In Vitro Comparative Skin Irritation Induced by Nano and Non-Nano Zinc Oxide
Abstract
:1. Introduction
2. Results and Discussion
2.1. ZnO Characterization
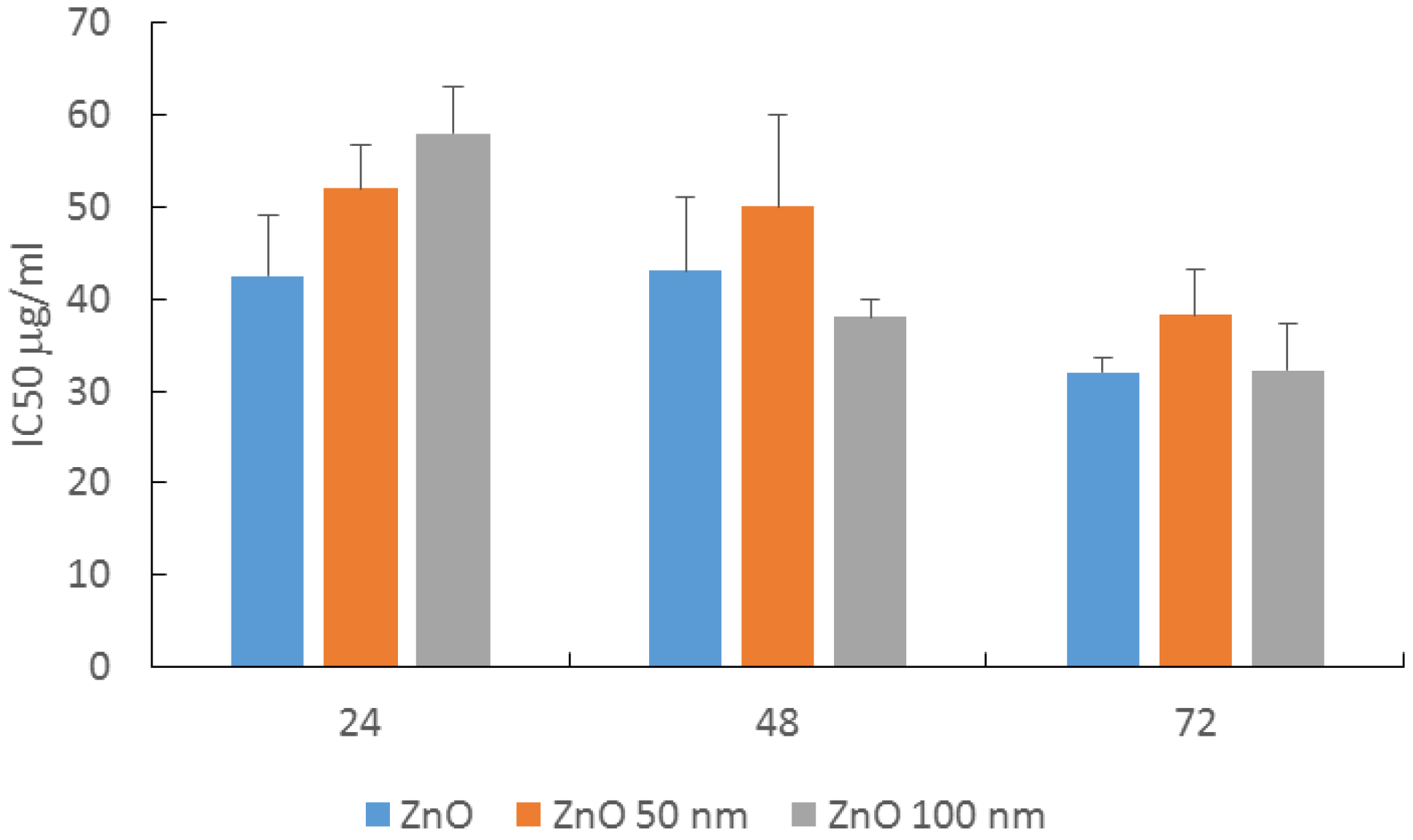
2.2. MTT Viability Assay in HaCaT Cells
2.3. Effect on HaCaT Morphology
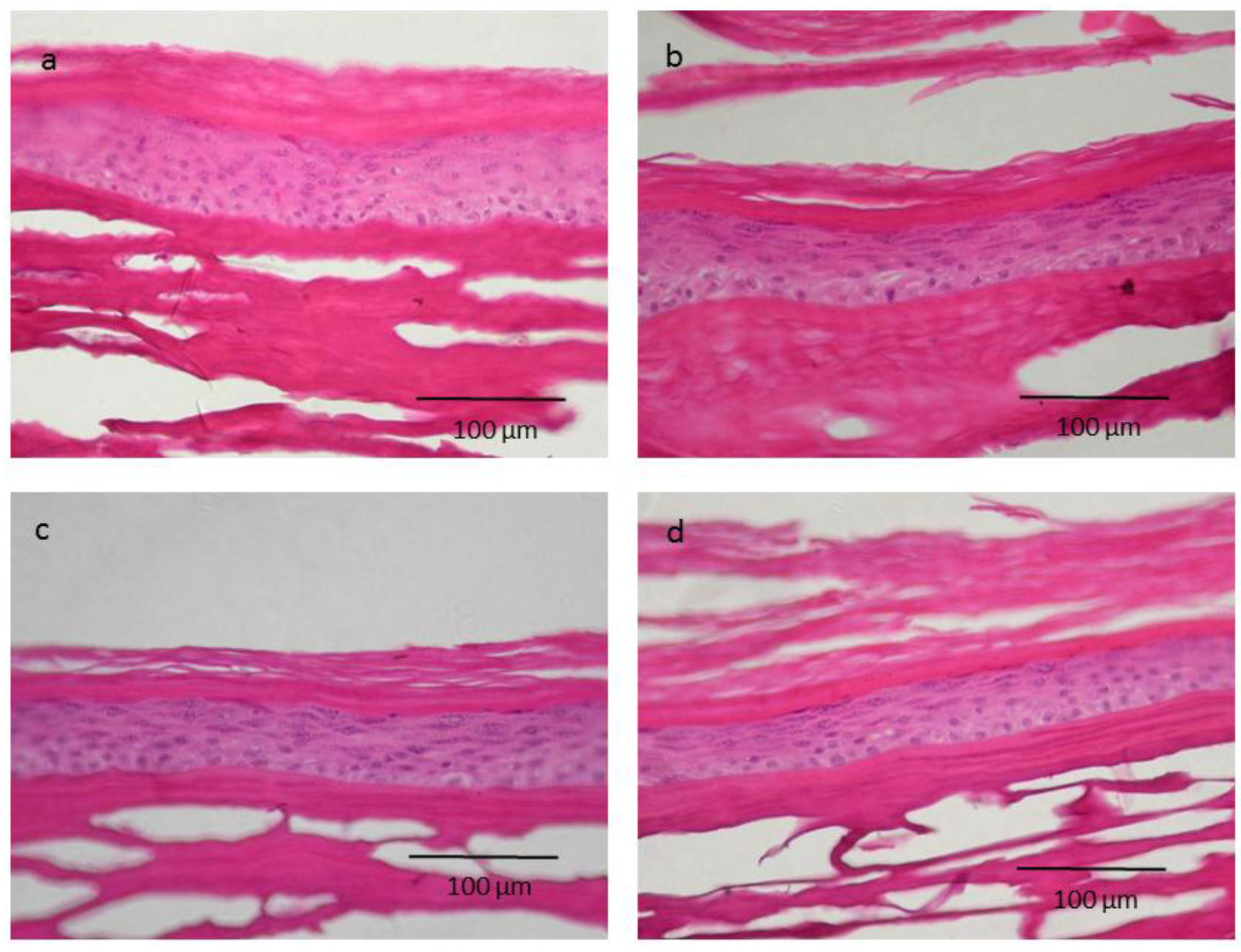
2.4. Skin Irritation on 3D Epidermis Model Study and Histology
3. Materials and Methods
3.1. ZnO Materials and Nanoparticles Characterization
3.2. Cell Culture and Treatment
3.3. MTT Vibility Assay
3.4. Effect on HaCaT Morphology
3.5. Skin Irritation on 3D Epidermal Model and Histology
3.6. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, W.T. Nanoparticles and their biological and environmental applications. J. Biosci. Bioeng. 2006, 102, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Dufour, E.K.; Roberts, M.S. Nanotechnology, cosmetics and the skin: Is there a health risk? Skin Pharmacol. Physiol. 2008, 21, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Warheit, D.B.; Ng, S.P.; Comfort, K.K.; Grabinski, C.M.; Braydich-Stolle, L.K. At the Crossroads of Nanotoxicology in vitro: Past Achievements and Current Challenges. Toxicol. Sci. 2015, 147, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, M.P.; Mitjans, M. Antitumor activities of metal oxides nanoparticles. Nanomaterials 2015, 5, 1004–1021. [Google Scholar] [CrossRef] [Green Version]
- Osmond, M.J.; McCall, M.J. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Kleinsasser, N. Dermal toxicity of ZnO nanoparticles: A worrying feature of sunscreen? Nanomedicine (Lond.) 2012, 7, 461–4613. [Google Scholar] [CrossRef] [PubMed]
- Gulson, B.; McCall, M.J.; Bowman, D.M.; Pinheiro, T. A review of critical factors for assessing the dermal absorption of metal oxide nanoparticles from sunscreens applied to humans, and a research strategy to address current deficiencies. Arch. Toxicol. 2015, 89, 1909–1930. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Dufour, E.K. Nano-sized cosmetic formulations or solid nanoparticles in sunscreens: A risk to human health? Arch. Toxicol. 2012, 86, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.M.; Song, Z.; Moghimi, H.R.; Roberts, M.S. Relative Penetration of Zinc Oxide and Zinc Ions into Human Skin after Application of Different Zinc Oxide Formulations. ACS Nano 2016, 10, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.J.; Seo, M.Y.; Jung, S.K.; Maeng, E.H.; Lee, S.Y.; Jang, D.H.; Lee, T.J.; Jo, K.Y.; Kim, Y.R.; Cho, K.B.; et al. Zinc oxide nanoparticles: A 90-day repeated-dose dermal toxicity study in rats. Int. J. Nanomed. 2014, 9 (Suppl. 2), 137–144. [Google Scholar]
- Everett, W.N.; Chern, C.; Sun, D.; McMahon, R.E.; Zhang, X.; Chen, W.J.; Hahn, M.S.; Sue, H.J. Phosphate-enhanced cytotoxicity of zinc oxide nanoparticles and agglomerates. Toxicol. Lett. 2014, 225, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.H.; Tso, C.P.; Tsai, Y.C.; Zhuang, C.M.; Shih, Y.H. The effect of electrolytes on the aggregation kinetics of three different ZnO nanoparticles in water. Sci. Total Environ. 2015, 530–531, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, H.R.; Kim, Y.R.; Kim, M.K. Toxic response of zinc oxide nanoparticles in human epidermal keratinocyte HaCaT cells. Toxicol. Environ. Health Sci. 2012, 4, 14–18. [Google Scholar] [CrossRef]
- Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Dwivedi, S.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Shin, H.S. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B 2014, 117, 267–276. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD Publishing: Paris, France, 2015. [Google Scholar]
- Choi, J.; Kim, H.; Choi, J.; Oh, S.M.; Park, J.; Park, K. Skin corrosion and irritation test of sunscreen nanoparticles using reconstructed 3D human skin model. Environ. Health Toxicol. 2014, 29, e2014004. [Google Scholar] [CrossRef] [PubMed]
- Surekha, P.; Kishore, A.S.; Srinivas, A.; Selvam, G.; Goparaju, A.; Reddy, P.N.; Murthy, P.B. Repeated dose dermal toxicity study of nano zinc oxide with Sprague-Dawley rats. Cutan. Ocul. Toxicol. 2012, 31, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, H.J.; Ryu, H.J.; Ryu, W.I.; Park, Y.H.; Bae, H.C.; Jang, Y.S.; Son, S.W. ZnO nanoparticles induce TNF-α expression via ROS-ERK-Egr 1 pathway in human keratinocytes. J. Dermatol. Sci. 2013, 72, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Leite-Silva, V.R.; Liu, D.C.; Sanchez, W.Y.; Studier, H.; Mohammed, Y.H.; Holmes, A.; Becker, W.; Grice, J.E.; Benson, H.A.; Roberts, M.S. Effect of flexing and massage on in vivo human skin penetration and toxicity of zinc oxide nanoparticles. Nanomedicine (Lond.) 2016, 11, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay to cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Inman, A.O.; Zhang, L.W. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol. Appl. Pharm. 2009, 234, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Kroll, A.; Pillukat, M.H.; Hahn, D.; Schnekenburger, J. Interference of engineered nanoparticles with in vitro toxicity assays. Arch. Toxicol. 2012, 86, 1123–1136. [Google Scholar] [CrossRef] [PubMed]



| Nanoparticles | Distilled Water | PBS | DMEM | |||
|---|---|---|---|---|---|---|
| Hydrodynamic Size (nm) | PDI | Hydrodynamic Size (nm) | PDI | Hydrodynamic Size (nm) | PDI | |
| ZnO 50 nm | 208.7 ± 6.8 | 0.3 ± 0.0 | 969.8 ± 275.9 | 1.0 ± 0.0 | 239.8 ± 1.6 | 0.3 ± 0.0 |
| ZnO 100 nm | 1008.7 ± 329.0 | 0.4 ± 0.4 | 1120.7 ± 118.9 | 1.0 ± 0.1 | 93.1 ± 1.3 | 0.3 ± 0.0 1 |
| % Viability | PBS | SDS | ZnO | ZnO 50 nm | ZnO 100 nm |
|---|---|---|---|---|---|
| Mean ± SD | 100.00 ± 6.20 | 21.12 ± 5.52 | 109.84 ± 3.37 | 100.50 ± 14.27 | 102.77 ± 11.32 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinardell, M.P.; Llanas, H.; Marics, L.; Mitjans, M. In Vitro Comparative Skin Irritation Induced by Nano and Non-Nano Zinc Oxide. Nanomaterials 2017, 7, 56. https://doi.org/10.3390/nano7030056
Vinardell MP, Llanas H, Marics L, Mitjans M. In Vitro Comparative Skin Irritation Induced by Nano and Non-Nano Zinc Oxide. Nanomaterials. 2017; 7(3):56. https://doi.org/10.3390/nano7030056
Chicago/Turabian StyleVinardell, Maria Pilar, Hector Llanas, Laura Marics, and Montserrat Mitjans. 2017. "In Vitro Comparative Skin Irritation Induced by Nano and Non-Nano Zinc Oxide" Nanomaterials 7, no. 3: 56. https://doi.org/10.3390/nano7030056







