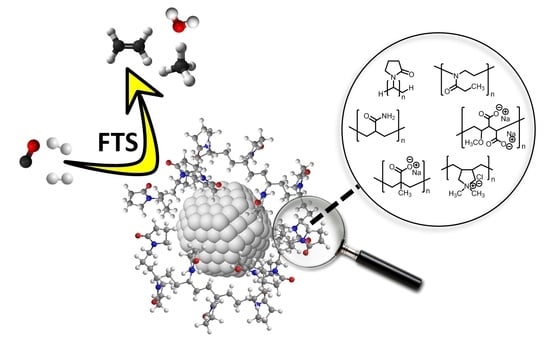
Effect of the Polymeric Stabilizer in the Aqueous Phase Fischer-Tropsch Synthesis Catalyzed by Colloidal Cobalt Nanocatalysts
Abstract
:1. Introduction
2. Results and Discussion
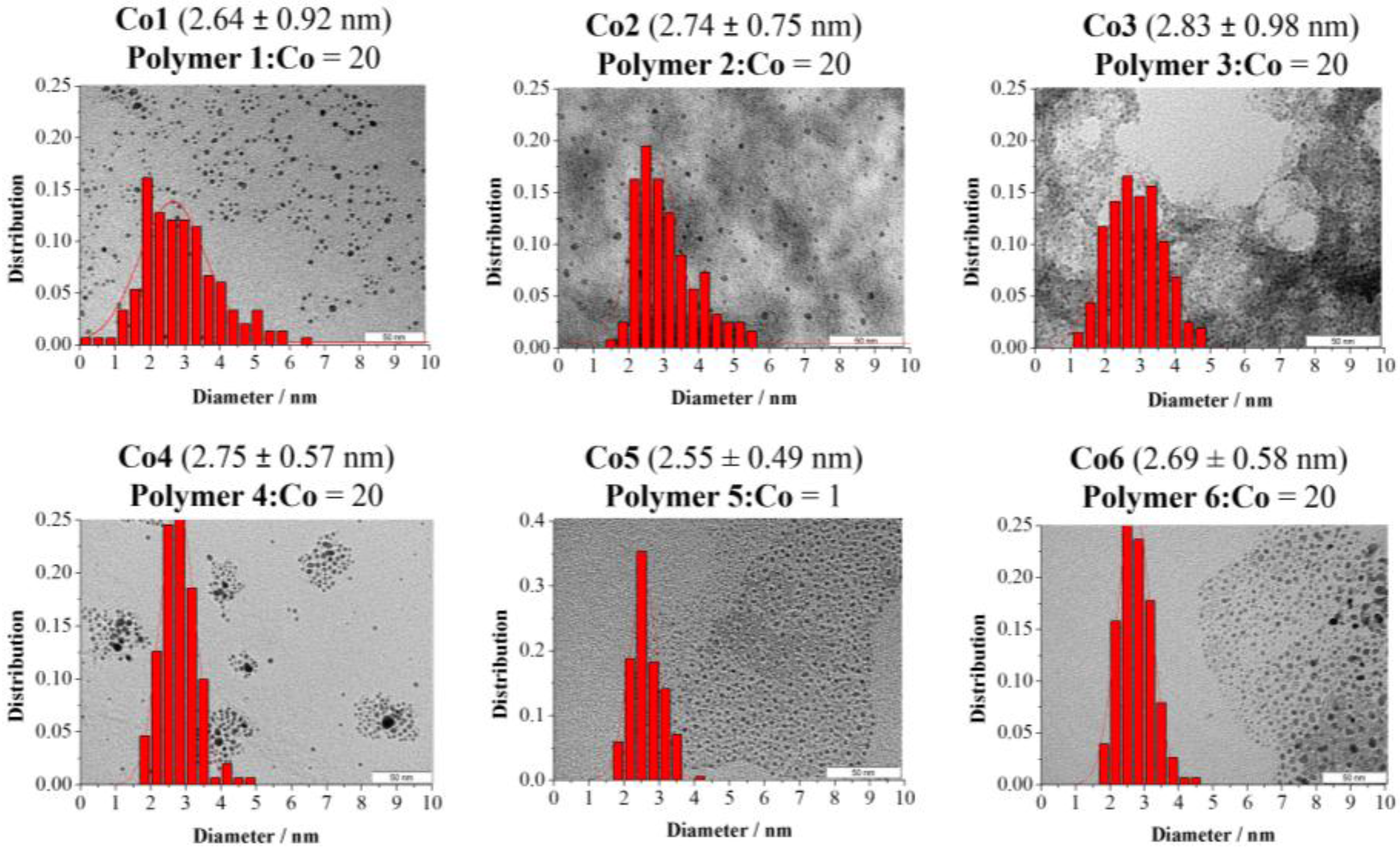
2.1. Synthesis and Characterization of CoNPs
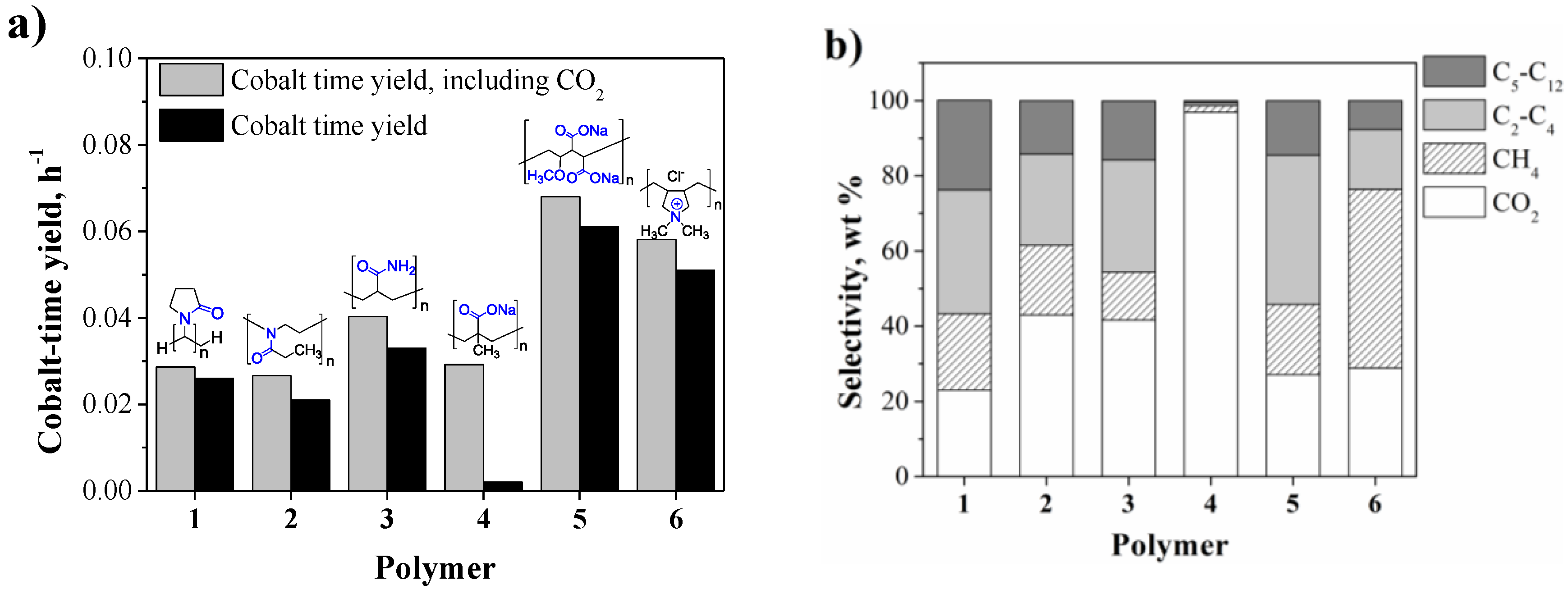
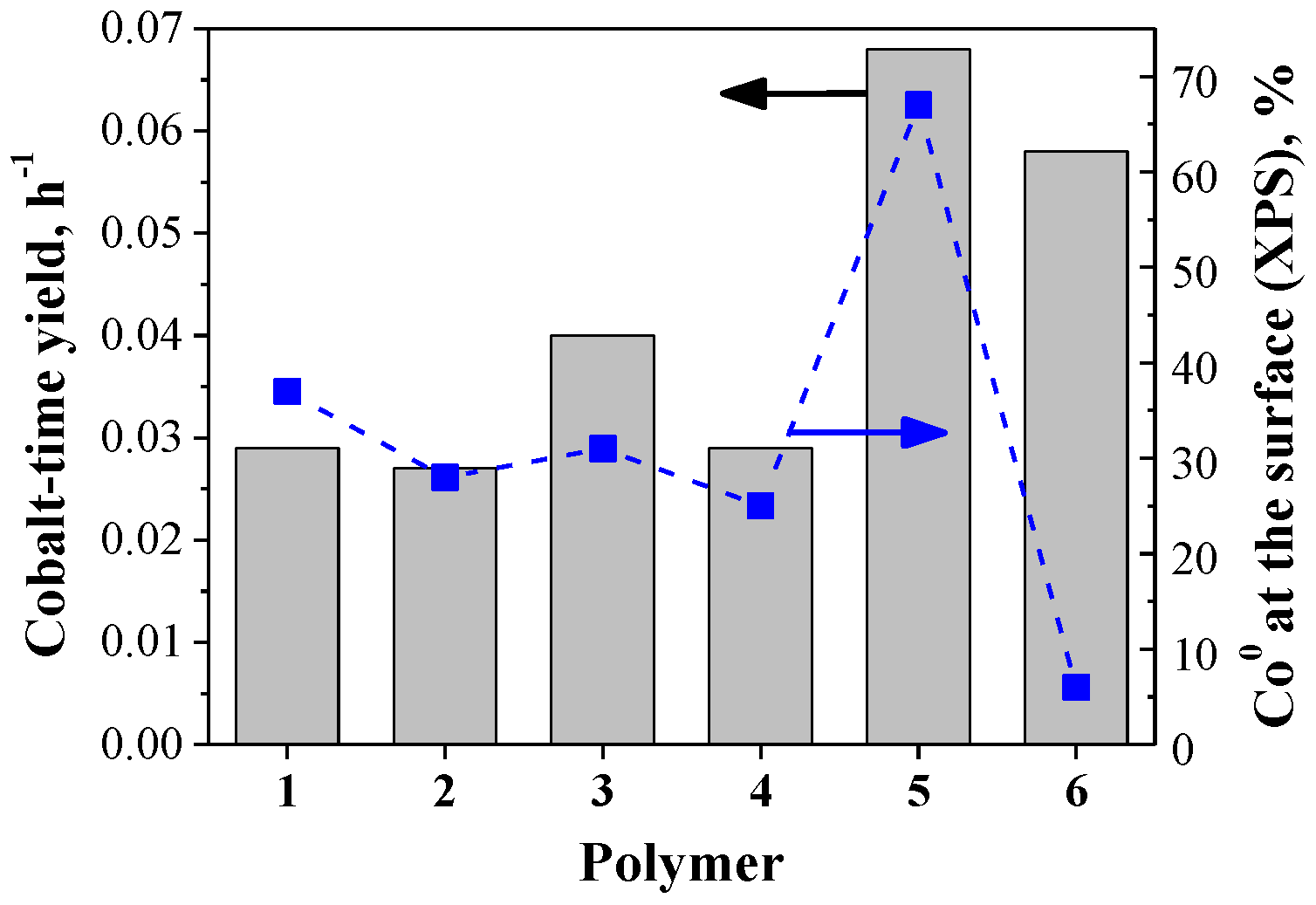
2.2. Aqueous Phase Fischer-Tropsch Catalytic Experiments
3. Materials and Methods
3.1. Synthesis of Polymeric Stabilized CoNPs, Co1–Co6
3.2. Aqueous Phase Fischer-Tropsch Experiments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AFTS | aqueous phase Fischer-Tropsch synthesis |
| WGS | water gas shift |
| NPs | nanoparticles |
| TEM | transmission electron microscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| FTIR | Fourier-transformed infrared spectroscopy |
| ICP | indouced coupled plasma |
References
- An, K.; Somorjai, G.A. Size and shape control of metal nanoparticles for reaction selectivity in catalysis. ChemCatChem 2012, 4, 1512–1524. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Bitter, J.H.; Kuipers, H.P.C.E.; Oosterbeek, H.; Holewijn, J.E.; Xu, X.; Kapteijn, F.; van Dillen, A.J.; de Jong, K.P. Cobalt particle size effects in the Fischer-Tropsch reaction studied with carbon nanofiber supported catalysts. J. Am. Chem. Soc. 2006, 128, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Steynberg, A. Fischer-Tropsch technology. Studies in surface science and catalysis. In Fischer-Tropsch Technology. Studies in Surface Science and Catalysis; Steynberg, A., Dry, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 152, pp. 1–3. [Google Scholar]
- Gates, B.D.; Xu, Q.; Stewart, M.; Ryan, D.; Willson, C.G.; Whitesides, G.M. New approaches to nanofabrication: Molding, printing, and other techniques. Chem. Rev. 2005, 105, 1171–1196. [Google Scholar] [CrossRef] [PubMed]
- Aguey-Zinsou, K.-F.; Ares-Fernandez, J.-R. Hydrogen in magnesium: New perspectives toward functional stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Vilé, G.; Almora-Barrios, N.; Mitchell, S.; López, N.; Pérez-Ramírez, J. From the Lindlar catalyst to supported ligand-modified palladium nanoparticles: Selectivity patterns and accessibility constraints in the continuous-flow three-phase hydrogenation of acetylenic compounds. Chem. Eur. J. 2014, 20, 5926–5937. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Prieto, L.M.; Carenco, S.; Wu, C.H.; Bonnefille, E.; Axnanda, S.; Liu, Z.; Fazzini, P.F.; Philippot, K.; Salmeron, M.; Chaudret, B. Organometallic ruthenium nanoparticles as model catalysts for CO hydrogenation: A nuclear magnetic resonance and ambient-pressure X-ray photoelectron spectroscopy study. ACS Catal. 2014, 4, 3160–3168. [Google Scholar] [CrossRef]
- Quek, X.Y.; Pestman, R.; van Santen, R.A.; Hensen, E.J.M. Effect of organic capping agents on ruthenium-nanoparticle-catalyzed aqueous-phase Fischer-Tropsch synthesis. ChemCatChem 2013, 5, 3148–3155. [Google Scholar] [CrossRef]
- Delgado, J.A.; Claver, C.; Castillón, S.; Curulla-Ferré, D.; Ordomsky, V.V.; Godard, C. Effect of polymeric stabilizers on Fischer-Tropsch synthesis catalyzed by cobalt nanoparticles supported on TiO2. J. Mol. Catal. A 2016, 417, 43–52. [Google Scholar] [CrossRef]
- Wang, H.; Kou, Y. Aqueous-phase Fischer–Tropsch synthesis catalyzed by cobalt nanoparticles. Chin. J. Catal. 2013, 34, 1914–1925. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, W.; Liu, J.X.; Si, R.; Sun, G.; Zhong, M.Q.; Su, H.Y.; Zhao, H.B.; Rodriguez, J.A.; Pennycook, S.J.; et al. Platinum-modulated cobalt nanocatalysts for low-temperature aqueous-phase Fischer-Tropsch synthesis. J. Am. Chem. Soc. 2013, 135, 4149–4158. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Zhang, J.G.; Tong, Y.; Yao, S.; Xiao, C.; Li, Z.; Kou, Y. Solubility adjustable nanoparticles stabilized by a novel PVP based family: Synthesis, characterization and catalytic properties. Chem. Commun. 2009, 4423–4425. [Google Scholar] [CrossRef] [PubMed]
- Scariot, M.; Silva, D.O.; Scholten, J.D.; Machado, G.; Teixeira, S.R.; Novak, M.A.; Ebeling, G.; Dupont, J. Cobalt nanocubes in ionic liquids: Synthesis and properties. Angew. Chem. Int. Ed. 2008, 47, 9075–9078. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.O.; Luza, L.; Gual, A.; Baptista, D.L.; Bernardi, F.; Zapata, M.J.M.; Morais, J.; Dupont, J. Straightforward synthesis of bimetallic Co/Pt nanoparticles in ionic liquid: Atomic rearrangement driven by reduction-sulfidation processes and Fischer–Tropsch catalysis. Nanoscale 2014, 6, 9085–9092. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.O.; Scholten, J.D.; Gelesky, M.A.; Teixeira, S.R.; Dos Santos, A.C.B.; Souza-Aguiar, E.F.; Dupont, J. Catalytic gas-to-liquid processing using cobalt nanoparticles dispersed in imidazolium ionic liquids. ChemSusChem 2008, 1, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.B.; Tao, Z.Y.; Xiao, C.X.; Liu, F.; Kou, Y. Liquid-phase Fischer–Tropsch synthesis over Fe nanoparticles dispersed in polyethylene glycol (PEG). Green Chem. 2010, 12, 795–797. [Google Scholar] [CrossRef]
- Meffre, A.; Mehdaoui, B.; Connord, V.; Carrey, J.; Fazzini, P.F.; Lachaize, S.; Respaud, M.; Chaudret, B. Complex nano-objects displaying both magnetic and catalytic properties: A proof of concept for magnetically induced heterogeneous catalysis. Nano Lett. 2015, 15, 3241–3248. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liew, K.Y.; Li, J. Size controlled synthesis of Co nanoparticles by combination of organic solvent and surfactant. Appl. Surf. Sci. 2009, 255, 4039–4044. [Google Scholar] [CrossRef]
- Chou, K.S.; Chang, Y.C.; Chiu, L.H. Studies on the continuous precipitation of silver nanoparticles. Ind. Eng. Chem. Res. 2012, 51, 4905–4910. [Google Scholar] [CrossRef]
- Seoudi, R.; Shabaka, A.; El Sayed, Z.A.; Anis, B. Effect of stabilizing agent on the morphology and optical properties of silver nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2011, 44, 440–447. [Google Scholar] [CrossRef]
- Lu, L.T.; Tung, L.D.; Robinson, I.; Ung, D.; Tan, B.; Long, J.; Cooper, A.I.; Fernig, D.G.; Thanh, N.T.K. Size and shape control for water-soluble magnetic cobalt nanoparticles using polymer ligands. J. Mater. Chem. 2008, 18, 2453–2458. [Google Scholar] [CrossRef]
- Shimmin, R.G.; Schoch, A.B.; Braun, P.V. Polymer size and concentration effects on the size of gold nanoparticles capped by polymeric thiols. Langmuir 2004, 20, 5613–5620. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.A.; Claver, C.; Castillón, S.; Curulla-Ferré, D.; Godard, C. Correlation between hydrocarbon product distribution and solvent composition in the Fischer–Tropsch synthesis catalyzed by colloidal cobalt nanoparticles. ACS Catal. 2015, 5, 4568–4578. [Google Scholar] [CrossRef]
- Delgado, J.A.; Castillón, S.; Curulla-Ferré, D.; Claver, C.; Godard, C. Effect of pH on catalyst activity and selectivity in the aqueous Fischer-Tropsch synthesis catalyzed by cobalt nanoparticles. Catal. Commun. 2015, 71, 88–92. [Google Scholar] [CrossRef]
- Garcia-Torres, J.; Vallés, E.; Gómez, E. Synthesis and characterization of Co@Ag core–shell nanoparticles. J. Nanopart. Res. 2010, 12, 2189–2199. [Google Scholar] [CrossRef]
- Petit, C.; Wang, Z.L.; Pileni, M.P. Seven-nanometer hexagonal close packed cobalt nanocrystals for high-temperature magnetic applications through a novel annealing process. J. Phys. Chem. B. 2005, 109, 15309–15316. [Google Scholar] [CrossRef] [PubMed]
- Doan, N.; Kontturi, K.; Johans, C. Directing oxidation of cobalt nanoparticles with the capping ligand. J. Colloid Interface Sci. 2010, 350, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Dobbrow, C.; Schmidt, A.M. Improvement of the oxidation stability of cobalt nanoparticles. Beilstein J. Nanotechnol. 2012, 3, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Ho, K.M.; Wu, A.H.; Wong, K.H.; Li, P. Hydrothermal microemulsion synthesis of oxidatively stable cobalt nanocrystals encapsulated in surfactant/polymer complex shells. Langmuir 2010, 26, 6009–6014. [Google Scholar] [CrossRef] [PubMed]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Borohydride reduction of cobalt ions in water. Chemistry leading to nanoscale metal, boride, or borate particles. Langmuir 1993, 9, 162–169. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Cobalt-based catalysts for the hydrolysis of NaBH4 and NH3BH3. Phys. Chem. Chem. Phys. 2014, 16, 6872–6885. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Coville, N.J. Effect of cobalt source on the catalyst reducibility and activity of boron-modified Co/TiO2 Fischer-Tropsch catalysts. S. Afr. J. Chem. 2003, 56, 1–4. [Google Scholar]
- Martens, J.H.A.; Van’t Blik, H.F.J.; Prins, R. Characterization of supported cobalt and cobalt-rhodium catalysts: II. Temperature-programmed reduction (TPR) and oxidation (TPO) of CoTiO2 and Co-Rh TiO2. J. Catal. 1986, 97, 200–209. [Google Scholar] [CrossRef]
- Van Steen, E.; Claeys, M.; Dry, M.E.; van de Loosdrecht, J.; Viljoen, E.L.; Visagie, J.L. Stability of nanocrystals: Thermodynamic analysis of oxidation and re-reduction of cobalt in water/hydrogen mixtures. J. Phys. Chem. B 2005, 109, 3575–3577. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Coville, N.J. The effect of boron on the catalyst reducibility and activity of Co/TiO2 Fischer-Tropsch catalysts. App. Catal. A 1999, 181, 201–208. [Google Scholar] [CrossRef]
- Saeys, M.; Tan, K.F.; Chang, J.; Borgna, A. Improving the stability of cobalt Fischer-Tropsch catalysts by boron promotion. Ind. Eng. Chem. Res. 2010, 49, 11098–11100. [Google Scholar] [CrossRef]
- Tan, K.F.; Chang, J.; Borgna, A.; Saeys, M. Effect of boron promotion on the stability of cobalt Fischer-Tropsch catalysts. J. Catal. 2011, 280, 50–59. [Google Scholar] [CrossRef]
- Delgado, J.A.; Claver, C.; Castillón, S.; Curulla-Ferré, D.; Ordomsky, V.V.; Godard, C. Fischer–Tropsch synthesis catalysed by small TiO2 supported cobalt nanoparticles prepared by sodium borohydride reduction. App. Catal. A 2016, 513, 39–46. [Google Scholar] [CrossRef]
- Lillebø, A.H.; Patanou, E.; Yang, J.; Blekkan, E.A.; Holmen, A. The effect of alkali and alkaline earth elements on cobalt based Fischer-Tropsch catalysts. Catal. Today 2013, 215, 60–66. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Pendyala, V.R.R.; Jacobs, G.; Sparks, D.E.; Shafer, W.D.; Davis, B.H. Fischer–Tropsch synthesis: Effect of halides and potassium addition on activity and selectivity of cobalt. Catal. Lett. 2014, 144, 1127–1133. [Google Scholar] [CrossRef]
- Gual, A.; Delgado, J.A.; Godard, C.; Castillón, S.; Curulla-Ferré, D.; Claver, C. Novel polymer stabilized water soluble Ru-nanoparticles as aqueous colloidal Fischer-Tropsch catalysts. Top. Catal. 2013, 56, 1208–1219. [Google Scholar] [CrossRef]

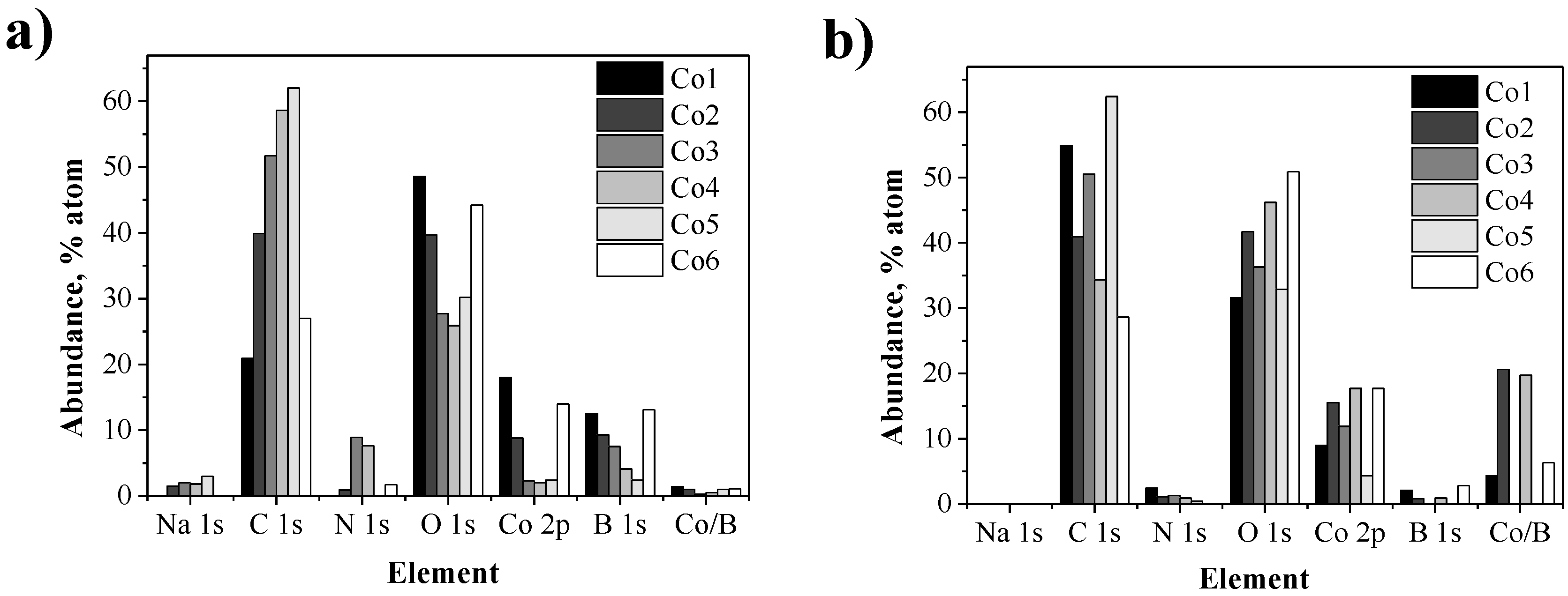
| NPs | Polymer | XPS Quantification Regions (mol %) | ICP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Na 1s | C 1s | N 1s | O 1s | Co 2p | B 1s | Co/B | Co/B | ||
| Co1 | 1 | 21 | 49 | 18 | 13 | 1.4 | 2.8 | ||
| Co2 | 2 | 2 | 40 | 1 | 40 | 9 | 9 | 1.0 | 2.6 |
| Co3 | 3 | 2 | 52 | 9 | 28 | 2 | 8 | 0.3 | 2.4 |
| Co4 | 4 | 2 | 59 | 8 | 26 | 2 | 4 | 0.5 | 6.4 |
| Co5 | 5 | 3 | 62 | 30 | 2 | 2 | 1.0 | 2.5 | |
| Co6 | 6 | 27 | 2 | 44 | 14 | 13 | 1.1 | 2.0 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, J.A.; Claver, C.; Castillón, S.; Curulla-Ferré, D.; Godard, C. Effect of the Polymeric Stabilizer in the Aqueous Phase Fischer-Tropsch Synthesis Catalyzed by Colloidal Cobalt Nanocatalysts. Nanomaterials 2017, 7, 58. https://doi.org/10.3390/nano7030058
Delgado JA, Claver C, Castillón S, Curulla-Ferré D, Godard C. Effect of the Polymeric Stabilizer in the Aqueous Phase Fischer-Tropsch Synthesis Catalyzed by Colloidal Cobalt Nanocatalysts. Nanomaterials. 2017; 7(3):58. https://doi.org/10.3390/nano7030058
Chicago/Turabian StyleDelgado, Jorge A., Carmen Claver, Sergio Castillón, Daniel Curulla-Ferré, and Cyril Godard. 2017. "Effect of the Polymeric Stabilizer in the Aqueous Phase Fischer-Tropsch Synthesis Catalyzed by Colloidal Cobalt Nanocatalysts" Nanomaterials 7, no. 3: 58. https://doi.org/10.3390/nano7030058