Effect of Nano-SiO2 on the Early Hydration of Alite-Sulphoaluminate Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Specimen Preparation
2.2.2. Determination of Heat of Hydration
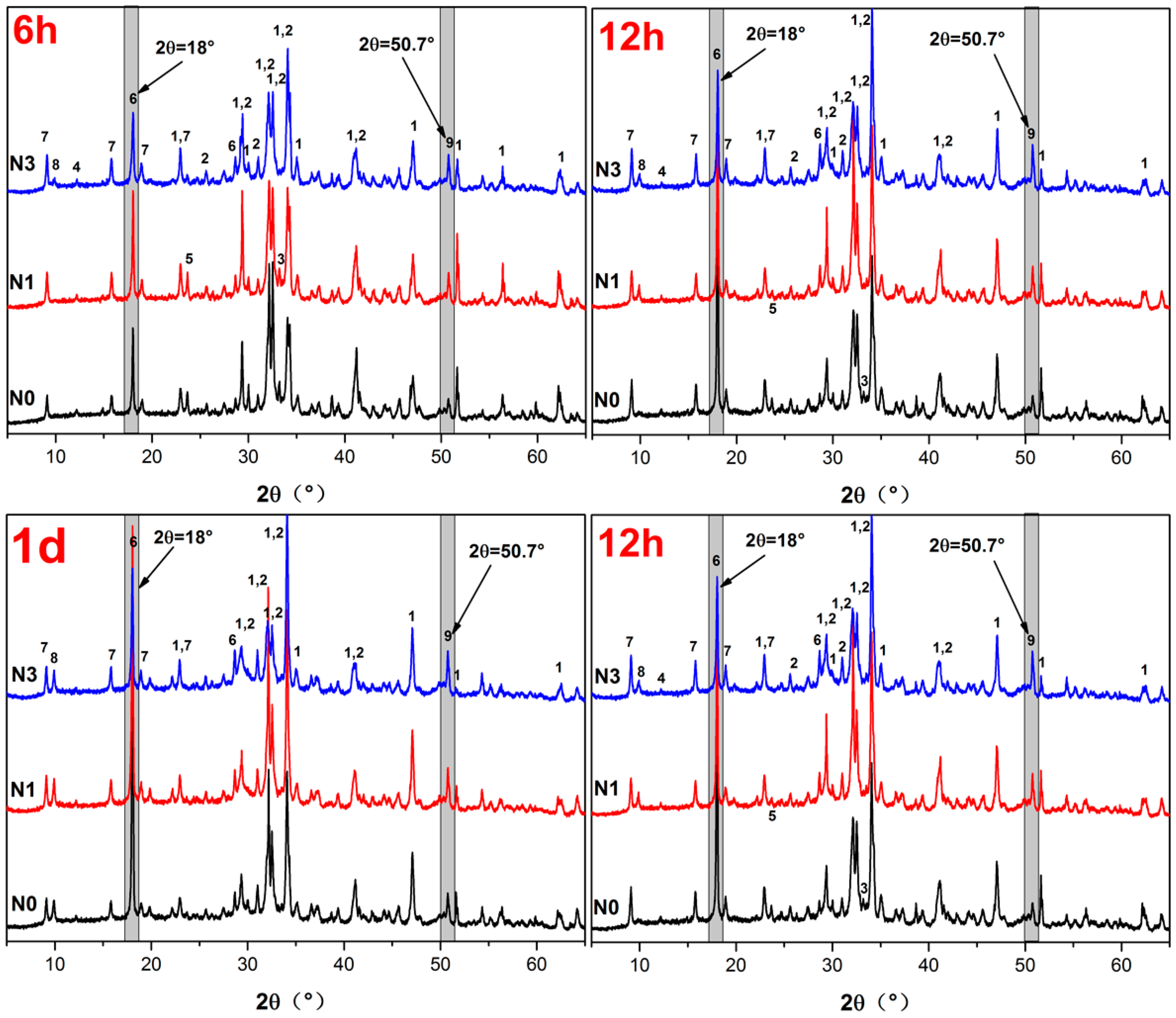
2.2.3. Determination of Phase Development
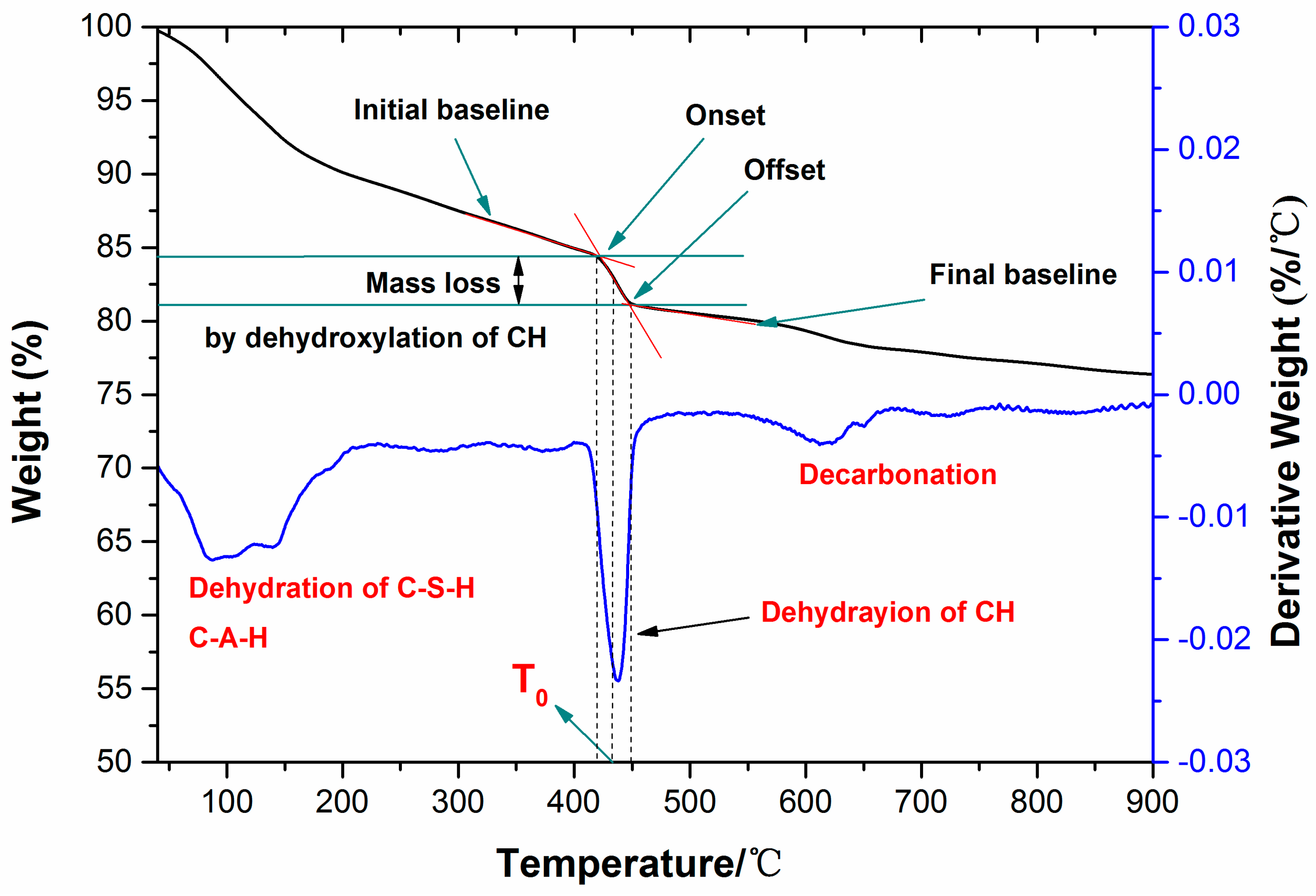
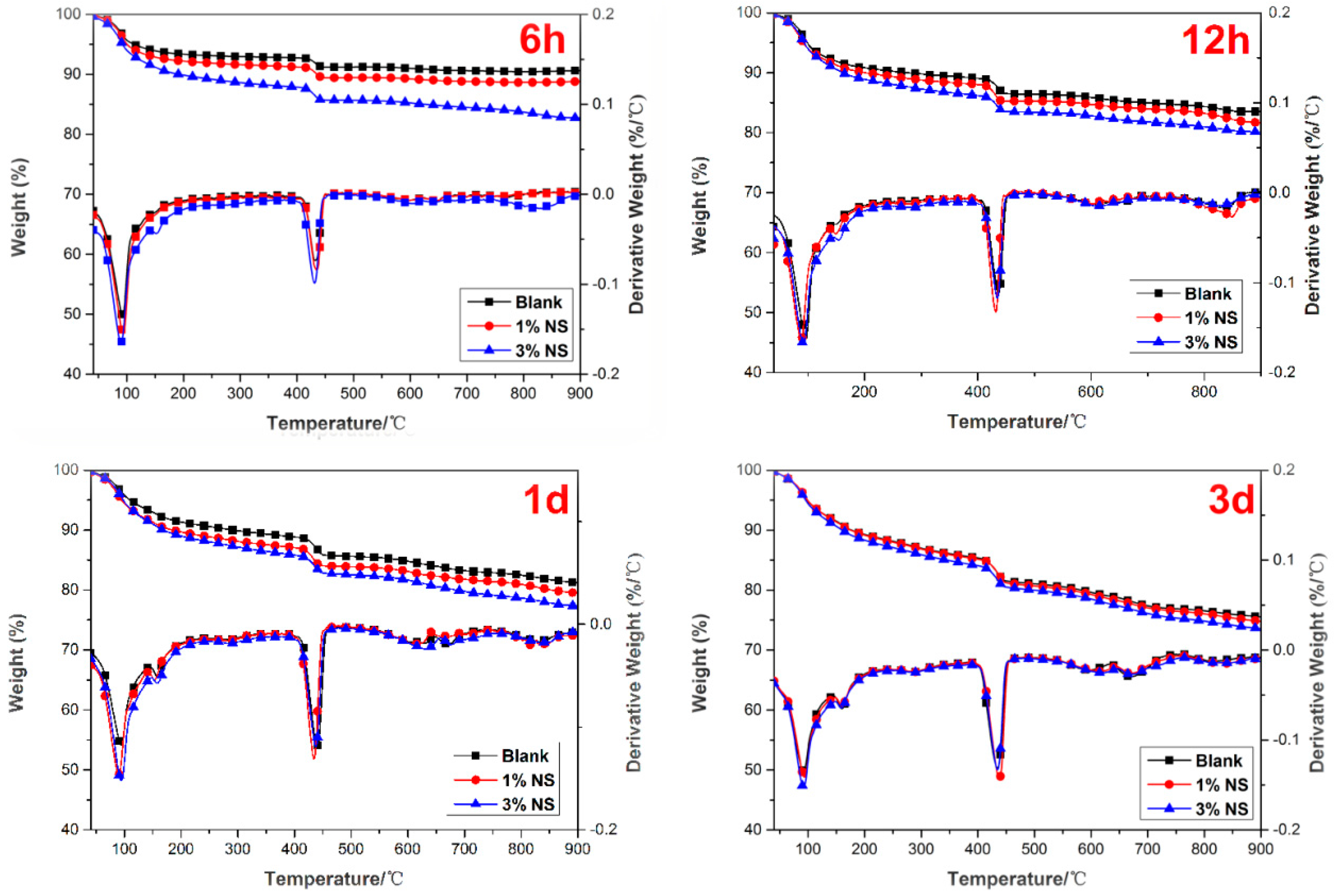
2.2.4. Determination of Calcium Hydroxide Content
2.2.5. Determination of Porosity and Pore Size Distribution
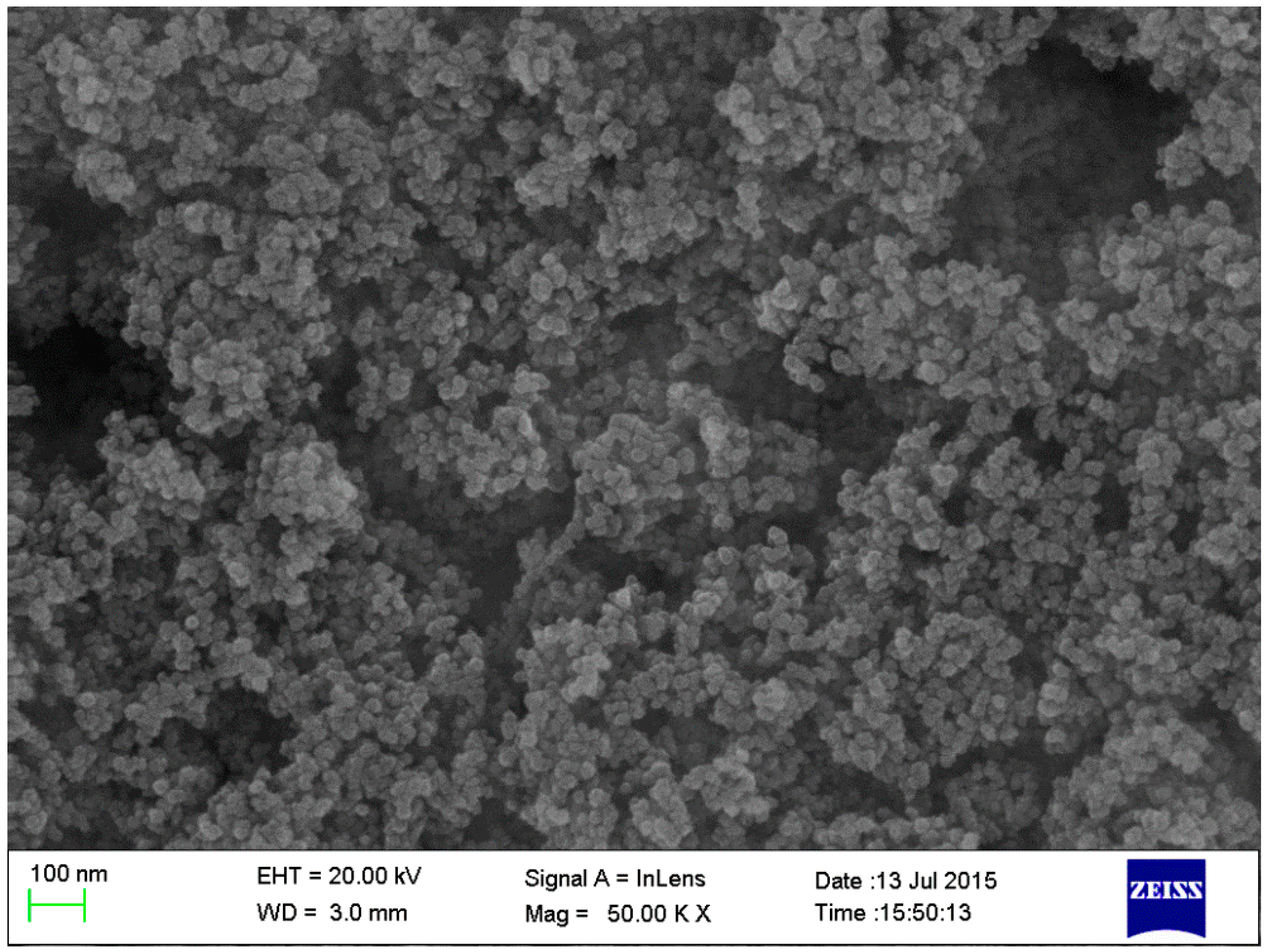
2.2.6. Determination of Microstructure and Morphology
3. Results and Discussion
3.1. Isothermal Calorimetric Analysis
3.2. X-ray Diffraction Analysis
3.3. TG/DTG Analysis
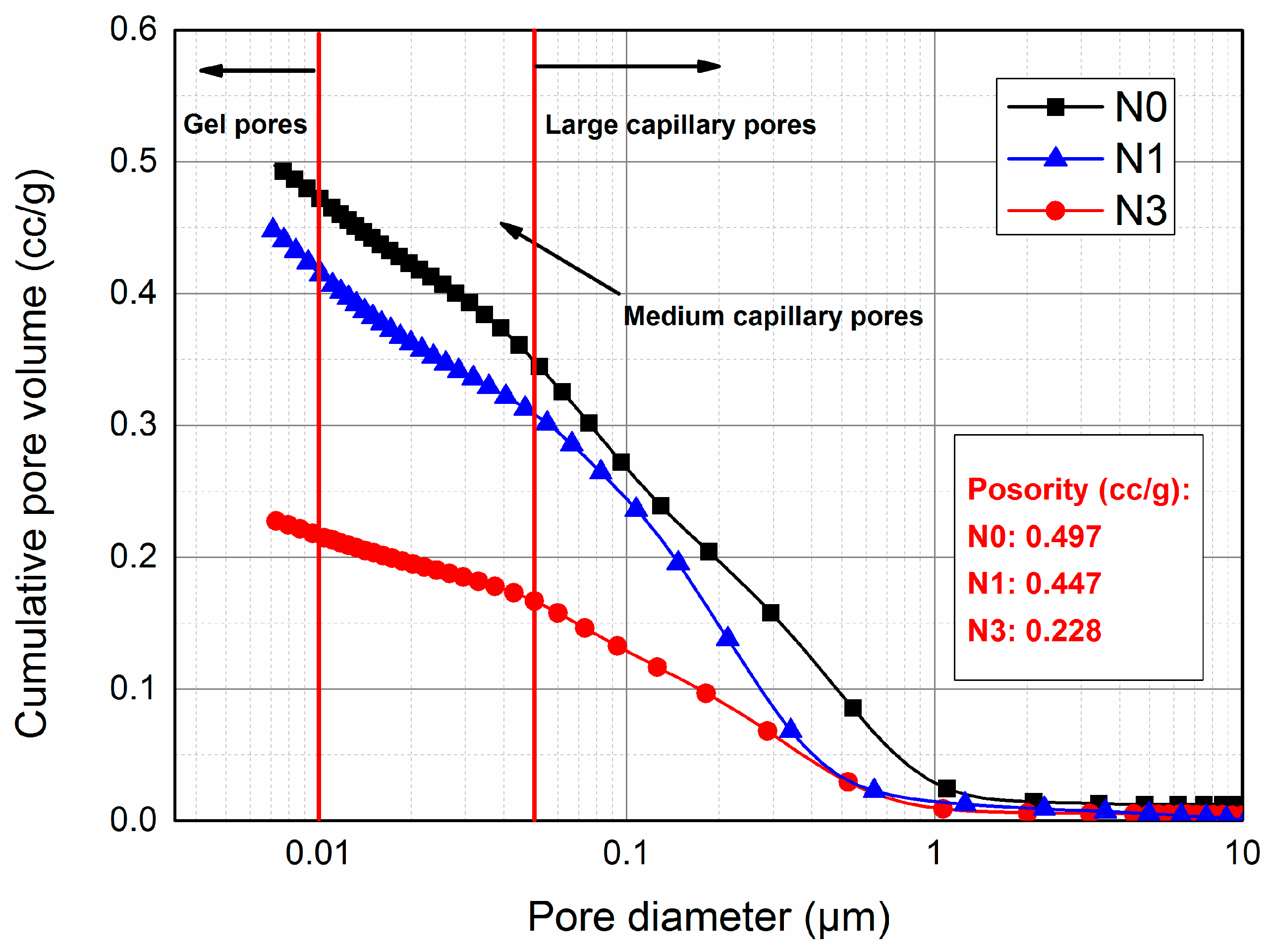
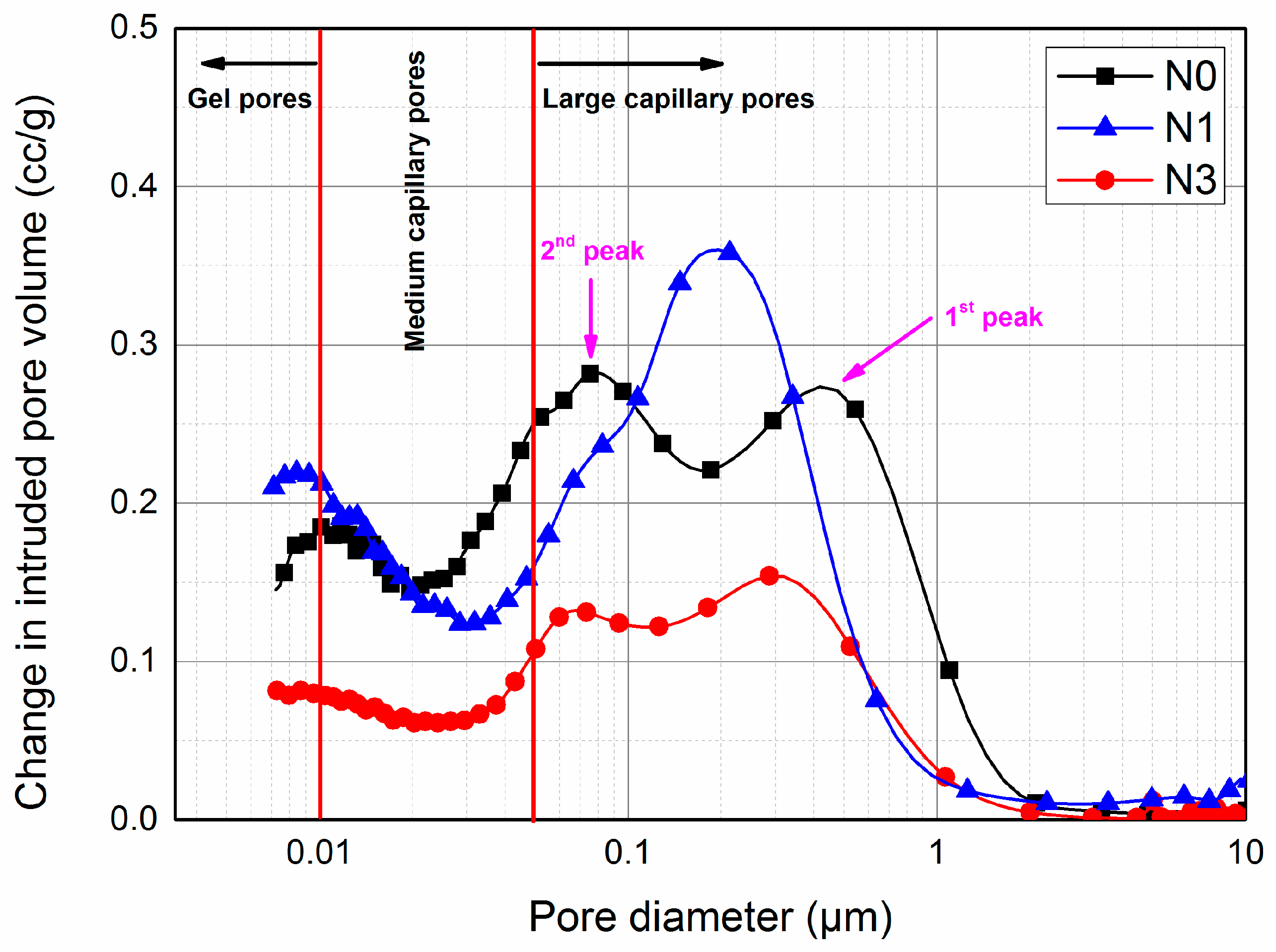
3.4. Pore Structure Analysis
3.5. Microstructure Analysis
4. Conclusions and Recommendations
- The incorporation of nano-SiO2 significantly accelerated the hydration process kinetics of AC$A cement, promoting C3S hydration which resulted in a greater cumulative heat release.
- Portlandite, ettringite, AFm, and C–S–H were found to be the major hydration products for all samples, indicating that nano-SiO2 had no effect on the kind of hydration product.
- The addition of nano-SiO2 led to a significant increase in CH content due to nucleation effect up to 6 h. However, since pozzolanic reactions are more dominant than the nucleation effects of nano-SiO2, an increased consumption of CH from 12 h to 3 days was more obvious in the nano-SiO2 incorporation pastes.
- The inclusion of nano-SiO2 led to a great improvement in microstructure. The total porosity and capillary pores decreased with increasing nano-SiO2 content. More refinement of the pore structure was achieved by increasing the nano-SiO2 content up to 3%.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, H.; Jeong, Y.; Jun, Y.; Jeong, J.H.; Oh, J.E. Strength enhancement and pore-size refinement in clinker-free CaO-activated GGBFS systems through substitution with gypsum. Cem. Concr. Compos. 2016, 68, 57–65. [Google Scholar] [CrossRef]
- Sobolev, K.; Gutiérrez, M.F. How nanotechnology can change the concrete world. Am. Ceram. Soc. Bull. 2005, 84, 14. [Google Scholar]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Hanus, M.J.; Harris, A.T. Nanotechnology innovations for the construction industry. Prog. Mater. Sci. 2013, 58, 1056–1102. [Google Scholar] [CrossRef]
- Motoyama, Y.; Appelbaum, R.; Parker, R. The National Nanotechnology Initiative: Federal support for science and technology, or hidden industrial policy? Technol. Soc. 2011, 33, 109–118. [Google Scholar] [CrossRef]
- Safiuddin, M.; Gonzalez, M.; Guo, J.; Tighe, S.L. State-of-the-art report on use of nano-materials in concrete. Int. J. Pavement Eng. 2014, 15, 940–949. [Google Scholar] [CrossRef]
- Kawashima, S.; Hou, P.; Corr, D.J.; Shah, S.P. Modification of cement-based materials with nanoparticles. Cem. Concr. Compos. 2013, 36, 8–15. [Google Scholar] [CrossRef]
- Rashad, A.M. Effects of ZnO2, ZrO2, Cu2O3, CuO, CaCO3, SF, FA, cement and geothermal silica waste nanoparticles on properties of cementitious materials—A short guide for Civil Engineer. Constr. Build. Mater. 2013, 48, 1120–1133. [Google Scholar] [CrossRef]
- Singh, L.P.; Karade, S.R.; Bhattacharyya, S.K.; Yousuf, M.M.; Ahalawat, S. Beneficial role of nanosilica in cement based materials—A review. Constr. Build. Mater. 2013, 47, 1069–1077. [Google Scholar] [CrossRef]
- Quercia, G.; Brouwers, H. Application of nano-silica (nS) in concrete mixtures. In Proceedings of the 8th fib International Ph. D. Symposium in Civil Engineering, Lyngby, Denmark, 20–23 June 2010; pp. 20–23. [Google Scholar]
- Zhang, R.; Cheng, X.; Hou, P.; Ye, Z. Influences of nano-TiO2 on the properties of cement-based materials: Hydration and drying shrinkage. Constr. Build. Mater. 2015, 81, 35–41. [Google Scholar] [CrossRef]
- Shaikh, F.U.A.; Supit, S.W.M. Chloride induced corrosion durability of high volume fly ash concretes containing nano particles. Constr. Build. Mater. 2015, 99, 208–225. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Shah, S.P. Highly dispersed carbon nanotube reinforced cement based materials. Cem. Concr. Res. 2010, 40, 1052–1059. [Google Scholar] [CrossRef]
- Brown, L.; Sanchez, F. Influence of carbon nanofiber clustering on the chemo-mechanical behavior of cement pastes. Cem. Concr. Compos. 2016, 65, 101–109. [Google Scholar] [CrossRef]
- Metaxa, Z.S.; Konsta-Gdoutos, M.S.; Shah, S.P. Carbon nanofiber cementitious composites: Effect of debulking procedure on dispersion and reinforcing efficiency. Cem. Concr. Compos. 2013, 36, 25–32. [Google Scholar] [CrossRef]
- Du, H.; Pang, S.D. Effect of colloidal nano-silica on the mechanical and durability performances of mortar. In Proceedings of the 10th International Symposium on High Performance Concrete—Innovation and Utilization, HPC 2014, Beijing, China, 16–18 September 2014; pp. 16–18. [Google Scholar]
- Senff, L.; Labrincha, J.A.; Ferreira, V.M.; Hotza, D.; Repette, W.L. Effect of nano-silica on rheology and fresh properties of cement pastes and mortars. Constr. Build. Mater. 2009, 23, 2487–2491. [Google Scholar] [CrossRef]
- Madani, H.; Bagheri, A.; Parhizkar, T. The pozzolanic reactivity of monodispersed nanosilica hydrosols and their influence on the hydration characteristics of Portland cement. Cem. Concr. Res. 2012, 42, 1563–1570. [Google Scholar] [CrossRef]
- Gaitero, J.J.; Campillo, I.; Guerrero, A. Reduction of the calcium leaching rate of cement paste by addition of silica nanoparticles. Cem. Concr. Res. 2008, 8, 1112–1118. [Google Scholar] [CrossRef]
- Hou, P.; Qian, J.; Cheng, X.; Shah, S.P. Effects of the pozzolanic reactivity of nano-SiO2 on cement-based materials. Cem. Concr. Res. 2015, 55, 250–258. [Google Scholar] [CrossRef]
- Said, A.; Zeidan, M.S.; Bassuoni, M.T.; Tian, Y. Properties of concrete incorporating nano-silica. Constr. Build. Mater. 2012, 36, 838–844. [Google Scholar] [CrossRef]
- Aly, M.; Hashmi, M.S.J.; Olabi, A.G.; Messeiry, M.; Abadir, E.F.; Hussain, A.I. Effect of colloidal nano-silica on the mechanical and physical behaviour of waste-glass cement mortar. Mater. Des. 2012, 33, 127–135. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Xu, S. Influence of nanoparticles on fluidity and mechanical properties of cement mortar. Constr. Build. Mater. 2015, 101, 892–901. [Google Scholar] [CrossRef]
- Ji, T. Preliminary study on the water permeability and microstructure of concrete incorporating nano-SiO2. Cem. Concr. Res. 2005, 35, 1943–1947. [Google Scholar] [CrossRef]
- Monteiro, P.J.M.; Kirchheim, A.P.; Chae, S.; Fischer, P.; MacDowell, A.A.; Schaible, E.; Wenk, H.R. Characterizing the nano and micro structure of concrete to improve its durability. Cem. Concr. Compos. 2009, 31, 577–584. [Google Scholar] [CrossRef]
- Rong, Z.; Sun, W.; Xiao, H.; Jiang, G. Effects of nano-SiO2 particles on the mechanical and microstructural properties of ultra-high performance cementitious composites. Cem. Concr. Compos. 2015, 56, 25–31. [Google Scholar] [CrossRef]
- Gesoglu, M.; Güneyisi, E.; Asaad, D.S.; Muhyaddin, G.F. Properties of low binder ultra-high performance cementitious composites: Comparison of nanosilica and microsilica. Constr. Build. Mater. 2016, 102, 706–713. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. Splitting tensile strength of concrete using ground granulated blast furnace slag and SiO2 nanoparticles as binder. Energy Build. 2011, 43, 864–872. [Google Scholar] [CrossRef]
- Tanaka, K.; Kurumisawa, K. Development of technique for observing pores in hardened cement paste. Cem. Concr. Res. 2002, 32, 1435–1441. [Google Scholar] [CrossRef]
- Kong, D.; Du, X.; Su, W.; Zhang, H.; Yang, Y.; Shah, S.P. Influence of nano-silica agglomeration on microstructure and properties of the hardened cement-based materials. Constr. Build. Mater. 2012, 37, 707–715. [Google Scholar] [CrossRef]
- He, X.; Shi, X. Chloride permeability and microstructure of Portland cement mortars incorporating nanomaterials. Transp. Res. Rec. 2008, 2070, 13–21. [Google Scholar] [CrossRef]
- Ardalan, R.B.; Jamshidi, N.; Arabameri, H.; Joshaghani, A.; Mehrinejad, M.; Sharafi, P. Enhancing the permeability and abrasion resistance of concrete using colloidal nano-SiO2 oxide and spraying nanosilicon practices. Constr. Build. Mater. 2017, 146, 128–135. [Google Scholar] [CrossRef]
- Adak, D.; Sarkar, M.; Mandal, S. Structural performance of nano-silica modified fly-ash based geopolymer concrete. Constr. Build. Mater. 2017, 135, 430–439. [Google Scholar] [CrossRef]
- Fallah, S.; Nematzadeh, M. Mechanical properties and durability of high-strength concrete containing macro-polymeric and polypropylene fibers with nano-silica and silica fume. Constr. Build. Mater. 2017, 132, 170–187. [Google Scholar] [CrossRef]
- Ma, B.; Li, H.; Mei, J.; Ouyang, P. Effect of Nano-TiO2 Addition on the Hydration and Hardening Process of Sulphoaluminate Cement. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 768–773. [Google Scholar] [CrossRef]
- Chitvoranund, N.; Winnefeld, F.; Hargis, C.W.; Lothenbach, B. Synthesis and hydration of alite-calcium sulfoaluminate cement. Adv. Cem. Res. 2017, 29, 101–111. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Xu, J.; Li, X.; Ma, S. Hydration properties of the alite–ye’elimite cement clinker synthesized by reformation. Constr. Build. Mater. 2015, 99, 254–259. [Google Scholar] [CrossRef]
- Ma, S.; Snellings, R.; Li, X.; Shen, X.; Scrivener, K.L. Alite–ye'elimite cement: Synthesis and mineralogical analysis. Cem. Concr. Res. 2013, 45, 15–20. [Google Scholar] [CrossRef]
- Yanjun, L.; Ning, Z.; Feng, L. Influence of CaF2 on the Formation and Hydration Properties of C4A3S. J. Build. Mater. 2001, 4, 217–221. [Google Scholar]
- Liu, X.; Li, Y. Effect of MgO on the composition and properties of alite-sulphoaluminate cement. Cem. Concr. Res. 2005, 35, 1685–1687. [Google Scholar] [CrossRef]
- Ma, S.; Shen, X.; Gong, X.; Zhong, B. Influence of CuO on the formation and coexistence of 3CaO center dot SiO2 and 3CaO·3Al2O3·CaSO4 minerals. Cem. Concr. Res. 2006, 36, 1784–1787. [Google Scholar] [CrossRef]
- Ma, S.; Shen, X.; Huang, Y.; Gong, X.; Zhong, B. Effect of zno on the formation and coexistence temperature range of alite and calcium sulphoaluminate. J. Chin. Ceram. Soc. 2006, 34, 1392–1396. [Google Scholar]
- Wild, S.; Khatib, J.M. Portlandite consumption in metakaolin cement pastes and mortars. Cem. Concr. Res. 1997, 27, 137–146. [Google Scholar] [CrossRef]
- Marsh, B.K.; Day, R.L. Pozzolanic and cementitious reactions of fly ash in blended cement pastes. Cem. Concr. Res. 1988, 18, 301–310. [Google Scholar] [CrossRef]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The use of thermal analysis in assessing the effect of temperature on a cement paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Silva, D.A.D.; Roman, H.R.; Gleize, P. Evidences of chemical interaction between EVA and hydrating Portland cement. Cem. Concr. Res. 2002, 32, 1383–1390. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Land, G.; Stephan, D. The influence of nano-silica on the hydration of ordinary Portland cement. J. Mater. Sci. 2012, 47, 1011–1017. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Shah, S.P.; Mishra, G.; Sharma, U. Studies on early stage hydration of tricalcium silicate incorporating silica nanoparticles: Part II. Constr. Build. Mater. 2016, 102, 943–949. [Google Scholar] [CrossRef]
- Zhu, J.; Feng, C.; Yin, H.; Zhang, Z.; Shah, S.P. Effects of colloidal nanoBoehmite and nano-SiO2 on fly ash cement hydration. Constr. Build. Mater. 2015, 101, 246–251. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Shah, S.P.; Mishra, G.; Sharma, U. Studies on early stage hydration of tricalcium silicate incorporating silica nanoparticles: Part I. Constr. Build. Mater. 2015, 74, 278–286. [Google Scholar] [CrossRef]
- Yu, Z.; Ye, G. The pore structure of cement paste blended with fly ash. Constr. Build. Mater. 2013, 45, 30–35. [Google Scholar] [CrossRef]
- Feldman, R.F. Significance of Porosity Measurements on Blended Cement Performance. In Fly Ash, Silica Fume, Slag and Other Mineral By-Products in Concrete, 1st ed.; American Concrete Institue: Detroit, MI, USA, 1983; Volume 79, pp. 415–434. [Google Scholar]
- Nunes, C.; Slížková, Z.; Stefanidou, M.; Němeček, J. Microstructure of lime and lime-pozzolana pastes with nanosilica. Cem. Concr. Res. 2016, 83, 152–163. [Google Scholar] [CrossRef]



| Oxide Content | Mineralogical Composition | ||
|---|---|---|---|
| Oxide | (% of Mass) | Phase | (% of Mass) |
| Calcium oxide, CaO | 63.9 | Tricalcium silicate, C3S | 48.16 |
| Silicon dioxide, SiO2 | 19.8 | Dicalcium silicate, C2S | 31.75 |
| Aluminium oxide, Al2O3 | 4.4 | Tricalcium aluminate, C3A | 5.70 |
| Sulphur trioxide, SO3 | 3.8 | Ferrite, C4AF | 4.61 |
| Ferric oxide, Fe2O3 | 3.1 | Free-lime, f-CaO | 1.48 |
| Magnesium oxide, MgO | 1.6 | Magnesium oxide, MgO | 1.35 |
| Potassium oxide, K2O | 0.4 | Calcium sulphoaluminate, C4A3S | 5.23 |
| Sodium oxide, Na2O | 0.1 | Gypsum, CS | 1.61 |
| Loss on ignition | 2.2 | ||
| Color | Diameter (nm) | Crystal Type | Surface Volume Ratio (m2/g) | Purity (%) | pH Value |
|---|---|---|---|---|---|
| White | 15 ± 5 | amorphous | 63 | 99.5 | 6 |
| Mix Designation | AC$A Cement (%) | Nano Silica (%) |
|---|---|---|
| N0 | 100 | 0 |
| N1 | 99 | 1 |
| N3 | 97 | 3 |
| Mass Loss (%) | |||
|---|---|---|---|
| Mix | Evaporable Water + Nonevaporable Water (C–S–H + Ettringite + Monosulfoaluminate) | Portlandite from TG (%) | Overall Weight Loss (%) |
| Control-6 h | 7.04 | 5.29 | 9.18 |
| Control-12 h | 10.20 | 8.64 | 16.39 |
| Control-1 d | 10.12 | 9.75 | 19.64 |
| Control-3 d | 13.00 | 11.15 | 25.51 |
| 1% NS-6 h | 8.37 | 6.19 | 11.06 |
| 1% NS-12 h | 11.23 | 8.31 | 18.72 |
| 1% NS-1 d | 11.88 | 9.53 | 21.64 |
| 1% NS-3 d | 13.13 | 11.08 | 26.18 |
| 3% NS-6 h | 11.38 | 6.41 | 17.22 |
| 3% NS-12 h | 12.70 | 8.43 | 19.79 |
| 3% NS-1 d | 12.82 | 9.58 | 23.42 |
| 3% NS-3 d | 14.08 | 10.55 | 27.36 |
| Mixture | Apparent Total Porosity (cc/g) | Volume of Large Capillary Pores (cc/g) | Volume of Medium Capillary Pores (cc/g) | Volume of Gel Pores (cc/g) |
|---|---|---|---|---|
| N0 | 49.75 | 33.6 | 12.39 | 2.49 |
| N1 | 44.82 | 30.64 | 10.80 | 3.37 |
| N3 | 22.84 | 16.27 | 4.93 | 1.20 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Xu, Z.; Li, W.; Shen, X. Effect of Nano-SiO2 on the Early Hydration of Alite-Sulphoaluminate Cement. Nanomaterials 2017, 7, 102. https://doi.org/10.3390/nano7050102
Sun J, Xu Z, Li W, Shen X. Effect of Nano-SiO2 on the Early Hydration of Alite-Sulphoaluminate Cement. Nanomaterials. 2017; 7(5):102. https://doi.org/10.3390/nano7050102
Chicago/Turabian StyleSun, Jinfeng, Zhiqiang Xu, Weifeng Li, and Xiaodong Shen. 2017. "Effect of Nano-SiO2 on the Early Hydration of Alite-Sulphoaluminate Cement" Nanomaterials 7, no. 5: 102. https://doi.org/10.3390/nano7050102






