Highly Enhanced Photoreductive Degradation of Polybromodiphenyl Ethers with g-C3N4/TiO2 under Visible Light Irradiation
Abstract
:1. Introduction
2. Results
2.1. Catalyst Characterization
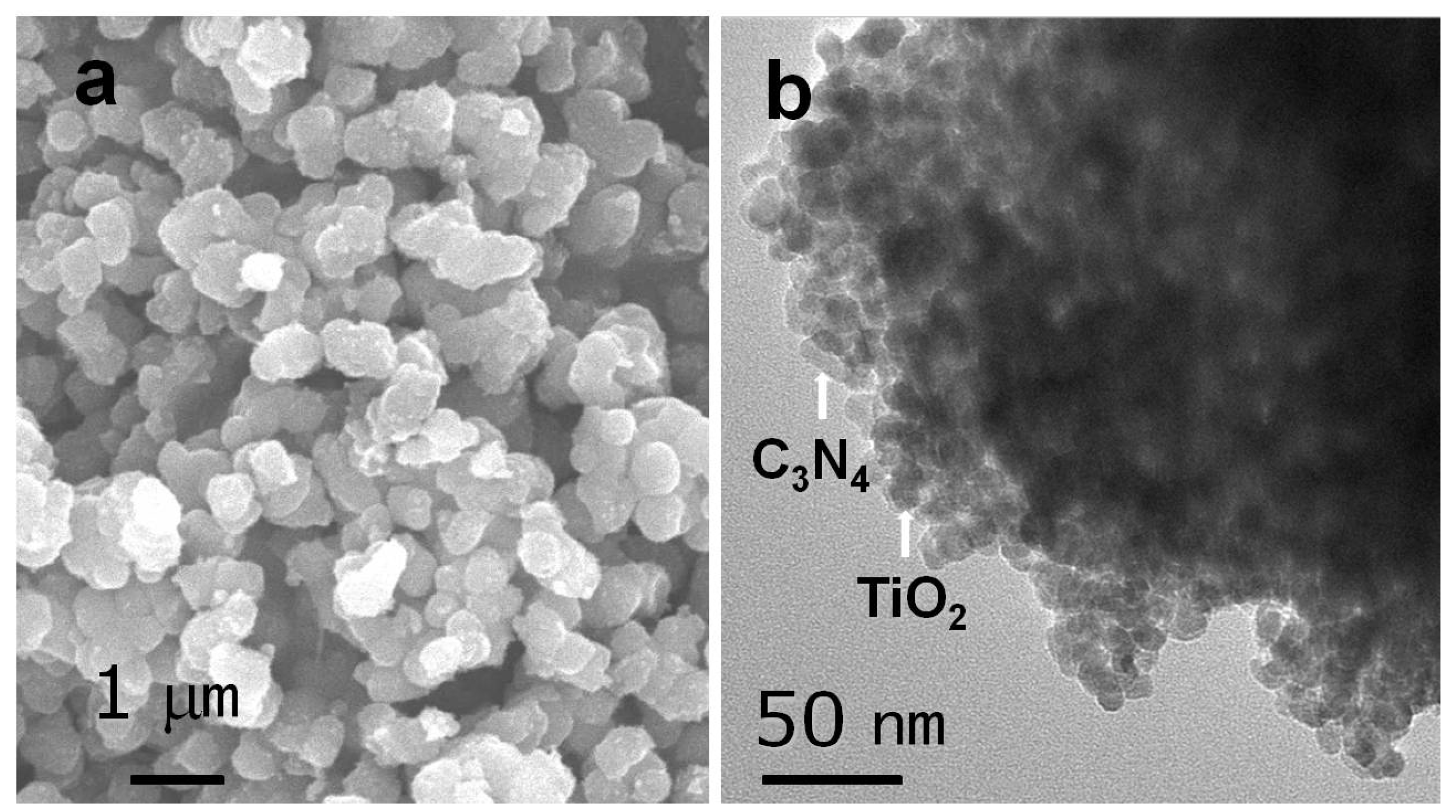
2.1.1. TEM and SEM
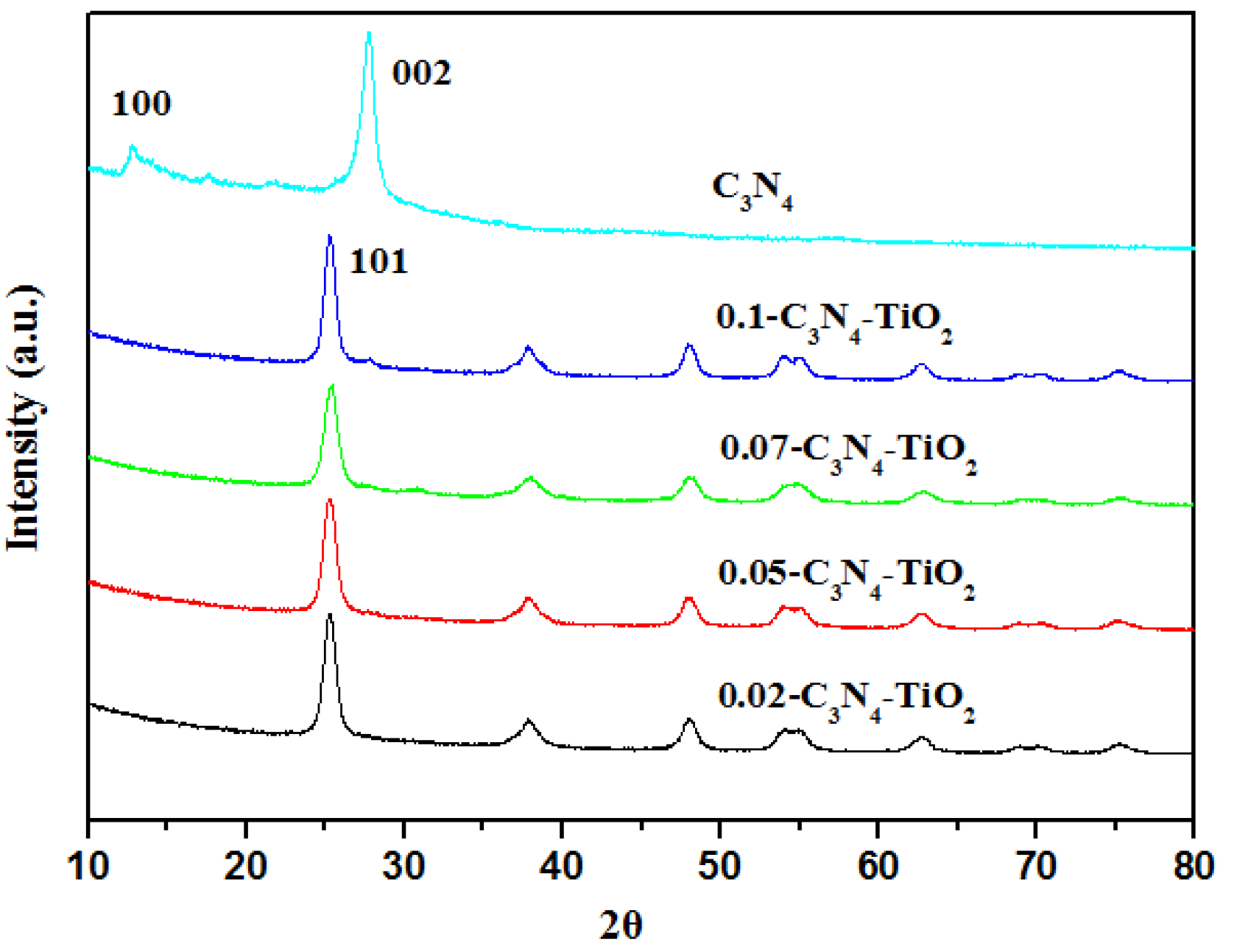
2.1.2. XRD
2.1.3. X-ray Photoelectron Spectroscopy (XPS)
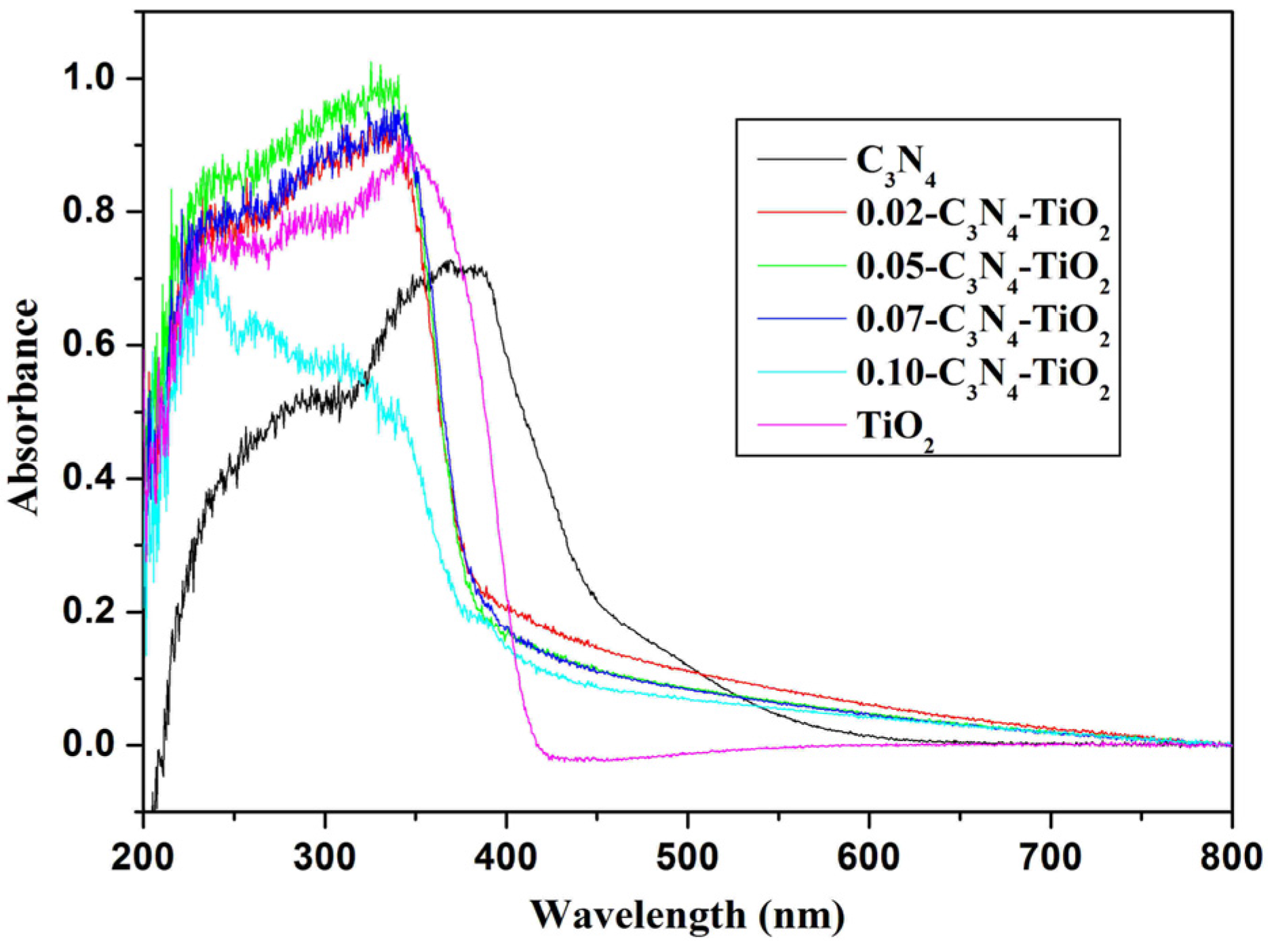
2.1.4. UV-Vis Absorption Spectra
2.2. Photocatalytic Reductive Performance Study
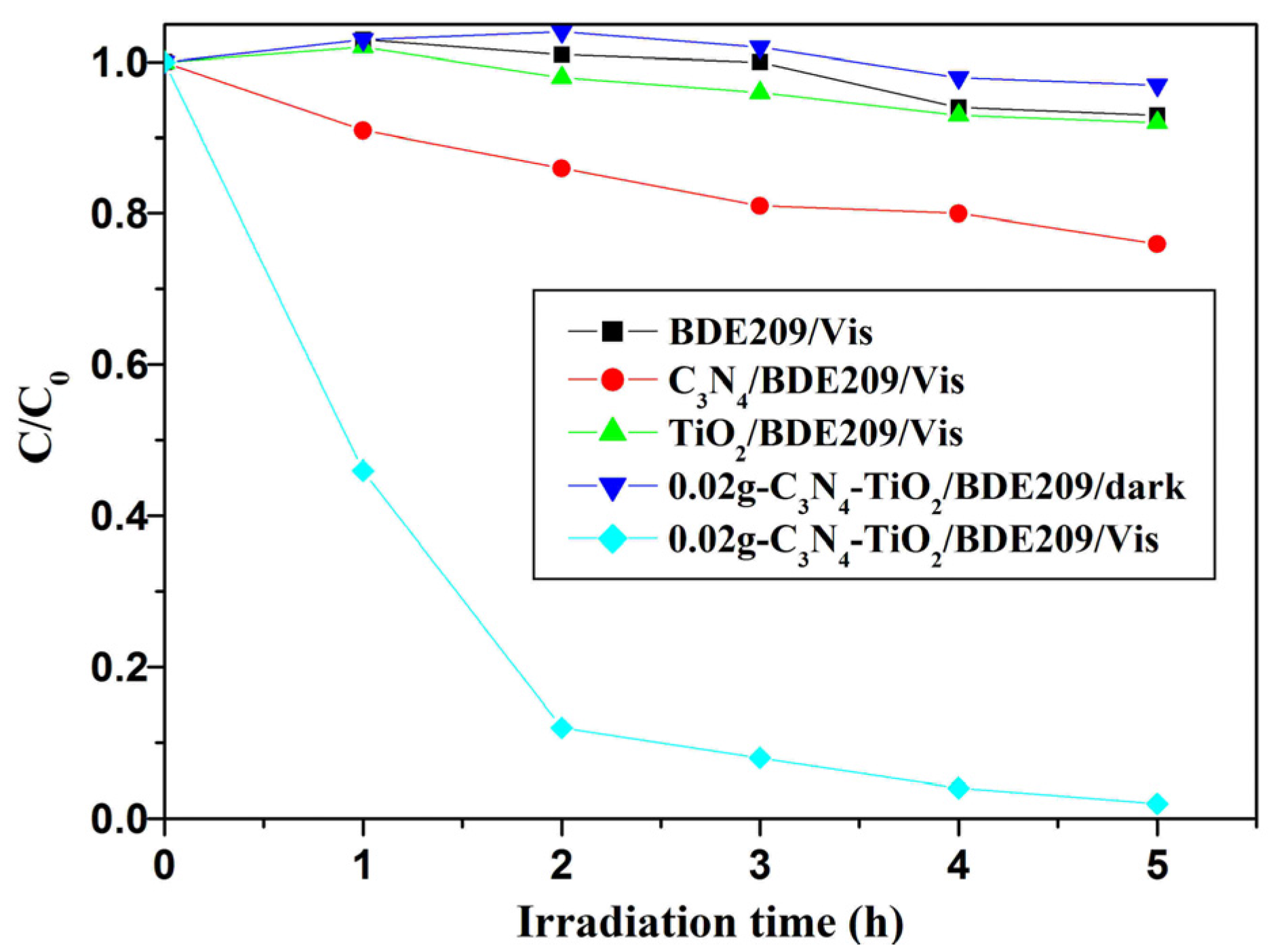
2.2.1. Degradation Kinetics
2.2.2. The Effects of Loaded Amount, Brunauer–Emmett–Teller (BET) Specific Surface Area, and Absorption Amount
2.2.3. Photostabilty of g-C3N4-TiO2
2.2.4. The Effect of Trapping Agents
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of g-C3N4-TiO2
4.2.2. Characterization
4.2.3. Experimental Setup
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Wit, C.A. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Mai, B.; Chen, S.; Luo, X.; Chen, L.; Yang, Q.; Sheng, G.; Peng, P.; Fu, J.; Zeng, E. Distribution of polybrominated diphenyl ethers in sediments of the Pearl River Delta and adjacent South China Sea. Environ. Sci. Technol. 2005, 39, 3521–3527. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Tai, C.; Zhao, Z.S.; Wang, Y.W.; Zhang, Q.H.; Jiang, G.B.; Hu, J.T. Debromination of decabrominated diphenyl ether by resin-bound iron nanoparticle. Environ. Sci. Technol. 2007, 41, 6841–6846. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Chang, W.; Ma, W.H.; Chen, C.C.; Zhao, J.C. Photoreductive Debromination of Decabromodiphenyl Ethers in the Presence of Carboxylates under Visible Light Irradiation. Environ. Sci. Technol. 2013, 47, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.R.; Martin, S.T.; Choi, W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Sun, C.Y.; Zhao, D.; Chen, C.C.; Ma, W.H.; Zhao, J.C. TiO2-mediated photocatalytic debromination of de-cabromodiphenyl ether: Kinetics and intermediates. Environ. Sci. Technol. 2009, 43, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.S.; Kau, J.H.; Huang, H.H.; Tseng, Y.H.; Wu, W.S.; Chang, H.H. Antibacterial Properties of Visible-Light-Responsive Carbon-Containing Titanium Dioxide Photocatalytic Nanoparticles against Anthrax. Nanomaterials 2016, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.C.; Antonietti, M. Polymeric graphitic carbon nitride as a heterogenous organocatalyst: From photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 2011, 50, 2–24. [Google Scholar]
- Liu, G.; Niu, P.; Sun, C.H.; Smith, S.C.; Chen, Z.G.; Lu, G.Q.; Cheng, H.M. Unique Electronic Structure Induced High Photoreactivity of Sulfur-Doped Graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.; Li, Q.Y.; Iwai, H.; Kako, T.; Ye, J.H. Hydrogen production using zinc-doped carbon nitride catalyst irradiated with visible light. Sci. Technol. Adv. Mater. 2011, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.J.; Ding, Z.G.; Liu, P.; Antonietti, M.; Fu, X.Z.; Wang, X.C. Metal-free activation of H2O2 by g-C3N4 under visible light irradiation for the degradation of organic pollutants. Phys. Chem. Chem. Phys. 2012, 14, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation of Rhodamine B and Methyl Orange over Boron-Doped g-C3N4 under Visible Light Irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Crittenden, J.C.; Hackney, S. Preparation of a novel TiO2-based p-n junction nanotube photocatalyst. Environ. Sci. Technol. 2005, 39, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.T.; Quan, X.; Chen, S. TiO2-multiwalled carbon nanotube heterojunction arrays and their charge separation capability. J. Phys. Chem. C 2007, 111, 12987–12991. [Google Scholar] [CrossRef]
- Zhao, S.S.; Chen, S.; Yu, H.T.; Quan, X. g-C3N4/TiO2 hybrid photocatalyst with wide absorption wavelength range and effective photogenerated charge separation. Sep. Purif. Technol. 2012, 99, 50–54. [Google Scholar] [CrossRef]
- Yan, H.J.; Yang, H.X. TiO2-g-C3N4 composite materials for photocatalytic H2 evolution under visible light irradiation. J. Alloys Compd. 2011, 509, L26–L29. [Google Scholar] [CrossRef]
- Chang, F.; Zhang, J.; Xie, Y.; Chen, J.; Li, C.; Wang, J.; Hu, X. Fabication, characterization and photocatalytic performance of exfoliated g-C3N4-TiO2 hybrids. Appl. Surf. Sci. 2014, 311, 574–581. [Google Scholar] [CrossRef]
- Sridharan, K.; Jang, E.Y.; Park, T.J. Novel visible light active graphitic C3N4-TiO2 composite photocatalyst: Synergistic synthesis, growth and photocatalytic treatment of hazardous pollutants. Appl. Catal. B Environ. 2013, 142, 718–728. [Google Scholar] [CrossRef]
- Mirandaa, C.; Mansillab, H.; Yánezb, J.; Obregóna, S.; Colóna, G. Improved photocatalytic activity of g-C3N4/TiO2 composites prepared by a simple impregnation method. J. Photochem. Photobiol. A Chem. 2013, 253, 16–21. [Google Scholar] [CrossRef]
- Sun, C.Y.; Chen, C.C.; Ma, W.H.; Zhao, J.C. Photocatalytic debromination of decabromodiphenyl ether by graphitic carbon nitride. Sci. China Chem. 2012, 55, 2532–2536. [Google Scholar] [CrossRef]
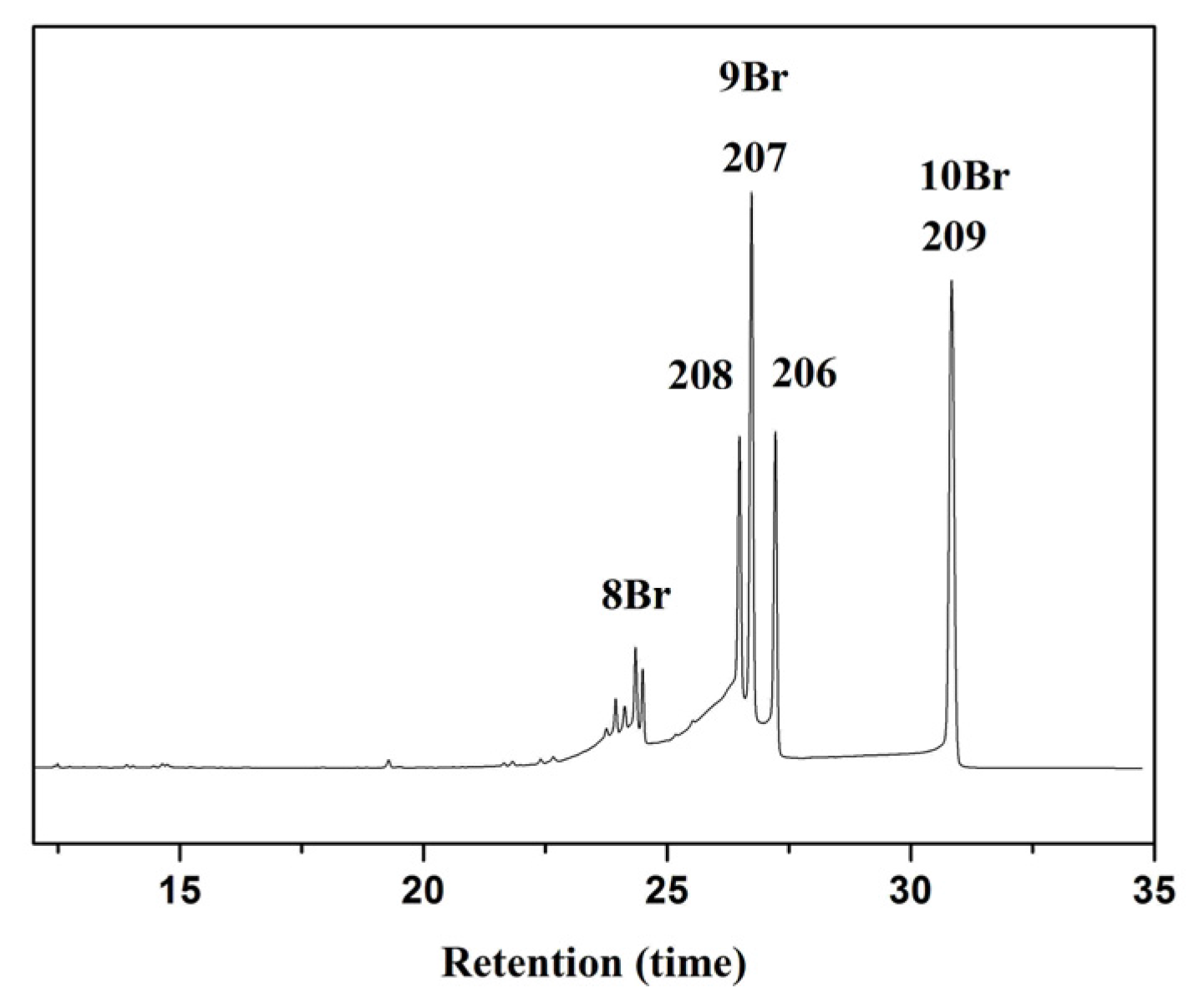
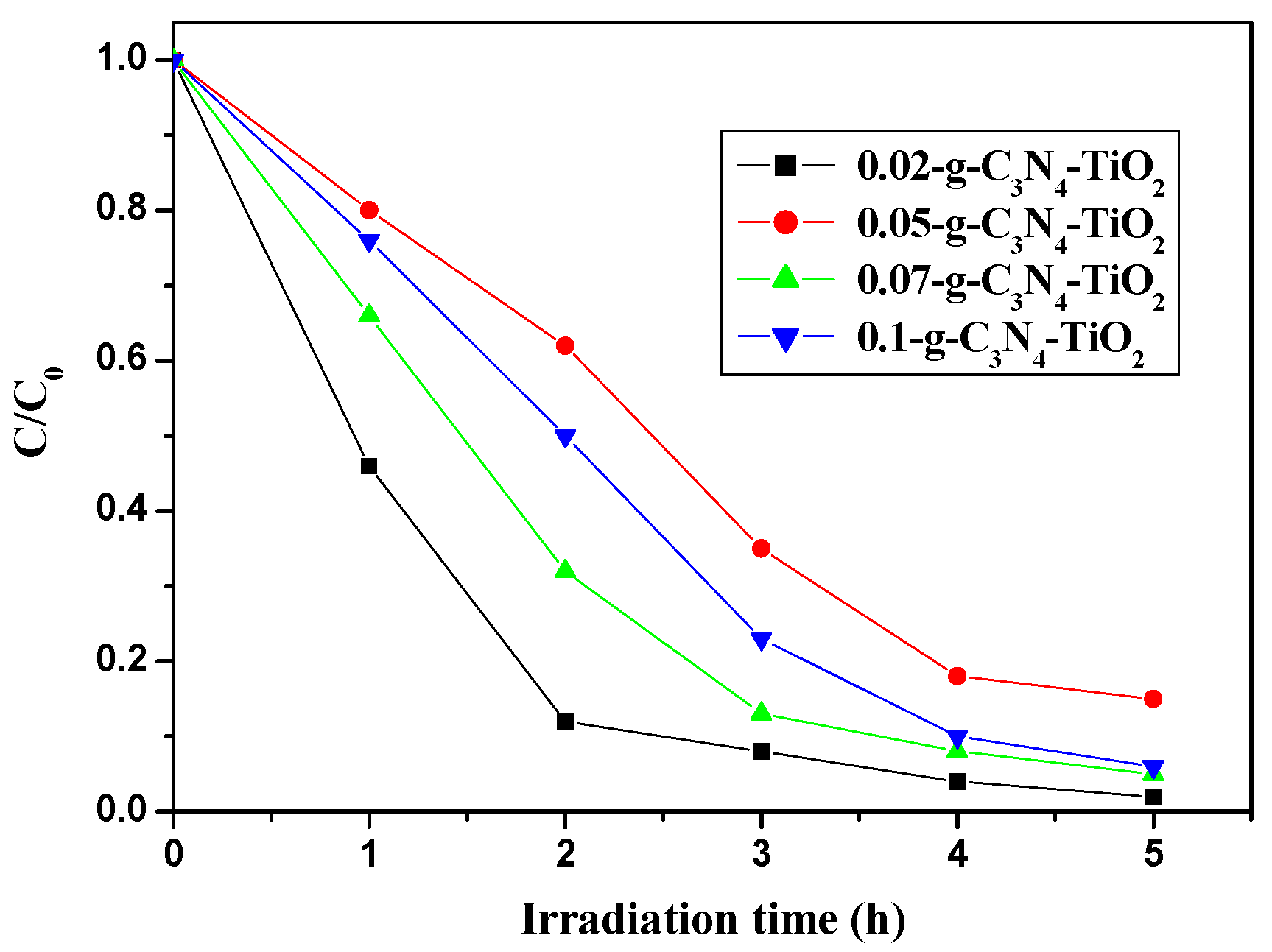
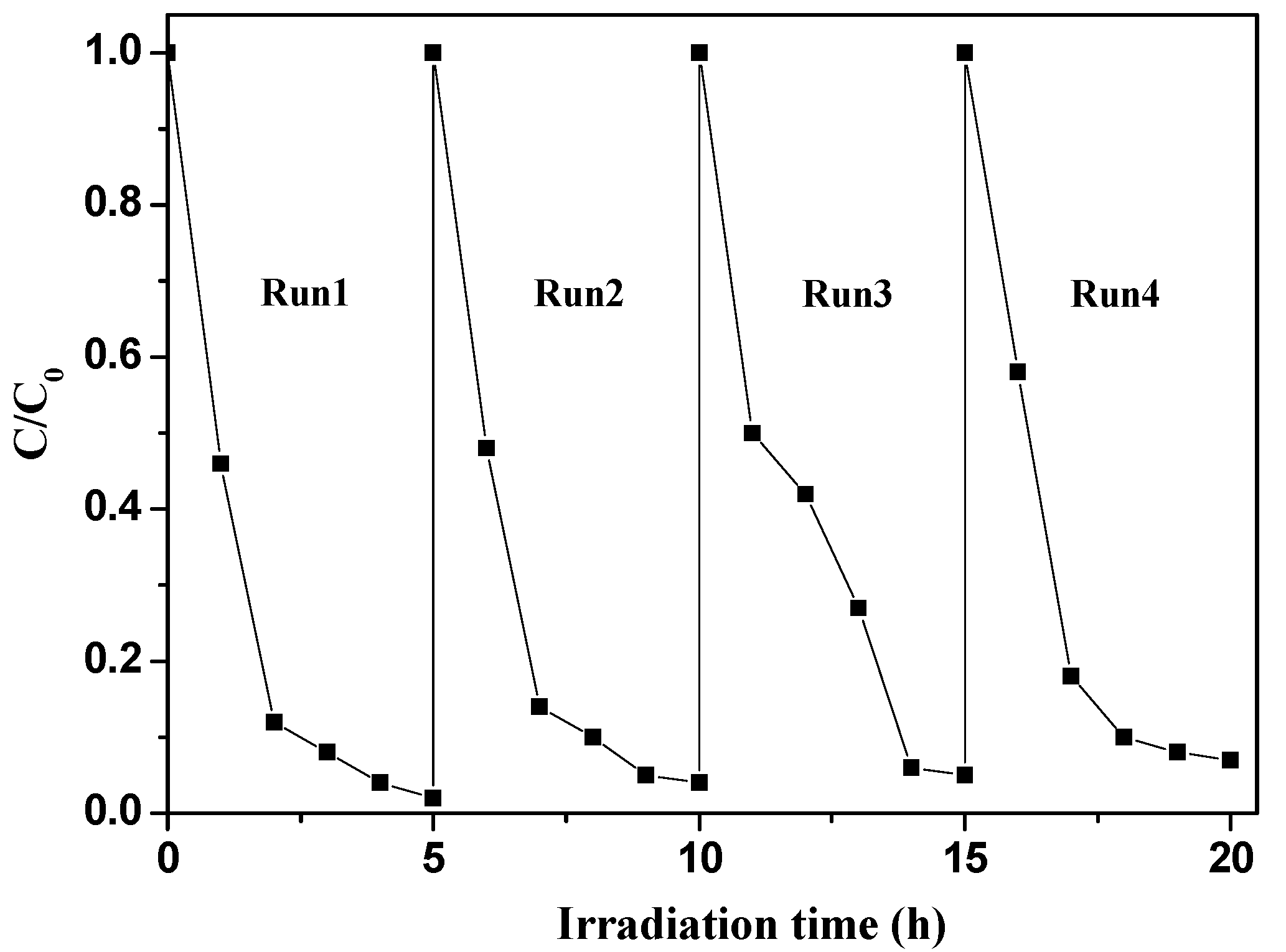
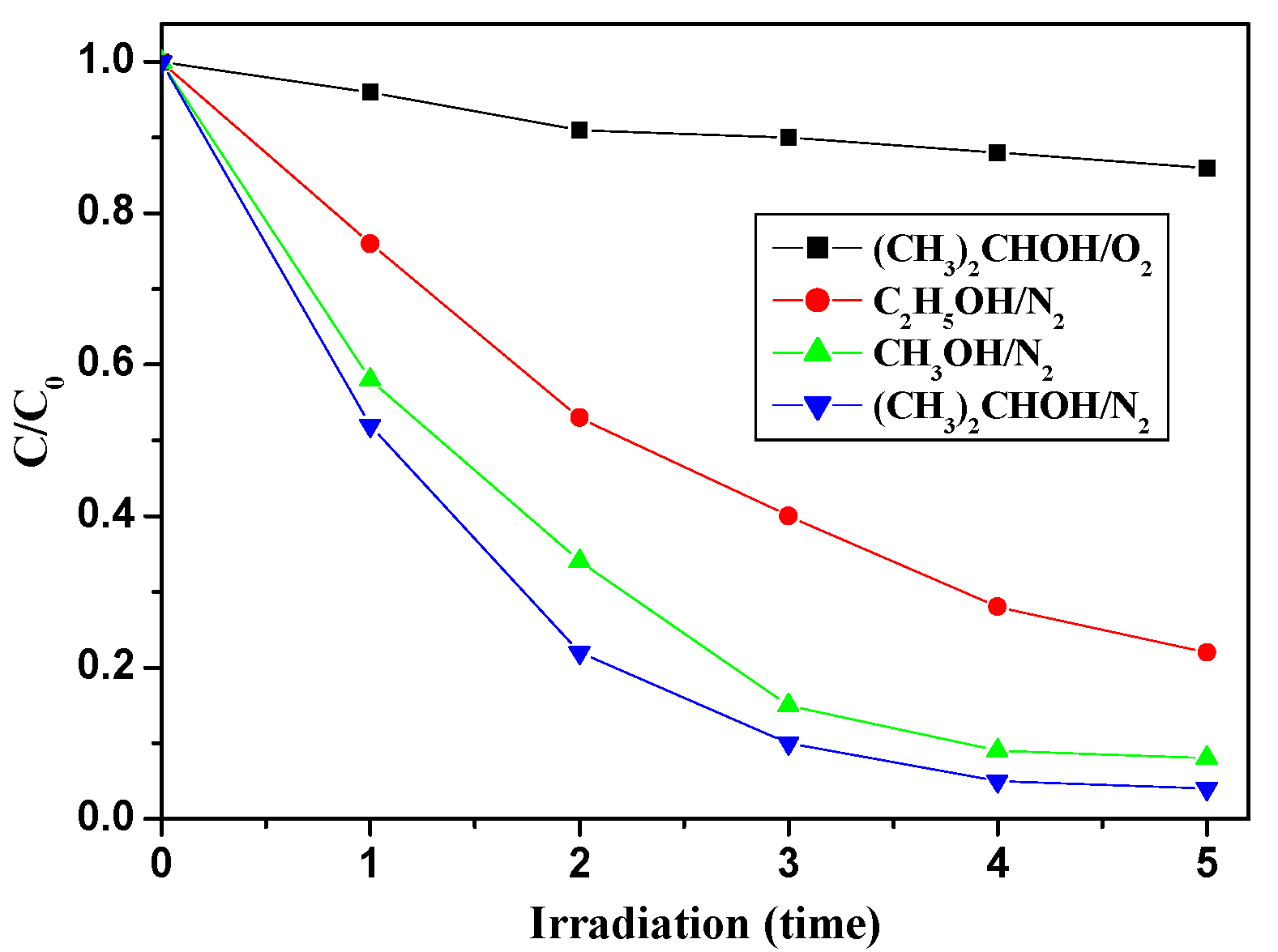

| Catalyst | Mass Percentage of C3N4 in C3N4-TiO2 | SBET (m2/g) | Absorption Amount | Kinetic Constant k (h−1) |
|---|---|---|---|---|
| g-C3N4 | 0 | 27.84 | 0.17 | 0.05 |
| 0.02-g-C3N4-TiO2 | 0.02 | 120.85 | 0.27 | 0.78 |
| 0.05-g-C3N4-TiO2 | 0.05 | 115.16 | 0.24 | 0.42 |
| 0.07-g-C3N4-TiO2 | 0.07 | 114.18 | 0.24 | 0.63 |
| 0.1-g-C3N4-TiO2 | 0.1 | 112.98 | 0.22 | 0.60 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, W.; Shao, Y.; Hu, X.; Liu, C.; Sun, C. Highly Enhanced Photoreductive Degradation of Polybromodiphenyl Ethers with g-C3N4/TiO2 under Visible Light Irradiation. Nanomaterials 2017, 7, 76. https://doi.org/10.3390/nano7040076
Ye W, Shao Y, Hu X, Liu C, Sun C. Highly Enhanced Photoreductive Degradation of Polybromodiphenyl Ethers with g-C3N4/TiO2 under Visible Light Irradiation. Nanomaterials. 2017; 7(4):76. https://doi.org/10.3390/nano7040076
Chicago/Turabian StyleYe, Weidong, Yingying Shao, Xuefeng Hu, Chulin Liu, and Chunyan Sun. 2017. "Highly Enhanced Photoreductive Degradation of Polybromodiphenyl Ethers with g-C3N4/TiO2 under Visible Light Irradiation" Nanomaterials 7, no. 4: 76. https://doi.org/10.3390/nano7040076





