Nanomaterials as Assisted Matrix of Laser Desorption/Ionization Time-of-Flight Mass Spectrometry for the Analysis of Small Molecules
Abstract
:1. Introduction
2. Nanomaterial-Assisted LDI Method Development
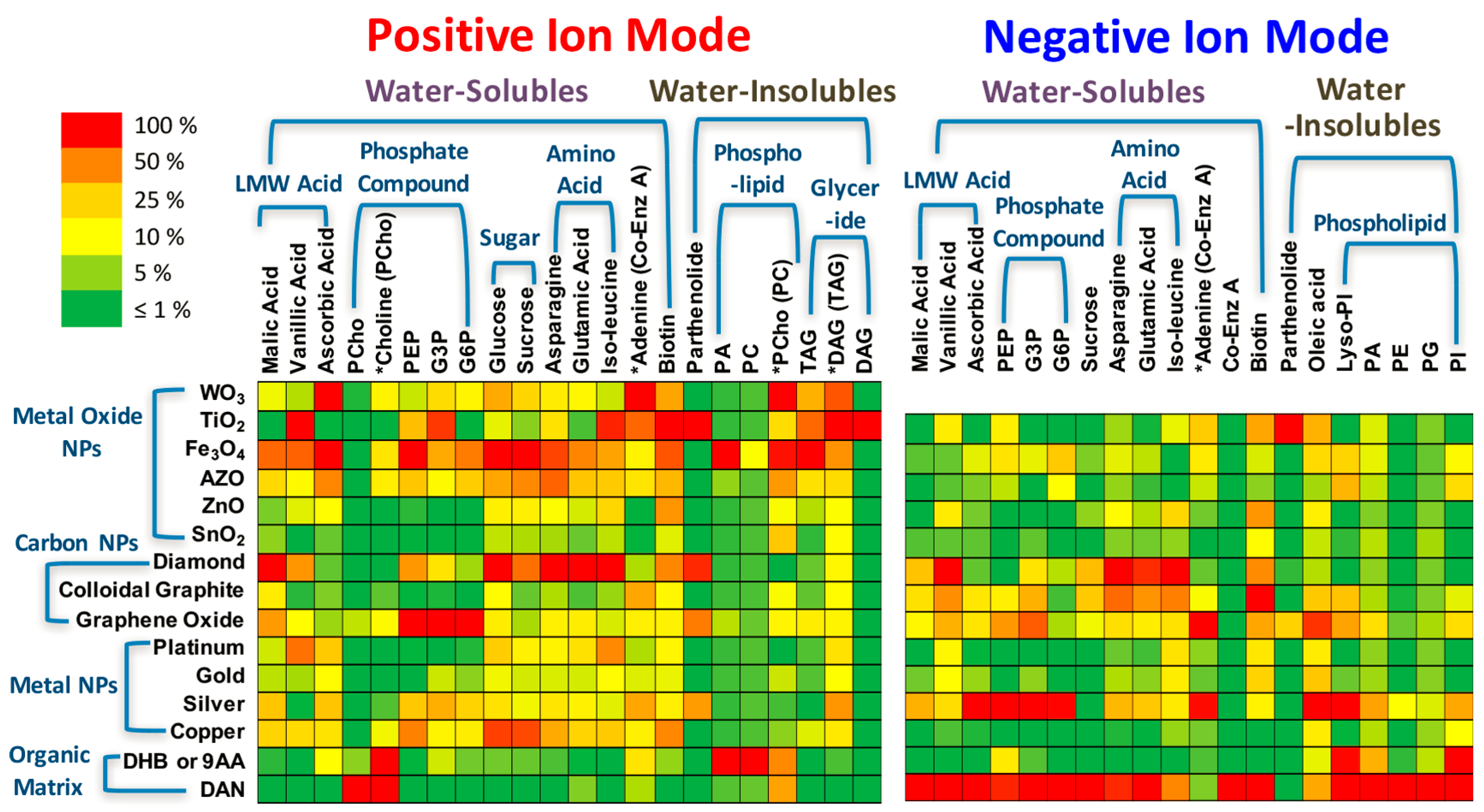
3. Nanomaterial-Assisted LDI for the Analysis of Small Biological Molecules
4. Nanomaterial-Assisted LDI for the Analysis of Environmental Pollutants
5. Nanomaterial-Assisted LDI for the Analysis of Other Small Molecules
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviation
| Arg | l-arginine |
| ATP | adenosine triphosphate |
| 9-AA | 9-aminoacridine |
| BaA | benzo[α]anthracene |
| BaP | benzo[α]pyrene |
| BDE-47 | 2,2′,4,4′-tetrabromodiphenyl ether |
| BPA | bisphenol A |
| CD | carbon dots |
| DHB | 2,5-dihydroxybenzoic acid |
| CHCA | α-cyano-4-hydroxycinnamic acid |
| CNT | carbon nanotube |
| ESI | electrospray ionization |
| E2 | estradiol |
| g-C3N4 | graphitic carbon nitride |
| Glu | l-glutamine |
| GO | graphene oxide |
| His | l-histidine |
| LBL | layer-by-layer |
| LDI | laser desorption/inoization |
| LOD | limits of detection |
| MALDI | matrix-assisted laser desorption/ionization |
| MFA | Mefenamic acid |
| MOF | metal-organic frameworks |
| Nanomaterial-assisted LDI | nanomaterial-assisted laser desorption/ionization |
| NAPA | silicon nanopost arrays |
| NP | nanoparticle |
| OCDD | octachlorodibenzo-p-dioxin |
| 1-OHP | 1-hydroxypyrene |
| PAHs | polycyclic aromatic hydrocarbons |
| PCP | pentachlorophenol |
| PFOS | perfluorooctanesulfonic acid |
| Phe | l-phenylalanine |
| ppt, | part per trillion |
| Rgo | reduced graphene oxide |
| SA | sinapic acid |
| SWNHs | single-walled carbon nanohorns |
| TBBPA | tetrabromobisphenol A |
| TCMs | traditional Chinese medicines |
| Trp | l-tryptophan |
| Tyr | l-tyrosine |
| 9-NA | 9-nitroanthracene |
| 2-NFL | 2-nitrofluorene |
| 1-NP | 1-nitropyrene |
References
- Tanaka, K.; Waki, H.; Ido, Y.; Akita, S.; Yoshida, Y.; Yoshida, T. Protein and polymer analyses up to m/z 100,000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1988, 2, 151–153. [Google Scholar] [CrossRef]
- Karas, M.; Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988, 60, 2299–2301. [Google Scholar] [CrossRef] [PubMed]
- Guinan, T.; Kirkbride, P.; Pigou, P.E.; Ronci, M.; Kobus, H.; Voelcker, N.H. Surface-assisted laser desorption ionization mass spectrometry techniques for application in forensics. Mass Spectrom. Rev. 2015, 34, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Cai, Z. Advances of MALDI-TOF MS in the analysis of traditional Chinese medicines. Top. Curr. Chem. 2013, 331, 143–164. [Google Scholar] [PubMed]
- Van Kampen, J.J.A.; Burgers, P.C.; de Groot, R.; Gruters, R.A.; Luider, T.M. Biomedical application of MALDI mass spectrometry for small-molecule analysis. Mass Spectrom. Rev. 2011, 30, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.J. Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: An update for 2009–2010. Mass Spectrom. Rev. 2015, 34, 268–422. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, T.R.; Goldstein, J.E.; Schumaker, S. MALDI TOF MS profiling of bacteria at the strain level: A review. Mass Spectrom. Rev. 2013, 32, 188–217. [Google Scholar] [CrossRef] [PubMed]
- Downard, K.M. Indirect study of non-covalent protein complexes by MALDI mass spectrometry: Origins, advantages, and applications of the “intensity-fading” approach. Mass Spectrom. Rev. 2016, 35, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, N.; Liu, S. Determination of peptide and protein disulfide linkages by MALDI mass spectrometry. Top. Curr. Chem. 2013, 331, 79–116. [Google Scholar] [PubMed]
- Montaudo, G.; Samperi, F.; Montaudo, M.S. Characterization of synthetic polymers by MALDI-MS. Prog. Polym. Sci. 2006, 31, 277–357. [Google Scholar] [CrossRef]
- Peterson, D.S. Matrix-free methods for laser desorption/ionization mass spectrometry. Mass Spectrom. Rev. 2007, 26, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Bergman, N.; Shevchenko, D.; Bergquist, J. Approaches for the analysis of low molecular weight compounds with laser desorption/ionization techniques and mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Chu, X.; Zhao, Z.-X.; He, X.-S.; Guo, Y.-L. Analysis of low molecular weight compounds by MALDI-FTICR-MS. J. Chromatogr. B 2011, 879, 1166–1179. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Hopfgartner, G. Laser-based methods for the analysis of low molecular weight compounds in biological matrices. Methods 2016, 104, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Y.; Deng, C.H. Recent advances in inorganic materials for LDI-MS analysis of small molecules. Analyst 2016, 141, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Q.; Liang, Y.; Jiang, G. Recent progress in application of carbon nanomaterials in laser desorption/ionization mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Silina, Y.E.; Volmer, D.A. Nanostructured solid substrates for efficient laser desorption/ionization mass spectrometry (LDI-MS) of low molecular weight compounds. Analyst 2013, 138, 7053–7065. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liu, B.; Girault, H.H. Nanomaterial-assisted laser desorption ionization for mass spectrometry-based biomedical analysis. Nanomedicine 2010, 5, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Sunner, J.; Dratz, E.; Chen, Y.-C. Graphite surface-assisted laser desorption/ionization time-of-flight mass spectrometry of peptides and proteins from liquid solutions. Anal. Chem. 1995, 67, 4335–4342. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Buriak, J.M.; Siuzdak, G. Desorption-ionization mass spectrometry on porous silicon. Nature 1999, 399, 243–246. [Google Scholar] [PubMed]
- Shen, Z.; Thomas, J.J.; Averbuj, C.; Broo, K.M.; Engelhard, M.; Crowell, J.E.; Finn, M.G.; Siuzdak, G. Porous silicon as a versatile platform for laser desorption/ionization mass spectrometry. Anal. Chem. 2001, 73, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Trauger, S.A.; Go, E.P.; Shen, Z.; Apon, J.V.; Compton, B.J.; Bouvier, E.S.P.; Finn, M.G.; Siuzdak, G. High sensitivity and analyte capture with desorption/ionization mass spectrometry on silylated porous silicon. Anal. Chem. 2004, 76, 4484–4489. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Geng, Z.; Shao, D.; Mei, Y.; Wang, Z. Single-crystalline EuF3 hollow hexagonal microdisks: Synthesis and application as a background-free matrix for MALDI-TOF-MS analysis of small molecules and polyethylene glycols. Anal. Chem. 2009, 81, 7625–7631. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, Z.; Yan, X.; Cai, Z. Zeolitic imidazolate framework nanocrystals for enrichment and direct detection of environmental pollutants by negative ion surface-assisted laser desorption/ionization time-of-flight mass spectrometry. RSC Adv. 2016, 6, 23790–23793. [Google Scholar] [CrossRef]
- Ma, Y.-R.; Zhang, X.-L.; Zeng, T.; Cao, D.; Zhou, Z.; Li, W.-H.; Niu, H.; Cai, Y.-Q. Polydopamine-coated magnetic nanoparticles for enrichment and direct detection of small molecule pollutants coupled with MALDI-TOF-MS. ACS Appl. Mater. Interfaces 2013, 5, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.-H.; Ho, K.-C.; Lin, Y.-S.; Chen, Y.-C. Gold nanoparticles as selective and concentrating probes for samples in MALDI MS analysis. Anal. Chem. 2004, 76, 4337–4342. [Google Scholar] [CrossRef] [PubMed]
- Yagnik, G.B.; Hansen, R.L.; Korte, A.R.; Reichert, M.D.; Vela, J.; Lee, Y.J. Large scale nanoparticle screening for small molecule analysis in laser desorption ionization mass spectrometry. Anal. Chem. 2016, 88, 8926–8930. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Z.; Vallant, R.M.; Takátsy, A.; Bakry, R.; Najam-ul-Haq, M.; Rainer, M.; Huck, C.W.; Bonn, G.K. Laser desorption/ionization mass spectrometric analysis of small molecules using fullerene-derivatized silica as energy-absorbing material. J. Mass Spectrom. 2010, 45, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Najam-ul-Haq, M.; Rainer, M.; Huck, C.W.; Hausberger, P.; Kraushaar, H.; Bonn, G.K. Nanostructured diamond-like carbon on digital versatile disc as a matrix-free target for laser desorption/ionization mass spectrometry. Anal. Chem. 2008, 80, 7467–7472. [Google Scholar] [CrossRef] [PubMed]
- Chitanda, J.M.; Zhang, H.; Pahl, E.; Purves, R.W.; El-Aneed, A. The development of novel nanodiamond based MALDI matrices for the analysis of small organic pharmaceuticals. J. Am. Soc. Mass Spectrom. 2016, 27, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Olesik, S.V. Electrospun nanofibers as substrates for surface-assisted laser desorption/ionization and matrix-enhanced surface-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2013, 85, 4384–4391. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, Y.; Zou, H.; Qiu, J.; Guo, Z.; Guo, B. Carbon nanotubes as assisted matrix for laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 2003, 75, 6191–6195. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xu, S.; Pan, C.; Yuan, C.; Zou, H.; Jiang, G. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry with a matrix of carbon nanotubes for the analysis of low-mass compounds in environmental samples. Environ. Sci. Technol. 2005, 39, 8442–8447. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Guo, Y. Oxidized carbon nanotubes as matrix for matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis of biomolecules. Rapid Commun. Mass Spectrom. 2005, 19, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Yao, S.; Guo, Y.; Xia, X. High-sensitivity matrix-assisted laser desorption/ionization fourier transform mass spectrometry analyses of small carbohydrates and amino acids using oxidized carbon nanotubes prepared by chemical vapor deposition as matrix. Anal. Chim. Acta 2007, 604, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-Y.; Lin, W.-D.; Hwu, W.-L.; Lai, C.-C.; Tsai, F.-J. Screening assay of very long chain fatty acids in human plasma with multiwalled carbon nanotube-based surface-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2010, 82, 6814–6820. [Google Scholar] [CrossRef] [PubMed]
- Cegłowski, M.; Schroeder, G. Laser desorption/ionization mass spectrometric analysis of surfactants on functionalized carbon nanotubes. Rapid Commun. Mass Spectrom. 2013, 27, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Cegłowski, M.; Jasiecki, S.; Schroeder, G. Laser desorption/ionization mass spectrometric analysis of folic acid, vancomycin and Triton® X-100 on variously functionalized carbon nanotubes. Rapid Commun. Mass Spectrom. 2013, 27, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zheng, H.; Wang, J.; Hou, J.; He, Q.; Liu, H.; Xiong, C.; Kong, X.; Nie, Z. Carbon nanodots as a matrix for the analysis of low-molecular weight molecules in both positive-and negative-ion matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and quantification of glucose and uric acid in real samples. Anal. Chem. 2013, 85, 6646–6652. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Cheng, J.; Li, J.; Wang, Y. Graphene as a novel matrix for the analysis of small molecules by MALDI-TOF MS. Anal. Chem. 2010, 82, 6208–6214. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Lai, Y.; Chen, G.; Cai, Z. Matrix interference-free method for the analysis of small molecules by using negative ion laser desorption/ionization on graphene flakes. Anal. Chem. 2011, 83, 3161–3169. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Min, D.-H. Mechanistic study of laser desorption/ionization of small molecules on graphene oxide multilayer films. Langmuir 2014, 30, 12675–12683. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Min, D.-H. The structural influence of graphene oxide on its fragmentation during laser desorption/ionization mass spectrometry for efficient small-molecule analysis. Chem. Eur. J. 2015, 21, 7217–7223. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Na, H.-K.; Kwack, S.-J.; Ryoo, S.-R.; Lee, Y.; Hong, S.; Hong, S.; Jeong, Y.; Min, D.-H. Synergistic effect of graphene oxide/MWCNT films in laser desorption/ionization mass spectrometry of small molecules and tissue imaging. ACS Nano 2011, 5, 4550–4561. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Min, D.-H. Fabrication of alternating multilayer films of graphene oxide and carbon nanotube and its application in mechanistic study of laser desorption/ionization of small molecules. ACS Appl. Mater. Interfaces 2012, 4, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Nasser, A.H.; Wu, B.S.; Wu, H.F. Graphene coated silica applied for high ionization matrix assisted laser desorption/ionization mass spectrometry: A novel approach for environmental and biomolecule analysis. Talanta 2014, 126, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bae, S.-E.; Huh, S.; Cha, S. Graphene oxide embedded sol–gel (GOSG) film as a SALDI MS substrate for robust metabolite fingerprinting. RSC Adv. 2015, 5, 56455–56459. [Google Scholar] [CrossRef]
- Lin, Z.; Zheng, J.; Lin, G.; Tang, Z.; Yang, X.; Cai, Z. Negative ion laser desorption/ionization time-of-flight mass spectrometric analysis of small molecules using graphitic carbon nitride nanosheet matrix. Anal. Chem. 2015, 87, 8005–8012. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Knapp, D.R. Matrix-free LDI mass spectrometry platform using patterned nanostructured gold thin film. Anal. Chem. 2010, 82, 7772–7778. [Google Scholar] [CrossRef] [PubMed]
- Kolářová, L.; Kučera, L.; Vaňhara, P.; Hampl, A.; Havel, J. Use of flower-like gold nanoparticles in time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-P.; Yu, C.-J.; Lin, C.-Y.; Lin, Y.-H.; Tseng, W.-L. Gold nanoparticles as assisted matrices for the detection of biomolecules in a high-salt solution through laser desorption/ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2009, 20, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Hinman, S.S.; Chen, C.-Y.; Duan, J.C.; Cheng, Q. Calcinated gold nanoparticle arrays for on-chip, multiplexed and matrix-free mass spectrometric analysis of peptides and small molecules. Nanoscale 2016, 8, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.T.; Furutani, H.; Oldenburg, S.J.; Darlington, T.K.; Prather, K.A. Gold nanoparticles as a matrix for visible-wavelength single-particle matrix-assisted laser desorption/ionization mass spectrometry of small biomolecules. J. Phys. Chem. C 2008, 112, 4083–4090. [Google Scholar] [CrossRef]
- Silina, Y.E.; Meier, F.; Nebolsin, V.A.; Koch, M.; Volmer, D.A. Novel galvanic nanostructures of Ag and Pd for efficient laser desorption/ionization of low molecular weight compounds. J. Am. Soc. Mass Spectrom. 2014, 25, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Ozawa, T.; Hisatomi, H.; Arakawa, R. Platinum vapor deposition surface-assisted laser desorption/ionization for imaging mass spectrometry of small molecules. Rapid Commun. Mass Spectrom. 2012, 26, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Osaka, I.; Ihozaki, T.; Hamada, S.; Kuroda, Y.; Murakami, T.; Miyazato, A.; Kawasakia, H.; Arakawa, R. Simultaneous detection of phosphatidylcholines and glycerolipids using matrix-enhanced surface-assisted laser desorption/ionization-mass spectrometry with sputter-deposited platinum film. J. Mass Spectrom. 2015, 50, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Agrawal, K.; Wu, H.-F. Application of platinum nanoparticles as affinity probe and matrix for direct analysis of small biomolecules and microwave digested proteins using matrix-assisted laser desorption/ionization mass spectrometry. Analyst 2011, 136, 2852–2857. [Google Scholar] [CrossRef] [PubMed]
- Piret, G.; Kim, D.; Drobecq, H.; Coffinier, Y.; Melnyk, O.; Schmuki, P.; Boukherroub, R. Surface-assisted laser desorption-ionization mass spectrometry on titanium dioxide (TiO2) nanotube layers. Analyst 2012, 137, 3058–3063. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-I.; Ryu, S.-Y.; Park, J.-M.; Noh, J.-Y.; Kang, M.-J.; Kwak, S.-Y.; Pyun, J.-C. Nylon nanoweb with TiO2 nanoparticles as a solid matrix for matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Han, S.W.; Jeong, B.; Park, S.H.; Kim, Y.-G.; Kim, Y.H.; Kim, Y.D. Effect of polydimethylsiloxane (PDMS) coating on TiO2-based MALDI matrix for dimethyl methylphosphonate (DMMP) analysis. Appl. Surf. Sci. 2015, 353, 342–349. [Google Scholar] [CrossRef]
- Shin, W.J.; Shin, J.H.; Song, J.Y.; Han, S.Y. Effects of ZnO nanowire length on surface-assisted laser desorption/ionization of small molecules. J. Am. Soc. Mass Spectrom. 2010, 21, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, M.; Wu, H.-F. Probing the fungicidal property of CdS quantum dots on Saccharomyces cerevisiae and Candida utilis using MALDI-MS. J. Nanopart. Res. 2013, 15, 1728–1740. [Google Scholar] [CrossRef]
- Primadona, I.; Lai, Y.-H.; Capangpangan, R.Y.; Obena, R.P.; Tseng, M.-C.; Huang, M.-F.; Chang, H.-T.; Li, S.-T.; Wu, C.-Y.; Chien, W.-T.; et al. Functionalized HgTe nanoparticles promote laser induced solid phase ionization/dissociation for comprehensive glycan sequencing. Analyst 2016, 141, 6093–6103. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Lu, W.; Fang, J.; Cole, R.B. Characterization of synthesized titanium oxide nanoclusters by MALDI-TOF mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.L.; Amorim Madeira, P.J.; Nunes, M.R.; Costa, F.M.; Helena Florêncio, M. Titanium dioxide anatase as matrix for matrix-assisted laser desorption/ionization analysis of small molecules. Rapid Commun. Mass Spectrom. 2008, 22, 3761–3766. [Google Scholar] [CrossRef] [PubMed]
- Sonderegger, H.; Rameshan, C.; Lorenz, H.; Klauser, F.; Klerks, M.; Rainer, M.; Bakry, R.; Huck, C.W.; Bonn, G.K. Surface-assisted laser desorption/ionization-mass spectrometry using TiO2-coated steel targets for the analysis of small molecules. Anal. Bioanal. Chem. 2011, 401, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Popović, I.; Nešić, M.; Vranješ, M.; Šaponjić, Z.; Petković, M. TiO2 nanocrystals-assisted laser desorption and ionization time-of-flight mass spectrometric analysis of steroid hormones, amino acids and saccharides. Validation and comparison of methods. RSC Adv. 2016, 6, 1027–1036. [Google Scholar] [CrossRef]
- Popović, I.; Milovanović, D.; Miletić, J.; Nešić, M.; Vranješ, M.; Šaponjić, Z.; Petković, M. Dependence of the quality of SALDI TOF MS analysis on the TiO2 nanocrystals’ size and shape. Opt. Quant. Electron. 2016, 48, 113–118. [Google Scholar] [CrossRef]
- Popović, I.A.; Nešić, M.; Vranješ, M.; Šaponjić, Z.; Petković, M. SALDI-TOF-MS analyses of small molecules (citric acid, dexasone, vitamins E and A) using TiO2 nanocrystals as substrates. Anal. Bioanal. Chem. 2016, 408, 7481–7490. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.A.; Stumpo, K.A.; Russell, D.H. Size-selected (2–10 nm) gold nanoparticles for matrix assisted laser desorption ionization of peptides. J. Am. Chem. Soc. 2005, 127, 5304–5305. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; Wu, H.-F. Gold nanoparticles assisted laser desorption/ionization mass spectrometry and applications: From simple molecules to intact cells. Anal. Bioanal. Chem. 2016, 408, 4485–4502. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar]
- Shih, Y.-H.; Chien, C.-H.; Singco, B.; Hsu, C.-L.; Lin, C.-H.; Huang, H.-Y. Metal-organic frameworks: New matrices for surface-assisted laser desorption-ionization mass spectrometry. Chem. Commun. 2013, 49, 4929–4931. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.-P.; Lirio, S.; Liu, W.-L.; Lin, C.-H.; Huang, H.-Y. A novel type of matrix for surface-assisted laser desorption-ionization mass spectrometric detection of biomolecules using metal-organic frameworks. Anal. Chim. Acta 2015, 888, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Chang, Y.J.; Fan, T.; Gu, Z.Y. Two-dimensional metal-organic framework nanosheets as a matrix for laser desorption/ionization of small molecules and monitoring enzymatic reactions at high salt concentrations. Chem. Commun. 2016, 52, 12984–12987. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ou, J.; Wang, H.; Liu, Z.; Ye, M.; Zou, H. Tailor-made stable Zr(IV)-based metal-organic frameworks for laser desorption/ionization mass spectrometry analysis of small molecules and simultaneous enrichment of phosphopeptides. ACS Appl. Mater. Interfaces 2016, 8, 20292–20300. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Bian, W.; Zheng, J.; Cai, Z. Magnetic metal–organic framework nanocomposites for enrichment and direct detection of small molecules by negative-ion matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Chem. Commun. 2015, 51, 8785–8788. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Fu, C.-P.; Liu, W.-L.; Lin, C.-H.; Huang, H.-Y.; Ma, S. Nanoporous carbons derived from metal-organic frameworks as novel matrices for surface-assisted laser desorption/ionization mass spectrometry. Small 2016, 12, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
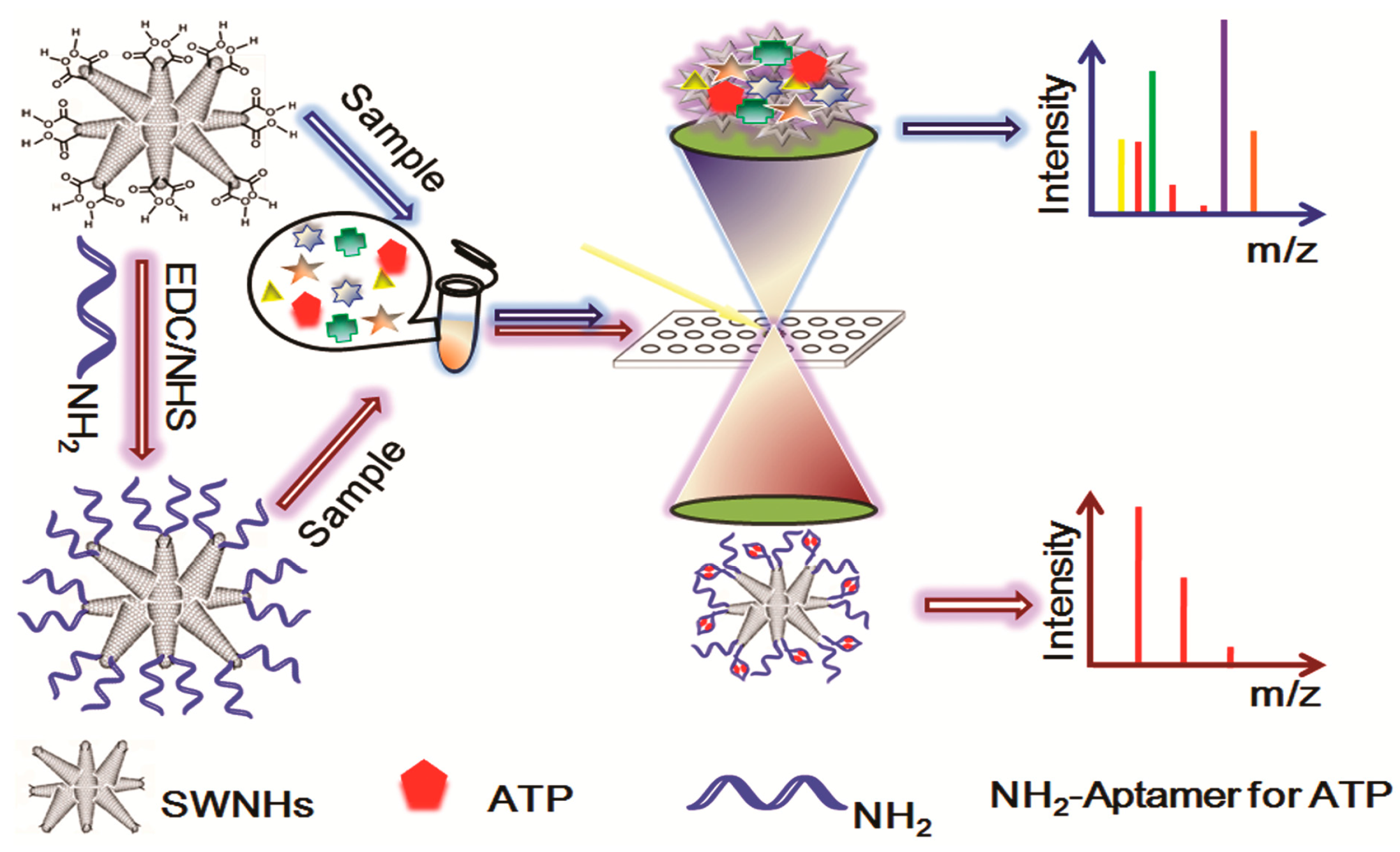
- Ma, R.; Lu, M.; Ding, L.; Ju, H.; Cai, Z. Surface-assisted laser desorption/ionization mass spectrometric detection of biomolecules by using functional single-walled carbon nanohorns as the matrix. Chem. Eur. J. 2013, 19, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Calandra, E.; Crotti, S.; Agostini, M.; Nitti, D.; Roverso, M.; Toffoli, G.; Marangon, E.; Posocco, B.; Traldi, P. Matrix-assisted laser desorption/ionization, nanostructure-assisted laser desorption/ionization and carbon nanohorns in the detection of antineoplastic drugs. 1. The cases of irinotecan, sunitinib and 6-alpha-hydroxy paclitaxel. Eur. J. Mass Spectrom. 2014, 20, 445–459. [Google Scholar]
- Khan, M.S.; Bhaisare, M.L.; Pandey, S.; Talib, A.; Wu, S.-M.; Kailasa, S.K.; Wu, H.-F. Exploring the ability of water soluble carbon dots as matrix for detecting neurological disorders using MALDI-TOF MS. Int. J. Mass Spectrom. 2015, 393, 25–33. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, D.; Chen, Y.; Hu, G.; Liu, H.; Jiang, Y. Development of N,S-doped carbon dots as a novel matrix for the analysis of small molecules by negative ion MALDI-TOF MS. RSC Adv. 2016, 6, 79043–79049. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.; Bai, H.; Liu, H.; Lin, S.; Jiang, Y. Carbon dots and 9AA as a binary matrix for the detection of small molecules by matrix-assisted laser desorption/ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2016, 27, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Sekula, J.; Niziol, J.; Misiorek, M.; Dec, P.; Wrona, A.; Arendowski, A.; Ruman, T. Gold nanoparticle-enhanced target for MS analysis and imaging of harmful compounds in plant, animal tissue and on fingerprint. Anal. Chim. Acta 2015, 895, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sekula, J.; Niziol, J.; Rode, W.; Ruman, T. Gold nanoparticle-enhanced target (AuNPET) as universal solution for laser desorption/ionization mass spectrometry analysis and imaging of low molecular weight compounds. Anal. Chim. Acta 2015, 875, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Niziol, J.; Ruman, T. Surface-transfer mass spectrometry imaging on a monoisotopic silver nanoparticle enhanced target. Anal. Chem. 2013, 85, 12070–12076. [Google Scholar] [CrossRef] [PubMed]
- Niziol, J.; Ossoliński, K.; Ossoliński, T.; Ossolińska, A.; Bonifay, V.; Sekula, J.; Dobrowolski, Z.; Sunner, J.; Beech, I.; Ruman, T. Surface-transfer mass spectrometry imaging of renal tissue on gold nanoparticle enhanced target. Anal. Chem. 2016, 88, 7365–7371. [Google Scholar] [CrossRef] [PubMed]
- Niziol, J.; Rode, W.; Zieliński, Z.; Ruman, T. Matrix-free laser desorption–ionization with silver nanoparticle-enhanced steel targets. Int. J. Mass Spectrom. 2013, 335, 22–32. [Google Scholar] [CrossRef]
- Wan, D.; Gao, M.; Wang, Y.; Zhang, P.; Zhang, X. A rapid and simple separation and direct detection of glutathione by gold nanoparticles and graphene-based MALDI-TOF-MS. J. Sep. Sci. 2013, 36, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-R.; Wang, D.-Y.; Chiu, Y.-C.; Yeh, Y.-C.; Chen, W.-T.; Chen, C.-H.; Chen, C.-W.; Chang, H.-C.; Hu, C.-C.; Chen, C.-C. Layer-by-layer thin film of reduced graphene oxide and gold nanoparticles as an effective sample plate in laser-induced desorption/ionization mass spectrometry. Anal. Chem. Acta 2014, 809, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Xu, L.; Wang, F.; Geng, Z.; Li, H.; Wang, H.; Li, C. A direct assay of carboxyl-containing small molecules by SALDI-MS on a AgNP/rGO-based nanoporous hybrid film. Analyst 2015, 141, 2712–2726. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-I.; Park, J.-M.; Hwang, S.-J.; Kang, M.-J.; Pyun, J.-C. Top-down synthesized TiO2 nanowires as a solid matrix for surface assisted laser desorption/ionization time-of-flight (SALDI-TOF) mass spectrometry. Anal. Chim. Acta 2014, 836, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Wu, H.-F. One-pot synthesis of dopamine dithiocarbamate functionalized gold nanoparticles for quantitative analysis of small molecules and phosphopeptides in SALDI-and MALDI-MS. Analyst 2012, 137, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wu, L.; Mungra, D.C.; Xia, S.; Zhu, J. Au@SiO2 core–shell nanoparticles for laser desorption/ionization time of flight mass spectrometry. Analyst 2012, 137, 2454–2458. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Wei, X.; Li, Y.; Wu, J.; Qian, K.; Liu, B. Designer SiO2@Au nanoshells towards sensitive and selective detection of small molecules in laser desorption ionization mass spectrometry. Nanomed. NBM 2015, 11, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Bibi, A.; Ju, H. Quantum dots assisted laser desorption/ionization mass spectrometric detection of carbohydrates: Qualitative and quantitative analysis. J. Mass Spectrom. 2016, 51, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, G.; Liu, X.; Sun, L.; Li, H.; Cheng, Q.; Xi, K.; Xu, D. MoS2/Ag nanohybrid: A novel matrix with synergistic effect for small molecule drugs analysis by negative-ion matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chim. Acta 2016, 937, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zheng, J.; Bian, W.; Cai, Z. CuFe2O4 magnetic nanocrystal clusters as a matrix for the analysis of small molecules by negative-ion matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Analyst 2015, 140, 5287–5294. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.N.; Antonakos, C.; Retterer, S.T.; Vertes, A. Metabolic differences in microbial cell populations revealed by nanophotonic ionization. Angew. Chem. Int. Ed. 2013, 52, 3650–3653. [Google Scholar] [CrossRef] [PubMed]
- Korte, A.R.; Stopla, S.A.; Morris, N.; Razunguzwa, T.; Vertes, T. Large-scale metabolite analysis of atandards and human serum by laser desorption Ionization mass spectrometry from silicon nanopost arrays. Anal. Chem. 2016, 88, 8989–8996. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.S.; Phan, N.T.N.; Fletcher, J.S.; Ewing, A.G. Intact lipid imaging of mouse brain samples: MALDI, nanoparticle-laser desorption ionization, and 40 keV argon cluster secondary ion mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 6857–6868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, X.; Cheng, J.; Li, J.; Wang, Y. Efficient analysis of non-polar environmental contaminants by MALDI-TOF MS with graphene as matrix. J. Am. Soc. Mass Spectrom. 2011, 22, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Li, R.; Hu, D.; Ma, N.; Shuang, S.; Cai, Z.; Dong, C. Magnetic graphene composites as both an adsorbent for sample enrichment and a MALDI-TOF MS matrix for the detection of nitropolycyclic aromatic hydrocarbons in PM2.5. Analyst 2015, 140, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Bian, W.; Li, R.; Geng, H.; Zhang, J.; Dong, C.; Shuang, S.; Cai, Z. Quantitative analysis of nitro-polycyclic aromatic hydrocarbons in PM2.5 samples with graphene as a matrix by MALDI-TOF MS. Anal. Methods 2015, 7, 3967–3971. [Google Scholar] [CrossRef]
- Lu, W.; Li, Y.; Li, R.; Shuang, S.; Dong, C.; Cai, Z. Facile synthesis of N-Doped carbon dots as a new matrix for detection of hydroxy-polycyclic aromatic hydrocarbons by negative-ion matrix-assisted laser desorption/ionization time-of flight mass spectrometry. ACS Appl. Mater. Interfaces 2016, 8, 12976–12984. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cheng, M.; Jiang, G. Mildly oxidized graphene: Facile synthesis, characterization, and application as a matrix in MALDI mass spectrometry. Chem. Eur. J. 2013, 19, 5561–5565. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cheng, M.; Wang, J.; Jiang, G. Graphene oxide nanoribbons: Improved synthesis and application in MALDI mass spectrometry. Chem. Eur. J. 2015, 21, 5594–5599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wei, Y.; He, Q.; Boey, F.; Zhang, Q.; Zhang, H. Reduced graphene oxide films used as matrix of MALDI-TOF-MS for detection of octachlorodibenzo-p-dioxin. Chem. Commun. 2010, 46, 6974–6976. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Deng, C.; Zhang, X.; Yang, P. Synthesis of highly water-dispersible polydopamine-modified multiwalled carbon nanotubes for matrix-assisted laser desorption/ionization mass spectrometry analysis. ACS Appl. Mater. Interfaces 2013, 5, 7770–7776. [Google Scholar] [CrossRef] [PubMed]
- He, X.-M.; Zhu, G.-T.; Yin, J.; Zhao, Q.; Yuan, B.-F.; Feng, Y.-Q. Electrospun polystyrene/oxidized carbon nanotubes film as both sorbent for thin film microextraction and matrix for matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Chromatogr. A 2014, 1351, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-S.; Wu, J.-H.; Xu, L.-D.; Zhao, Q.; Luo, Y.-B.; Yuan, B.-F.; Feng, Y.-Q. A magnetite/oxidized carbon nanotube composite used as an adsorbent and a matrix of MALDI-TOF-MS for the determination of benzo[a]pyrene. Chem. Commun. 2011, 47, 9816–9818. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, X.; Deng, C. Enrichment and determination of crotonaldehyde using magnetic multiwalled carbon nanotubes as an adsorbent and a matrix for matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Wang, S.; Tan, Y.; Song, X.; Cai, Y. Simultaneous and direct analysis of multiple types of organic contaminants in water based on a MOF decorated with a suitable quantity of Au nanoparticles, using SALDI-TOF MS. RSC Adv. 2016, 6, 99919–99923. [Google Scholar] [CrossRef]
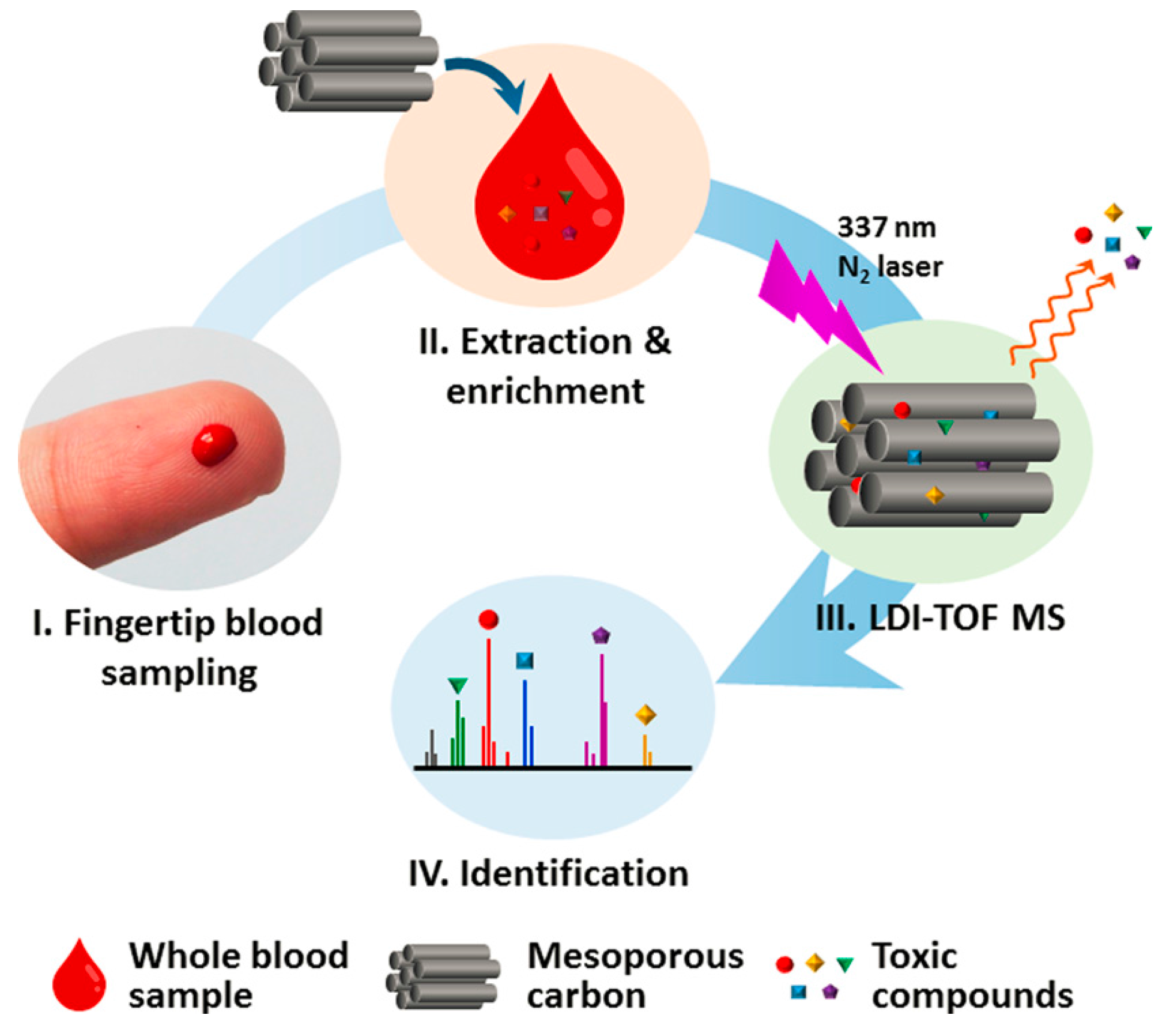
- Huang, X.; Liu, Q.; Fu, J.; Nie, Z.; Gao, K.; Jiang, G. Screening of toxic chemicals in a single drop of human whole blood using ordered mesoporous carbon as a mass spectrometry probe. Anal. Chem. 2016, 88, 4107–4113. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Yin, P.; Gao, M.; Deng, C.; Zhang, X. High throughput identification of components from traditional Chinese medicine herbs by utilizing graphene or graphene oxide as MALDI-TOF MS matrix. J. Mass Spectrom. 2011, 46, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-W.; Chien, M.-W.; Su, C.-Y.; Chen, H.-Y.; Li, L.-J.; Lai, C.-C. Analysis of flavonoids by graphene-based surface-assisted laser desorption/ionization time-of-flight mass spectrometry. Analyst 2012, 137, 5809–5816. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Gao, M.; Zhang, X. High throughput detection of tetracycline residues in milk using graphene or graphene oxide as MALDI-TOF MS matrix. J. Am. Soc. Mass Spectrom. 2012, 23, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-I.; Park, J.-M.; Noh, J.-Y.; Hwang, S.-J.; Kang, M.-J.; Pyun, J.-C. Analysis of benzylpenicillin in milk using MALDI-TOF mass spectrometry with top-down synthesized TiO2 nanowires as the solid matrix. Chemosphere 2016, 143, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Lai, Y.; Chen, G.; Cai, Z. Laser desorption/ionization on the layer of graphene nanoparticles coupled with mass spectrometry for characterization of polymers. Chem. Commun. 2011, 47, 12807–12809. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Zhang, X.; Chen, X.; Li, S.; Zhu, J. N-doped graphene: An alternative carbon-based matrix for highly efficient detection of small molecules by negative ion MALDI-TOFMS. Anal. Chem. 2014, 86, 9122–9130. [Google Scholar] [CrossRef] [PubMed]
- Gedda, G.; Pandey, S.; Bhaisare, M.L.; Wu, H.-F. Carbon dots as nanoantennas for anti-inflammatory drug analysis using surface-assisted laser desorption/ionization time-of-flight mass spectrometry in serum. RSC Adv. 2014, 4, 38027–38033. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Yang, X.; Yang, Y.; Qin, P.; Wu, X.; Cai, Z. Nanomaterials as Assisted Matrix of Laser Desorption/Ionization Time-of-Flight Mass Spectrometry for the Analysis of Small Molecules. Nanomaterials 2017, 7, 87. https://doi.org/10.3390/nano7040087
Lu M, Yang X, Yang Y, Qin P, Wu X, Cai Z. Nanomaterials as Assisted Matrix of Laser Desorption/Ionization Time-of-Flight Mass Spectrometry for the Analysis of Small Molecules. Nanomaterials. 2017; 7(4):87. https://doi.org/10.3390/nano7040087
Chicago/Turabian StyleLu, Minghua, Xueqing Yang, Yixin Yang, Peige Qin, Xiuru Wu, and Zongwei Cai. 2017. "Nanomaterials as Assisted Matrix of Laser Desorption/Ionization Time-of-Flight Mass Spectrometry for the Analysis of Small Molecules" Nanomaterials 7, no. 4: 87. https://doi.org/10.3390/nano7040087




