nano7020031
Chicago/Turabian StyleShow, Yoshiyuki, and Yutaro Ueno. 2017. "Formation of Platinum Catalyst on Carbon Black Using an In‐Liquid Plasma Method for Fuel Cells" Nanomaterials 7, no. 2: 31. https://doi.org/10.3390/nano7020031




