Synthesis of Carbon Nanotubes in Thermal Plasma Reactor at Atmospheric Pressure
Abstract
:1. Introduction
2. CNT Synthesis Methods
3. CVD Reactors for the Synthesis of CNTs from the Gas Phase Working at Atmospheric Pressure
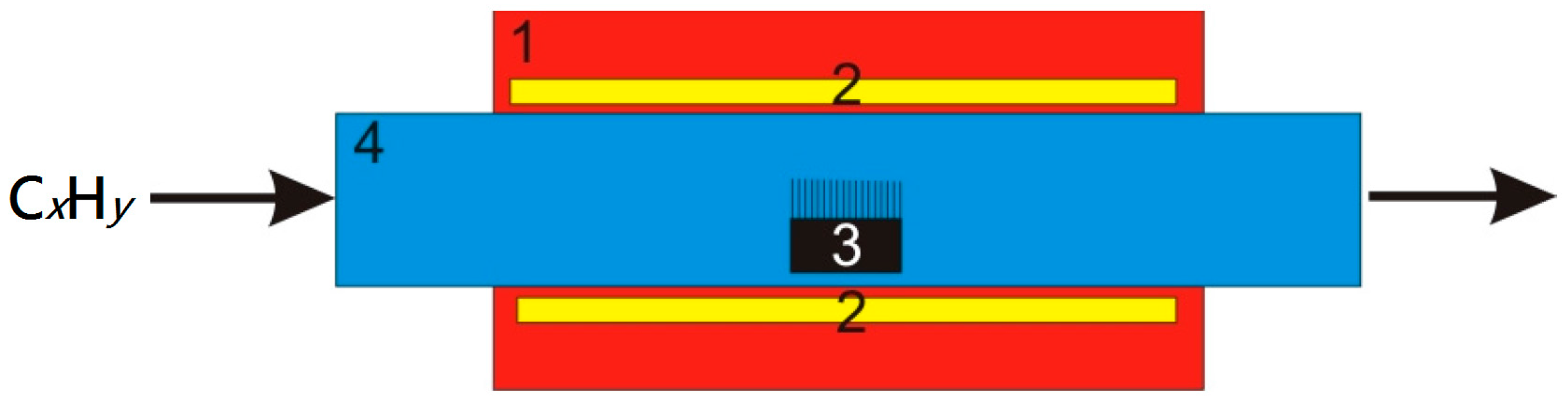
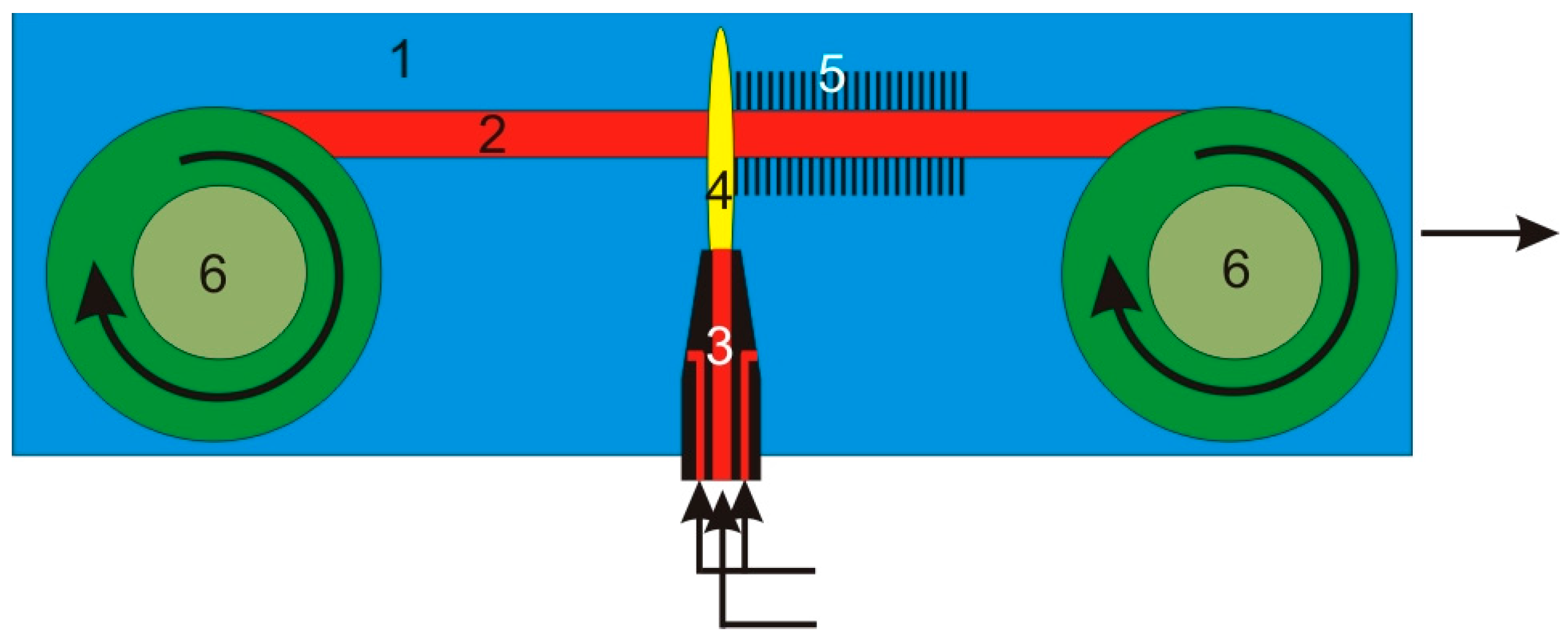
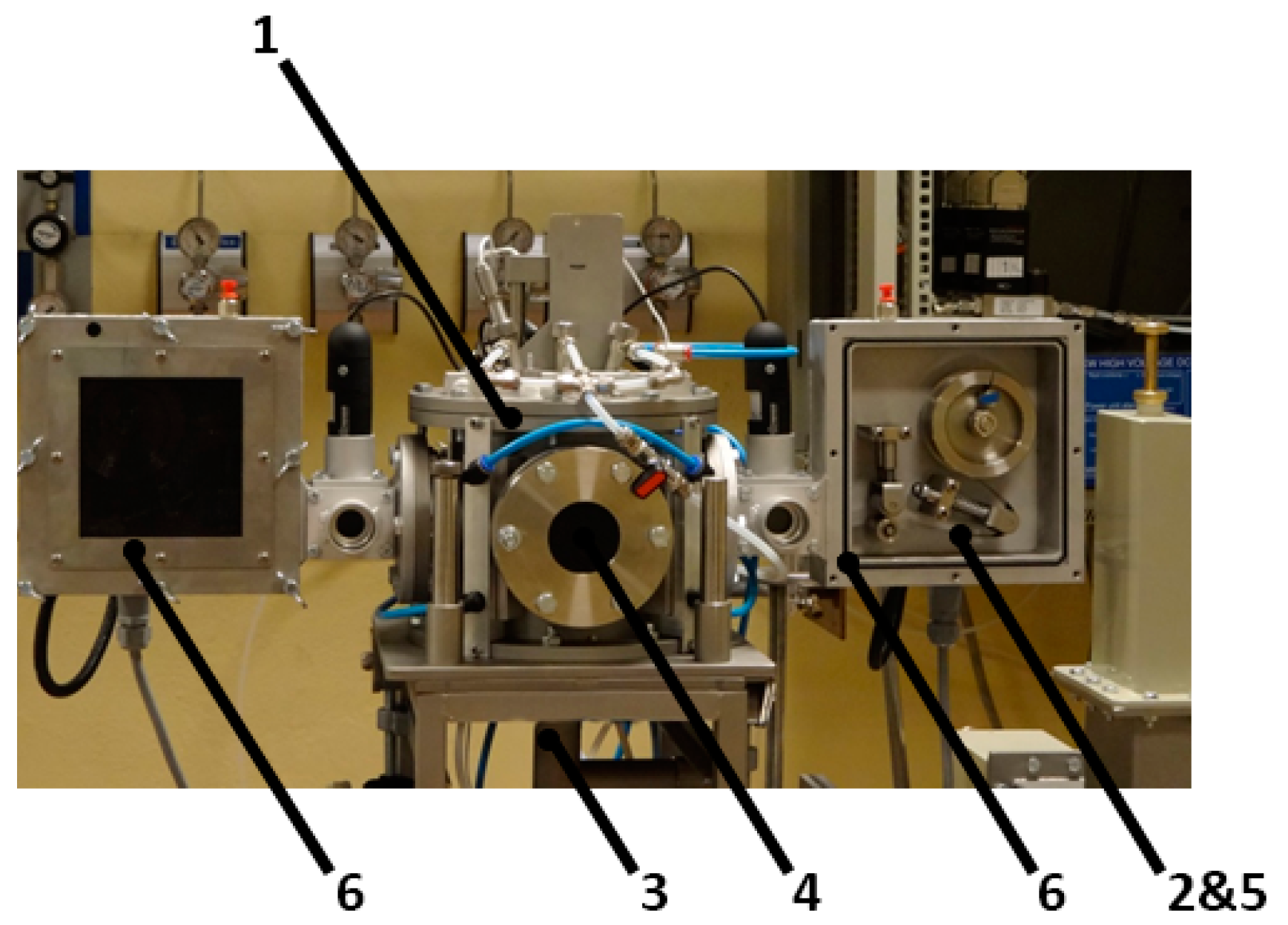
4. Experimental Setup for CNT Plasma Synthesis
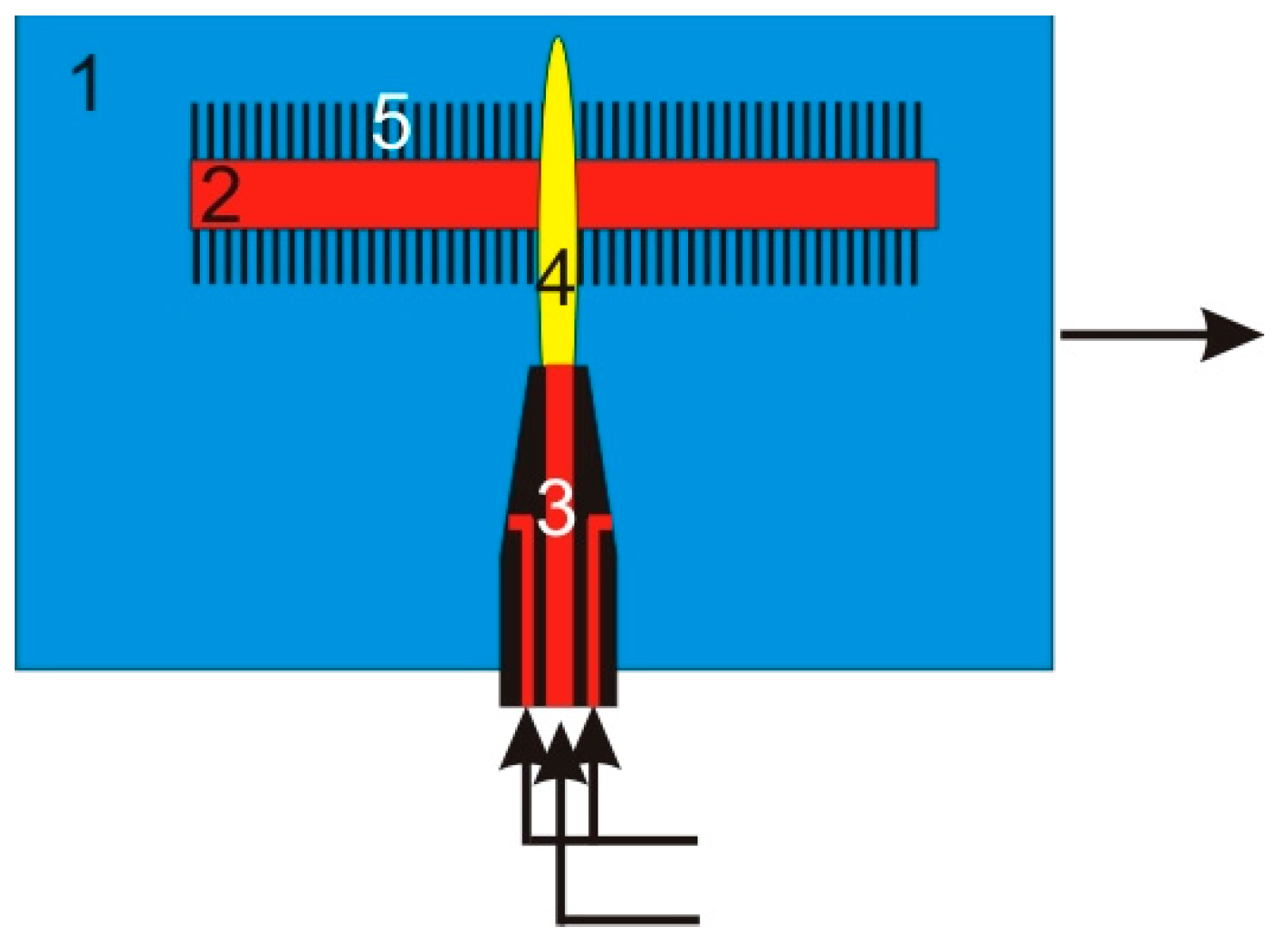
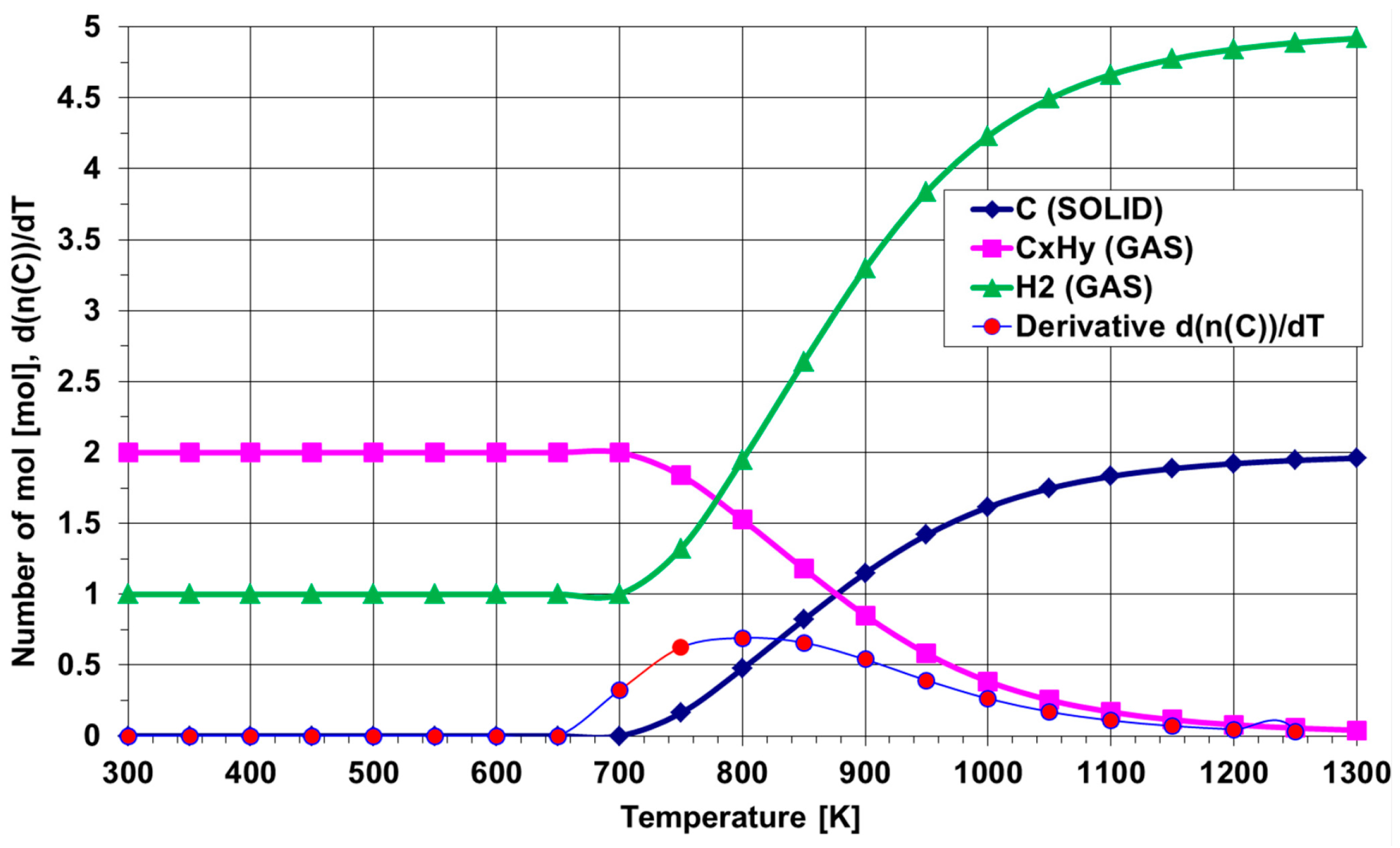
5. Thermal Decomposition of Gaseous Reagents
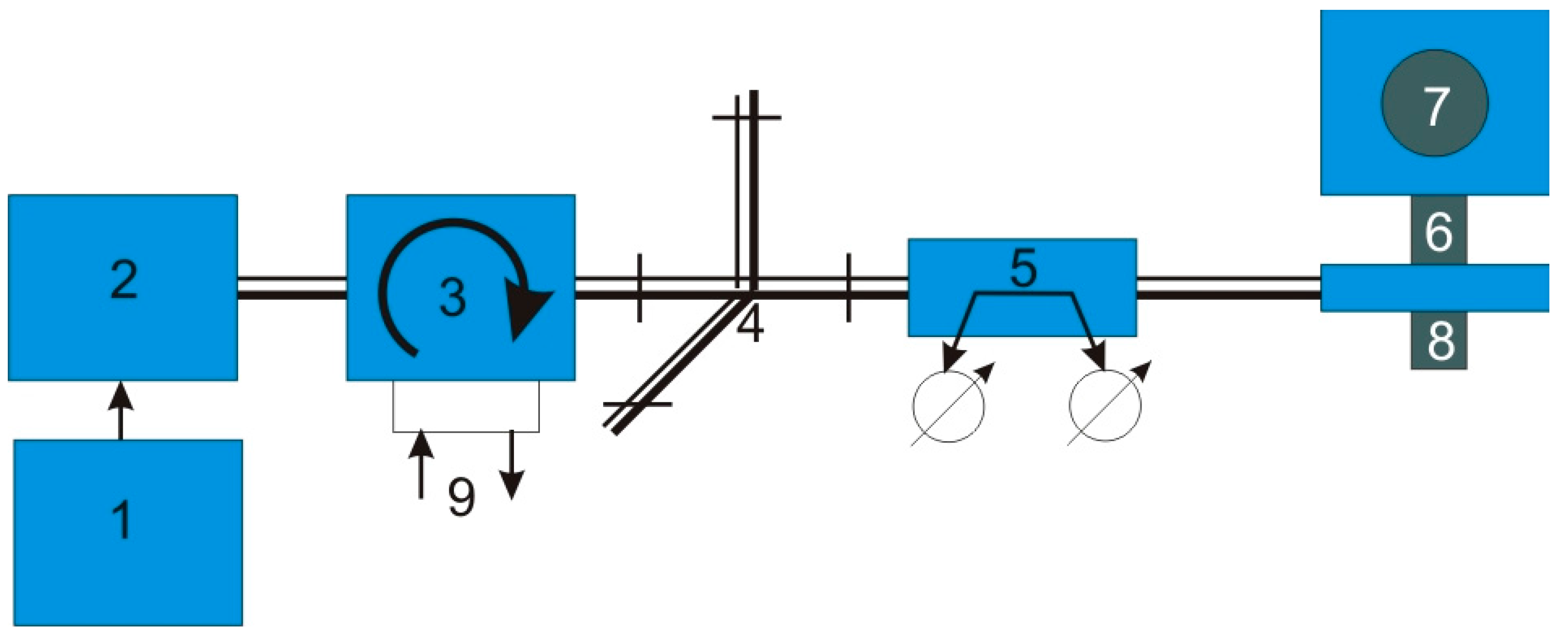
6. Experimental Setup for Continuous CNT Synthesis
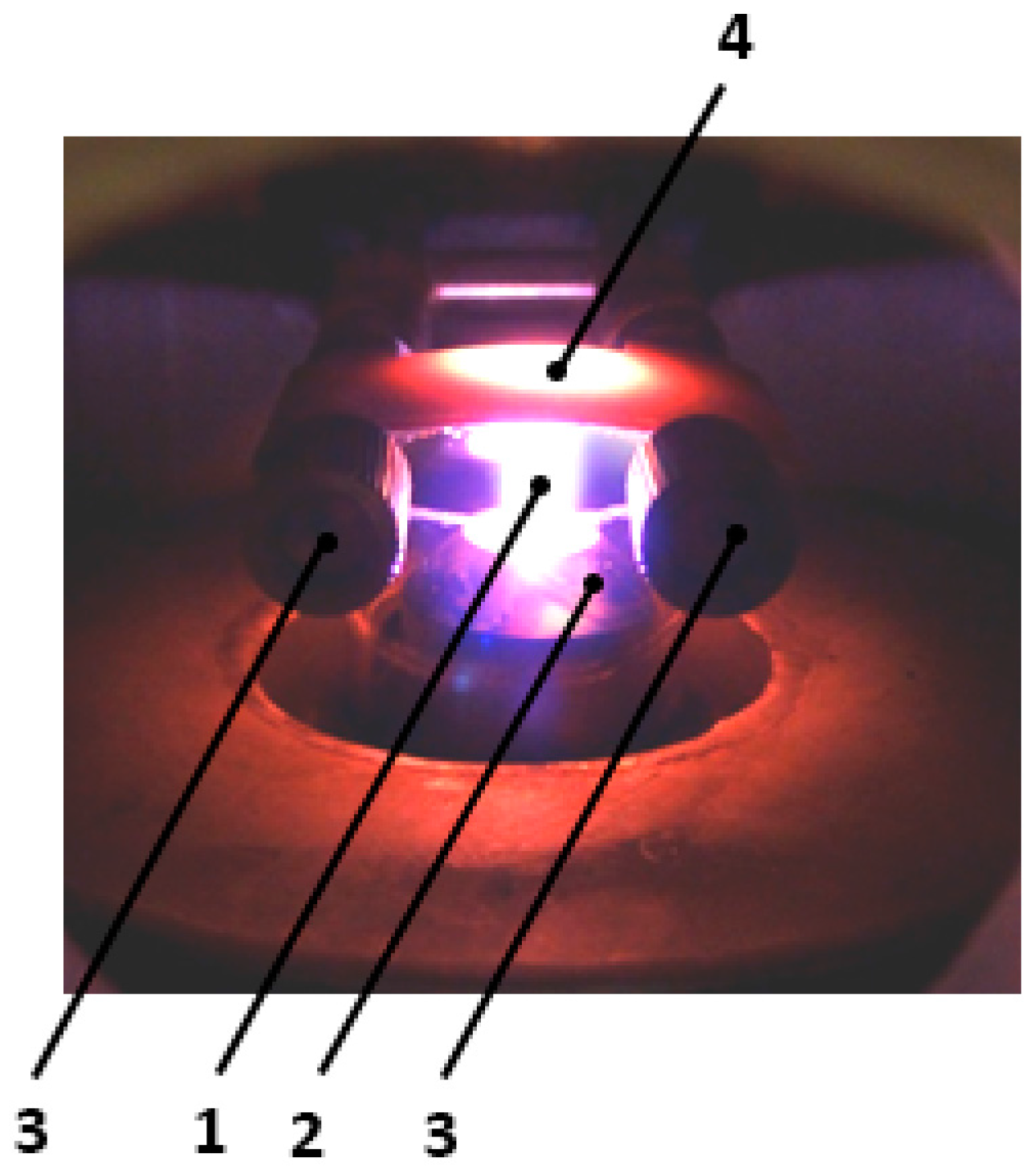
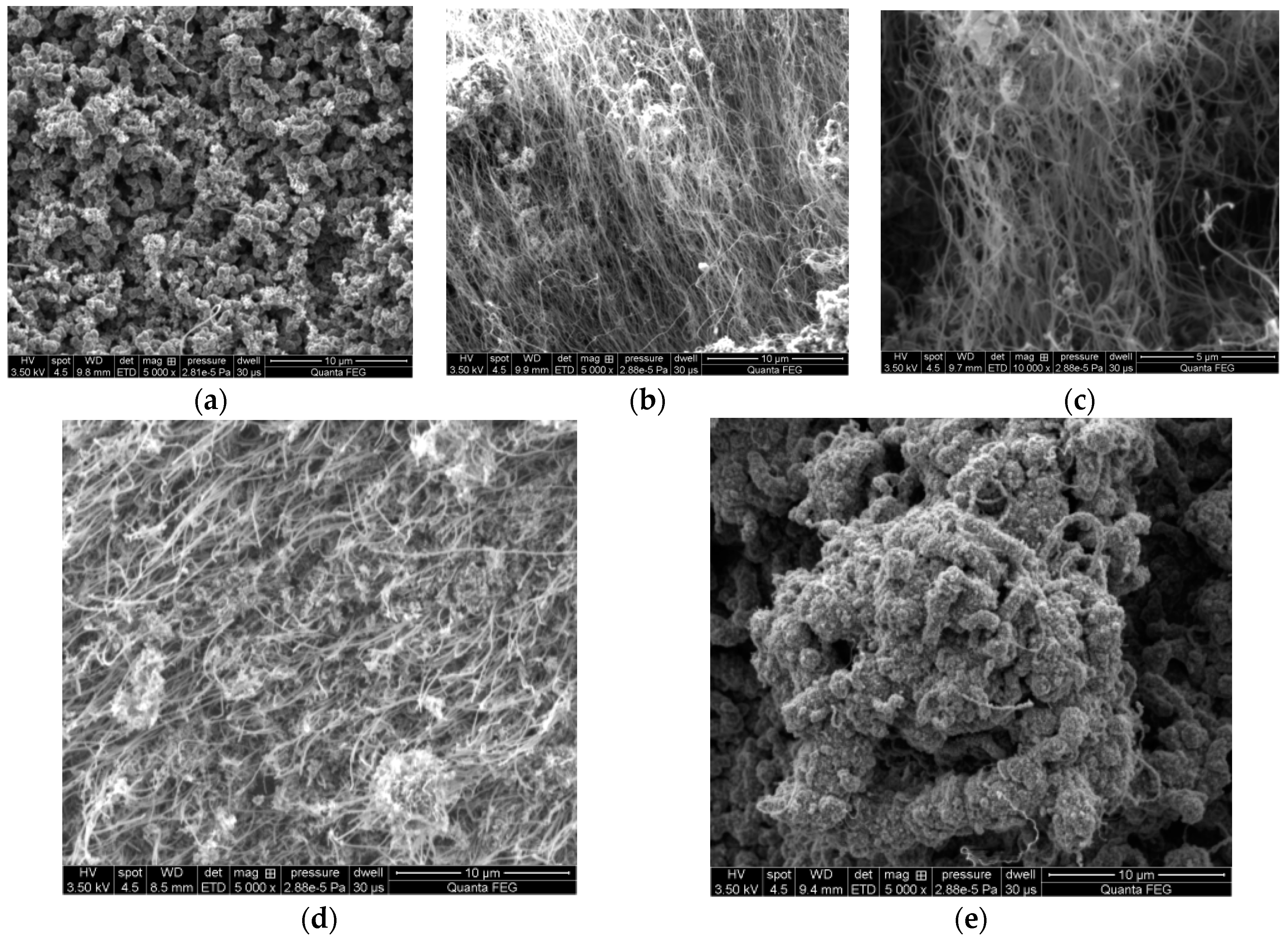
7. Results
- chemical methods: coating the steel strip (substrate) with a layer of an antioxidant, e.g., Al2O3 applied in a dip-coating manner, followed by the application of an oxide catalyst layer such a Fe2O3 on the top,
- treatment of the substrate surface with a ferrocene catalyst. Ferrocene evaporates from the source placed inside the reactor chamber which is a currently patented method by the paper authors.
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–356. [Google Scholar] [CrossRef]
- Kolacinski, Z.; Szymanski, L.; Raniszewski, G. Anode plasma jet behavior in CNTs deposit growth. In Proceedings of the 17th International Collegium on Plasma Processes, Marseille, France, June 2009.
- Raniszewski, G.; Szymanski, L.; Kolacinski, Z. Carbon nanotubes synthesis by electric arc plasma with external magnetic field. In Proceedings of the 4th International Conference Nanocon, Brno, Czech, 23–25 October 2012.
- Szymanski, L.; Kolacinski, Z.; Raniszewski, G. Reaktor plazmowy z łukiem wirującym [The plasma reactor using rotating arc]. Prz. Elektrotech. 2012, 88, 118–122, [in Polish]. [Google Scholar]
- Szymanski, L.; Raniszewski, G.; Kolacinski, Z. Magnetic Field Controlled CNTs Synthesis. In An Electrical Arc, Book of Contributed Papers—Hakone XIII; Lublin University of Technology: Kazimierz Dolny, Poland, 2012; pp. 268–270. [Google Scholar]
- Anazawa, K.; Shimotani, K.; Manabe, C.; Watanabe, H.; Shimizu, M. High-purity carbon nanotubes synthesis method by an arc discharging in magnetic field. Appl. Phys. Lett. 2002, 81, 739. [Google Scholar] [CrossRef]
- Keidar, M.; Levchenko, I.; Arbel, T.; Alexander, M.; Waas, A.M.; Ostrikov, K. Increasing the length of single-wall carbon nanotubes in a magnetically enhanced arc discharge. Appl. Phys. Lett. 2008, 92, 043129. [Google Scholar] [CrossRef]
- Gruenberger, T.M.; Gonzalez-Aguilar, J.; Fabry, F.; Fulchieri, L.; Grivei, E.; Probst, N.; Flamand, G.; Okuno, H.; Charlier, J.C. Production of carbon nanotubes and other nanostructures via continuous 3-phase AC plasma processing. Fuller. Nanotub. Carbon Nanostruct. 2004, 12, 571–581. [Google Scholar] [CrossRef]
- Matsuura, T.; Watanabe, T. A new type of arc plasma reactor with 12-phase alternating current discharge for synthesis of carbon nanotubes. Thin Solid Films 2007, 515, 4240–4246. [Google Scholar] [CrossRef]
- Okuno, H.; Grivel, E.; Fabry, F.; Gruenberger, T.M.; Gonzalez-Aguilar, J.J.; Palnichenko, A.; Fulchieri, L.; Probst, N.; Chalier, J.C. Synthesis of carbon nanotubes and nano-necklaces by thermal plasma process. Carbon 2004, 42, 2543–2549. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Rinzler, A.G.; Tomanek, D.; Colbert, D.T.; Smalley, R.E. Self assembly of tubular fullerenes. J. Phys. Chem. 1995, 99, 10694. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic growth of single-walled nanotubes by laser vaporization. Chem. Phys. Lett. 1995, 243, 49–54. [Google Scholar] [CrossRef]
- Hernandez, E.; Ordejon, P.; Boustani, I.; Rubio, A.; Alonso, J.A. Tight binding molecular dynamics studies of boron assisted nanotube growth. J. Chem. Phys. 2000, 113, 3814–3821. [Google Scholar] [CrossRef]
- Sun, L.F.; Mao, J.M.; Pan, Z.W.; Chang, B.H.; Zhou, W.Y.; Wang, G.; Qian, L.X.; Xie, S.S. Growth of straight nanotubes with a cobalt-nickel catalyst by chemical vapor deposition. Appl. Phys. Lett. 1999, 74, 644–646. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.H.; Kim, S.G.; Andrew, G.; et al. Crystalline ropes of metallic carbon nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Yudasaka, M.; Komatsu, T.; Ichihashi, T.; Iijima, S. Single-wall carbon nanotube formation by laser ablation using double-targets of carbon and metal. Chem. Phys. Lett. 1997, 278, 102–106. [Google Scholar] [CrossRef]
- Yudasaka, M.; Sensui, N.; Takizawa, M.; Bandow, S.; Ichihashi, T.; Iijima, S. Formation of single-wall carbon nanotubes catalyzed by Ni separating from Y in laser ablation or in arc discharge using a C target containing a NiY catalyst. Chem. Phys. Lett. 1999, 312, 155–160. [Google Scholar] [CrossRef]
- Yudasaka, M.; Yamada, R.; Sensui, N.; Wilkins, T.; Ichihashi, T.; Iijima, S. Mechanism of the effect of NiCo, Ni and Co catalysts on the yield of singlewall carbon nanotubes formed by pulsed Nd:YAG laser ablation. J. Phys. Chem. B 1999, 103, 6224–6229. [Google Scholar] [CrossRef]
- Yudasaka, M.; Zhang, M.; Iijima, S. Porous target enhances production of single-wall carbon nanotubes by laser ablation. Chem. Phys. Lett. 2000, 323, 549–553. [Google Scholar] [CrossRef]
- Bernier, P.; Laplaze, D.; Auriol, J.; Barbedette, L.; Flamant, G.; Lebrun, M.; Brunelle, A.; Della-Negra, S. Production of fullerenes from solar energy. Synth. Met. 1995, 70, 1455–1456. [Google Scholar] [CrossRef]
- Chibante, L.P.F.; Thess, A.; Alford, J.M.; Diener, M.D.; Smalley, R.E. Solar generation of the fullerenes. J. Phys. Chem. 1993, 97, 8696–8700. [Google Scholar] [CrossRef]
- Fields, C.L.; Pitts, J.R.; Hale, M.J.; Bingham, C.; Lewandowski, A.; King, D.E. Formation of fullerenes in highly concentrated solar flux. J. Phys. Chem. 1993, 97, 8701–8702. [Google Scholar] [CrossRef]
- Fields, C.L.P., Jr.; Mischler, D.; Bingham, C.; Lewandowski, A.; Schultz, D.L.; Bekkedahl, T.A.; Jones, K.M.; Heben, M.J. An update on solar fullerene production at the national renewable energy Laboratory. In Proceedings of the 8th International Symposium on Solar Thermal Concentrating Technologies, Köln, Germany, 6–11 October 1996.
- Heben, M.J.; Bekkedahl, T.A.; Schultz, D.L.; Jones, K.M.; Dillon, A.C.; Curtis, C.J.; Bingham, C.; Pitts, J.R.; Lewandowski, A.; Fields, C.L. Production of single-wall carbon nanotubes using concentrated sunlight. In Proceedings of the Symposium on Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials; Electrochemical Society Inc.: Pennington, UK, November 1996. [Google Scholar]
- Hsu, W.K.; Hare, J.P.; Terrones, M.; Kroto, H.W.; Walton, D.R.M.; Harris, P.J.F. Condensed-phase nanotubes. Nature 1995, 377, 687. [Google Scholar] [CrossRef]
- Hsu, W.K.; Terrones, M.; Hare, J.P.; Terrones, H.; Kroto, H.W.; Walton, D.R.M. Electrolytic formation of carbon nanostructures. Chem. Phys. Lett. 1996, 262, 161–166. [Google Scholar] [CrossRef]
- Laplaze, D.; Bernier, P.; Barbedette, L.; Lambert, J.M.; Brunelle, A.; Della-Negra, S.; Flamant, G.; Lebrun, M. Production of fullerenes from solar energy: The Odeillo experiment. Comptes Rendus de l'Acad. des Sci. Ser. 2. 1994, 318, 733–738. [Google Scholar] [CrossRef]
- Laplaze, D.; Bernier, P.; Flamant, G.; Lebrun, M.; Brunelle, A.; Della-Negra, S. Preparation of fullerenes using a solar furnace. Synth. Met. 1996, 77, 67–71. [Google Scholar] [CrossRef]
- Laplaze, D.; Bernier, P.; Journet, C.; Sauvajol, J.L.; Bormann, D.; Flamant, G.; Lebrun, M. The use of solar energy for the production of fullerenes and porous silicon. J. Phys. 1997, 7, 463–472. [Google Scholar] [CrossRef]
- Laplaze, D.; Bernier, P.; Journet, C.; Vie, V.; Flamant, G.; Lebrun, M. Carbon sublimation using a solar furnace. Synth. Met. 1997, 86, 2295–2296. [Google Scholar] [CrossRef]
- Laplaze, D.; Bernies, J.; Journet, C.P.; Vie, V.; Flamant, G.; Philippot, E.; Lebrun, M. Evaporation of graphite using a solar furnace: Production of fullerenes. In Proceedings of the 8th International Symposium on Solar Thermal Concentrating Technologies, Köln, Germany, 6–11 October 1996.
- Jeong, S.-H.; Lee, O.-J.; Lee, K.-H.; Oh, S.H.; Park, C.-G. Preparation of aligned carbon nanotubes with prescribed dimensions, template synthesis and sonication cutting approach. Chem. Mater. 2002, 14, 1859–1862. [Google Scholar] [CrossRef]
- Katoh, R.; Tasaka, Y.; Sekreta, E.; Yumura, M.; Ikazaki, F.; Kakudate, Y.; Fujiwara, S. Sonochemical production of a carbon nanotube. Ultrason. Sonochem. 1999, 6, 185–187. [Google Scholar] [CrossRef]
- Cho, W.S.; Hamada, E.; Kondo, Y.; Takayanagi, K. Synthesis of carbon nanotubes from bulk polymer. Appl. Phys. Lett. 1996, 69, 278–279. [Google Scholar] [CrossRef]
- HulicÏovµ, D.; Hosoi, K.; Kuroda, S.I.; Abe, H.; Oya, A. Carbon nanotubes prepared by spinning and carbonizing fine core±shell polymer microspheres. Adv. Mater. 2002, 14, 452–455. [Google Scholar]
- Jiang, J.; Feng, T.; Cheng, X.; Dai, L.; Cao, G.; Jiang, B.; Wang, X.; Liu, X.; Zou, S. Synthesis and growth mechanism of Fe-catalyzed carbon nanotubes by plasma-enhanced chemical vapor deposition. Nucl. Instrum. Methods Phys. Res. 2006, B244, 327–332. [Google Scholar] [CrossRef]
- Meyyappan, M.; Delzeit, L.; Cassell, A.; Hash, D. Carbon nanotube growth by PECVD: A review. Plasma Sour. Sci. Technol. 2003, 12, 205–216. [Google Scholar] [CrossRef]
- Minea, T.M.; Point, S.; Gohier, A.; Granier, A.; Godon, C.; Alvarez, F. Single chamber PVD/PECVD process for in situ control of the catalyst activity on carbon nanotubes growth. Surf. Coat. Technol. 2005, 200, 1101–1105. [Google Scholar] [CrossRef]
- Blase, X.; Charlier, J.C.; De Vita, A.; Car, R.; Redlich, P.; Terrones, M.; Hsu, W.K.; Terrones, H.; Carroll, D.L.; Ajayan, P.M. Boron-mediated growth of long helicity-selected carbon nanotubes. Phys. Rev. Lett. 1999, 83, 5078–5081. [Google Scholar] [CrossRef]
- Yabe, Y.; Ohtake, Y.; Ishitobi, T.; Show, Y.; Izumi, T.; Yamauchib, H. Synthesis of well-aligned carbon nanotubes by radio frequency plasma enhanced CVD method. Diam. Relat. Mater. 2004, 1292–1295. [Google Scholar] [CrossRef]
- Ebbesen, T.W. Carbon Nanotubes: Preparation and Properties; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Huczko, A. Nanorurki Węglowe: Czarne diamenty XXI wieku [Carbon Nanotubes - Black Diamonds of XXI century]; BEL Studio: Warszawa, Poland, 2004; [in Polish]. [Google Scholar]
- Iijima, S. Carbon nanotubes: Past, present, and future. Physica B 2002, 323, 1–5. [Google Scholar] [CrossRef]
- Kong, J.; Cassell, A.M.; Dai, H. Chemical vapor depositin of methane for single walled carbon nanotubes. Chem. Phys. Lett. 1998, 292, 567–574. [Google Scholar] [CrossRef]
- Moisala, A.; Nasibulin, A.G.; Kauppinen, E.I. The role of metal nanoparticles in the catalytic production of single-walled carbon nanotubes—A review. J. Phys. Condens. Matter 2003, 15, 3011. [Google Scholar] [CrossRef]
- Nagy, J.B.; Bister, G.; Fonseca, A.; Mehn, D.; Konya, Z.; Kiricsi, I.; Horvath, Z.E.; Biro, L.P. On the growth mechanism of single-walled carbon nanotubes by catalytic carbon vapor deposition on supported metal catalysts. J. Nanosci. Nanotechnol. 2004, 4, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Przygocki, W.; Lochowicz, A. Fulereny i Nanorurki—Właściwości i Zastosowanie [Fulerens and Nanotubes – Properties and Application]; Wydawnictwo Naukowo-Techniczne: Warszawa, Poland, 2001; [in Polish]. [Google Scholar]
- Reich, S.; Thomsen, C.; Maultzsch, J. Carbon Nanotubes, 1st ed.; Wiley-Vch: Weinheim Germany, 2004. [Google Scholar]
- Rotkin, S.; Subramoney, S. Applied Physiks of Carbon Nanotubes. Fundamentals of Theory, Optics and Transport Devices; Springer: New York, NY, USA, 2005. [Google Scholar]
- Wang, Y.; Rybczynski, J.; Wang, D.Z.; Kempa, K.; Ren, Z.F.; Li, W.Z.; Kimball, B. Periodicity and alignment of large-scale carbon nanotubes arrays. Appl. Phys. Lett. 2004, 85, 4741–4743. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Chen, X.; Chen, Y. Preparation of carbon nanotubes and nano-particles by microwave plasma-enhanced chemical vapor deposition. Scr. Mater. 2001, 44, 1567–1570. [Google Scholar] [CrossRef]
- Kondo, H.; Fukuoka, F.; Maruyama, T. Low temperature growth of single-walled carbon nanotubes from Pt catalysts under low ethanol pressure by alcohol gas source method. J. Nanotechnol. 2012, 2012, 690304. [Google Scholar] [CrossRef]
- Atiyah, M.R.; Awang Black, D.R.; Achamadun, F.; Achamad, I.S.; Mohd Yasin, F.; Yusoff, H.M. Low temperature growth of vertically aligned carbon nanotubes via floating catalyst chemical vapor depositon method. J. Mater. Sci. Technol. 2011, 27, 290–300. [Google Scholar] [CrossRef]
- Halonen, N.; Sápi, A.; Nagy, L.; Puskás, R.; Leino, A.; Mäklin, J.; Kukkola, J.; Tóth, G.; Wu, M.; Liao, H.; et al. Low-temperature growth of multi-walled carbon nanotubes by thermal CVD. Phys. Statut Solidi B 2011, 248, 2500–2503. [Google Scholar] [CrossRef]
- Lee, C.J.; Park, J.; Kim, J.M.; Huh, Y.; Lee, J.Y.; No, K.S. Low-temperature growth of carbon nanotubes by thermal chemical vapor deposition using Pd, Cr, and Pt as co-catalyst. Chem. Phys. Lett. 2000, 327, 277–283. [Google Scholar] [CrossRef]
- Mahajan, A.; Kingon, A.I.; Kukovecz, K.; Vilarinho, P. Studies on the thermal decomposition of multiwall carbon nanotubes under different atmospheres. Mater. Lett. 2013, 90, 165–168. [Google Scholar] [CrossRef]
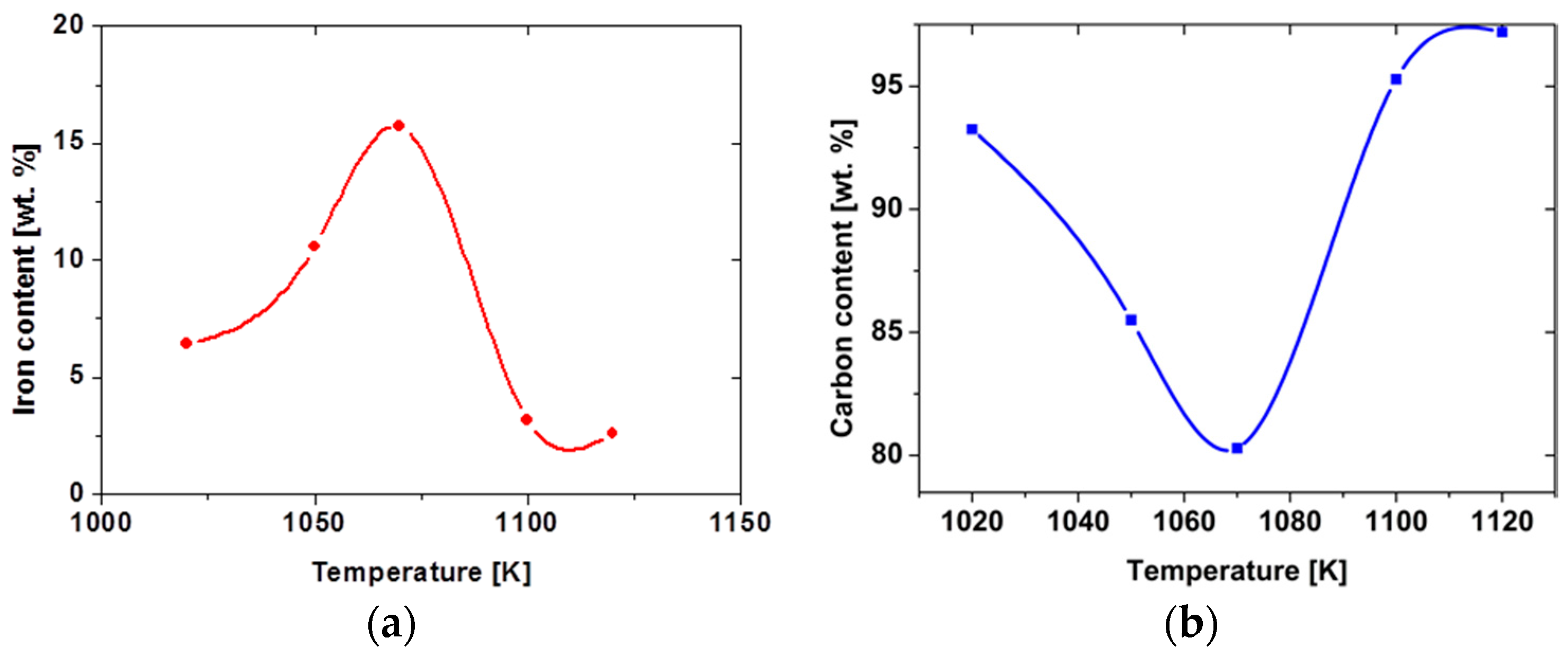
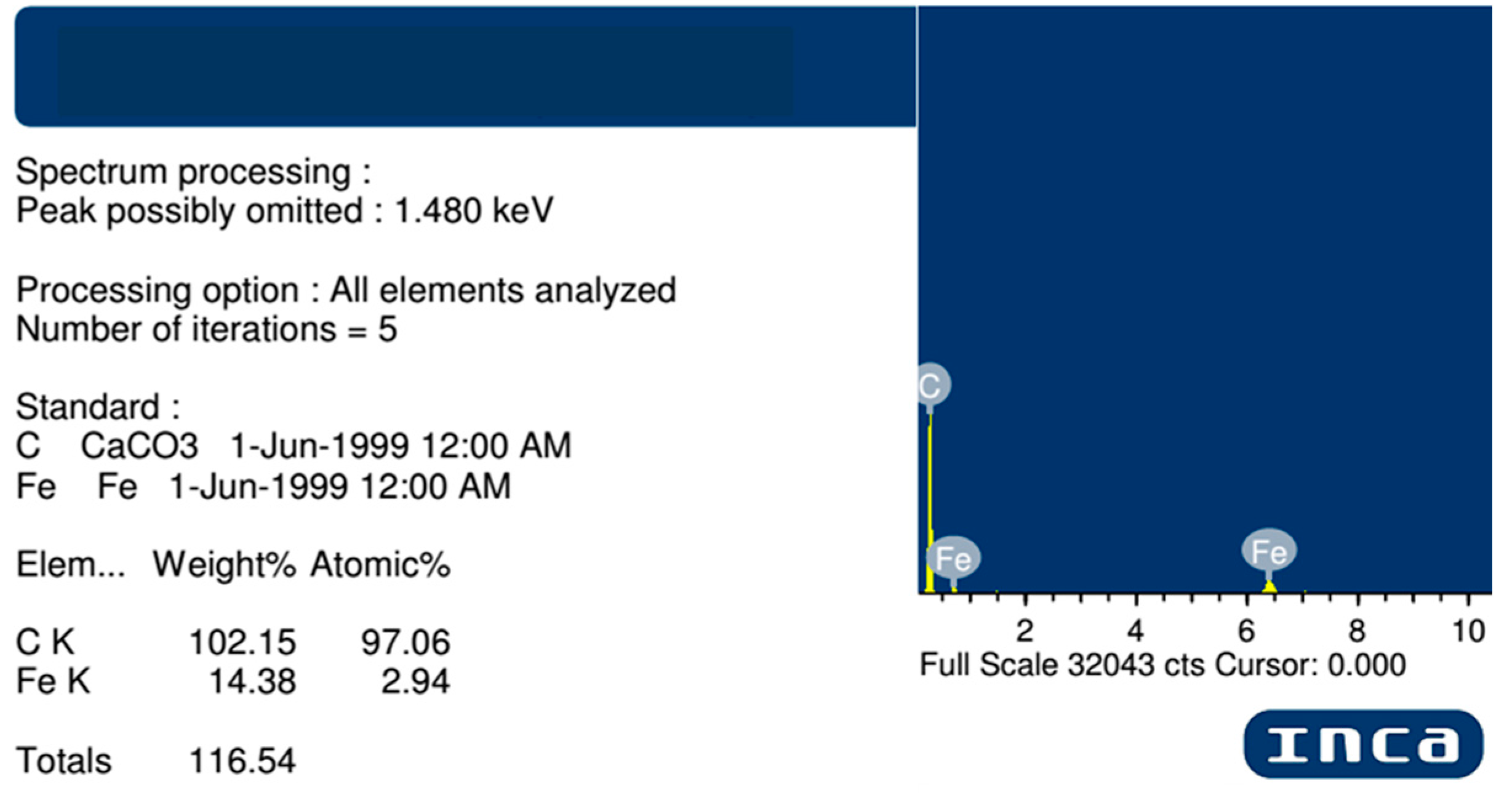
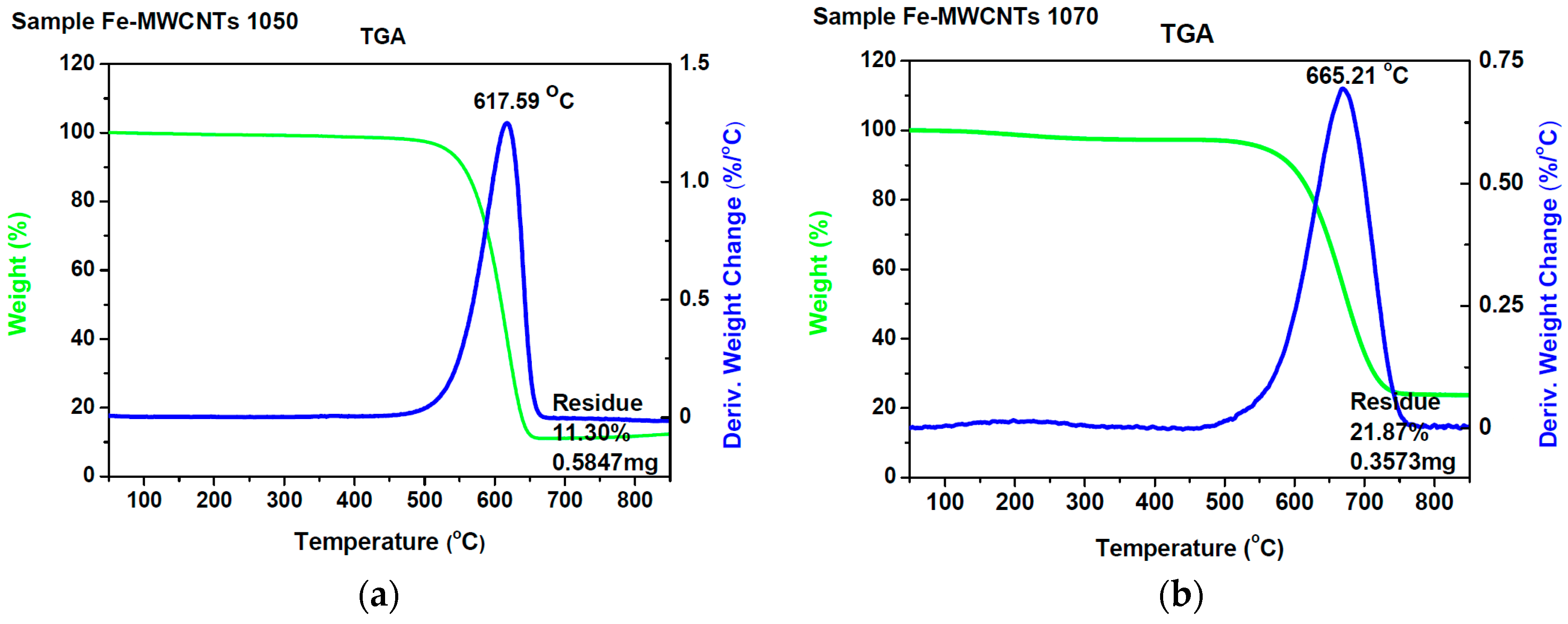
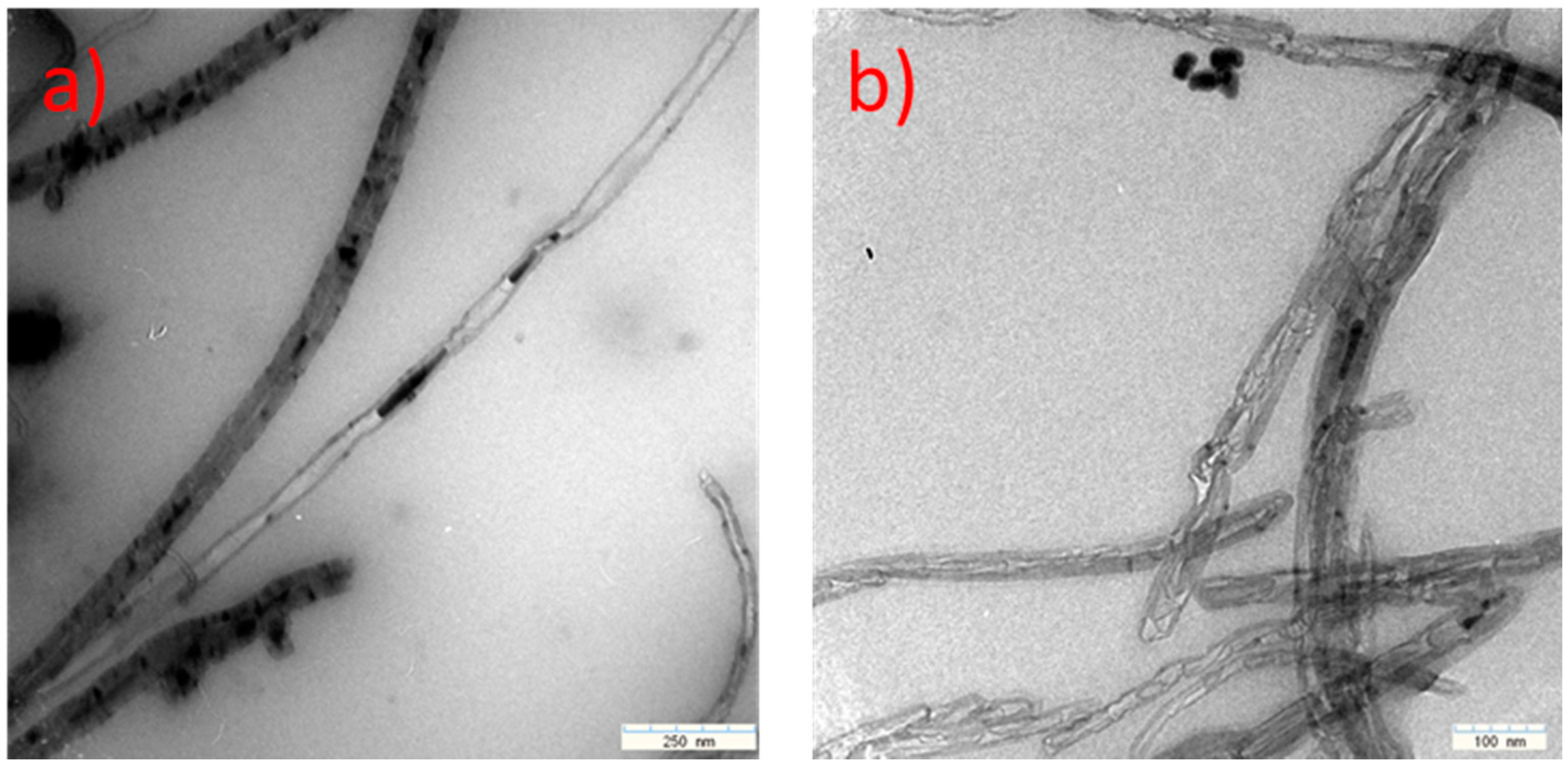
| Methods | Short Description of the Synthesis Conditions | Temperature | Reference |
|---|---|---|---|
| PECVD | Pressure of 5–10 torr, Ni catalyst, reagents: methane and ethane | 770 K | [49] |
| PECVD | Reagents methane + hydrogen | 790 K | [50] |
| PECVD | Reaction with alcohol | 720 K | [51] |
| CVD | Reaction with benzene/ferrocene | 670/840 K | [52] |
| CVD | The reaction with methanol, acetylene, and Co, Co/Fe, Ni/Co/Fe/Al2O3 | 770 K | [53] |
| CVD | The reaction of acetylene with NH3/H2, Fe/Ni/Co | 770–820 K | [54,55] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymanski, L.; Kolacinski, Z.; Wiak, S.; Raniszewski, G.; Pietrzak, L. Synthesis of Carbon Nanotubes in Thermal Plasma Reactor at Atmospheric Pressure. Nanomaterials 2017, 7, 45. https://doi.org/10.3390/nano7020045
Szymanski L, Kolacinski Z, Wiak S, Raniszewski G, Pietrzak L. Synthesis of Carbon Nanotubes in Thermal Plasma Reactor at Atmospheric Pressure. Nanomaterials. 2017; 7(2):45. https://doi.org/10.3390/nano7020045
Chicago/Turabian StyleSzymanski, Lukasz, Zbigniew Kolacinski, Slawomir Wiak, Grzegorz Raniszewski, and Lukasz Pietrzak. 2017. "Synthesis of Carbon Nanotubes in Thermal Plasma Reactor at Atmospheric Pressure" Nanomaterials 7, no. 2: 45. https://doi.org/10.3390/nano7020045







