Electrodeposition of Rhodium Nanowires Arrays and Their Morphology-Dependent Hydrogen Evolution Activity
Abstract
:1. Introduction
2. Results and Discussion
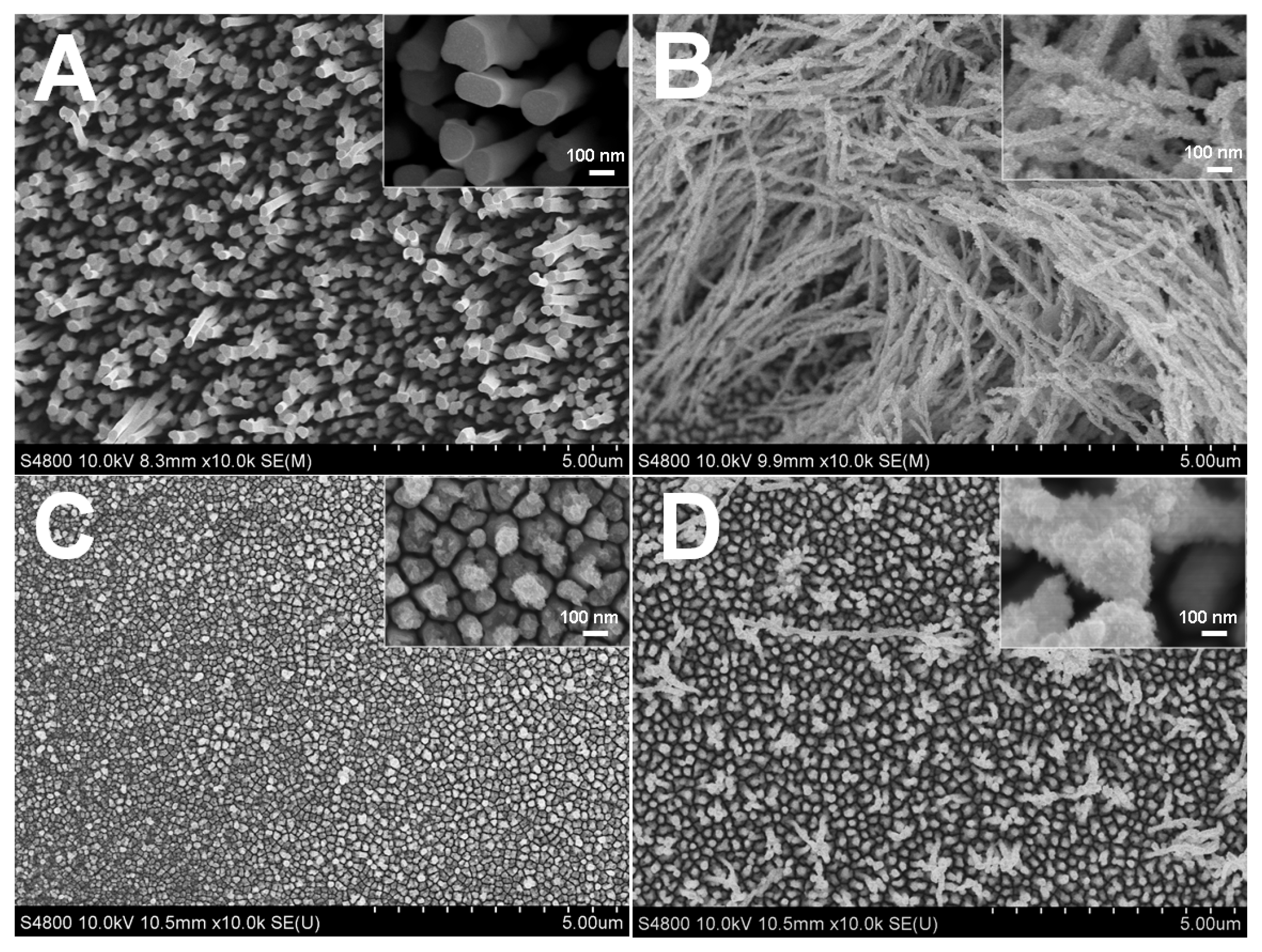
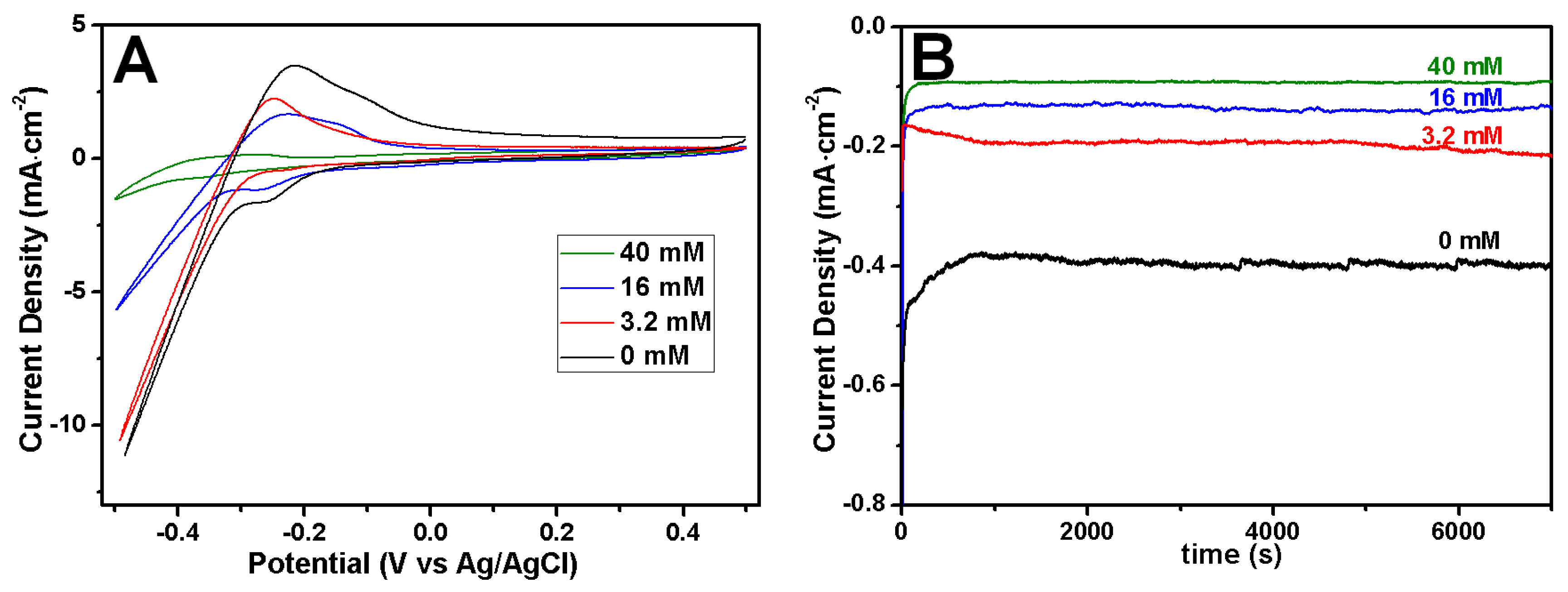
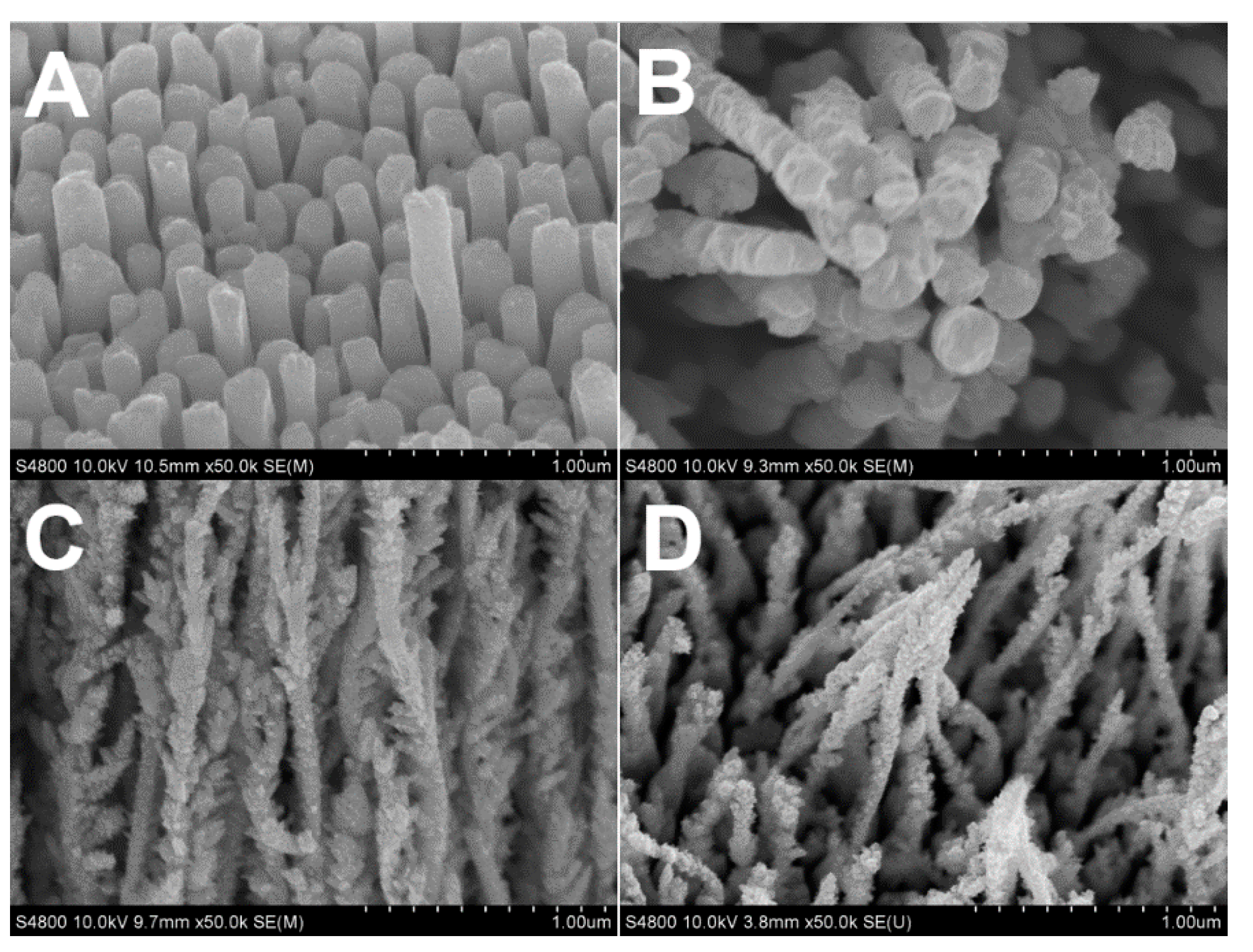
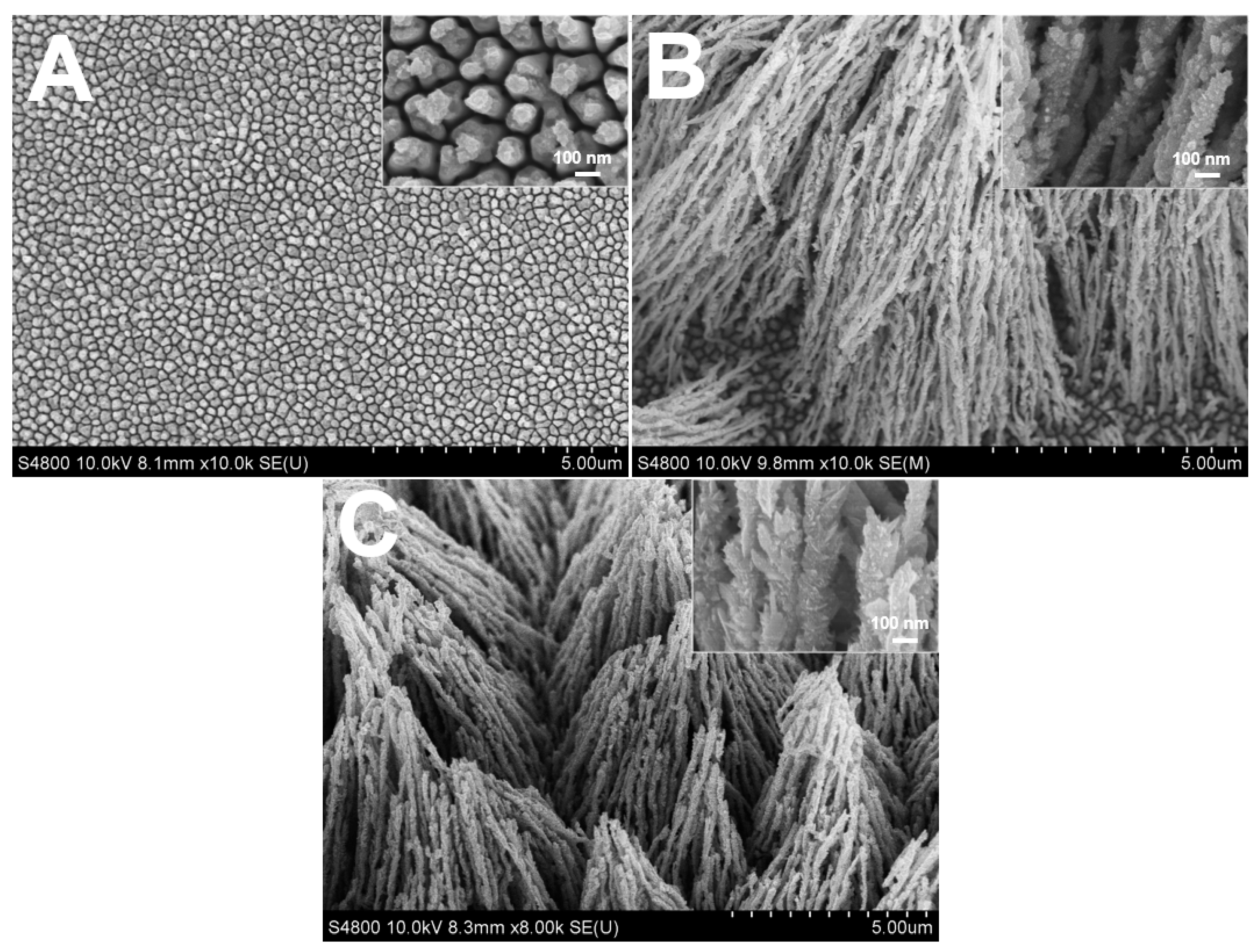
2.1. Influence of Citrate on the Morphology of Rh Nanowires
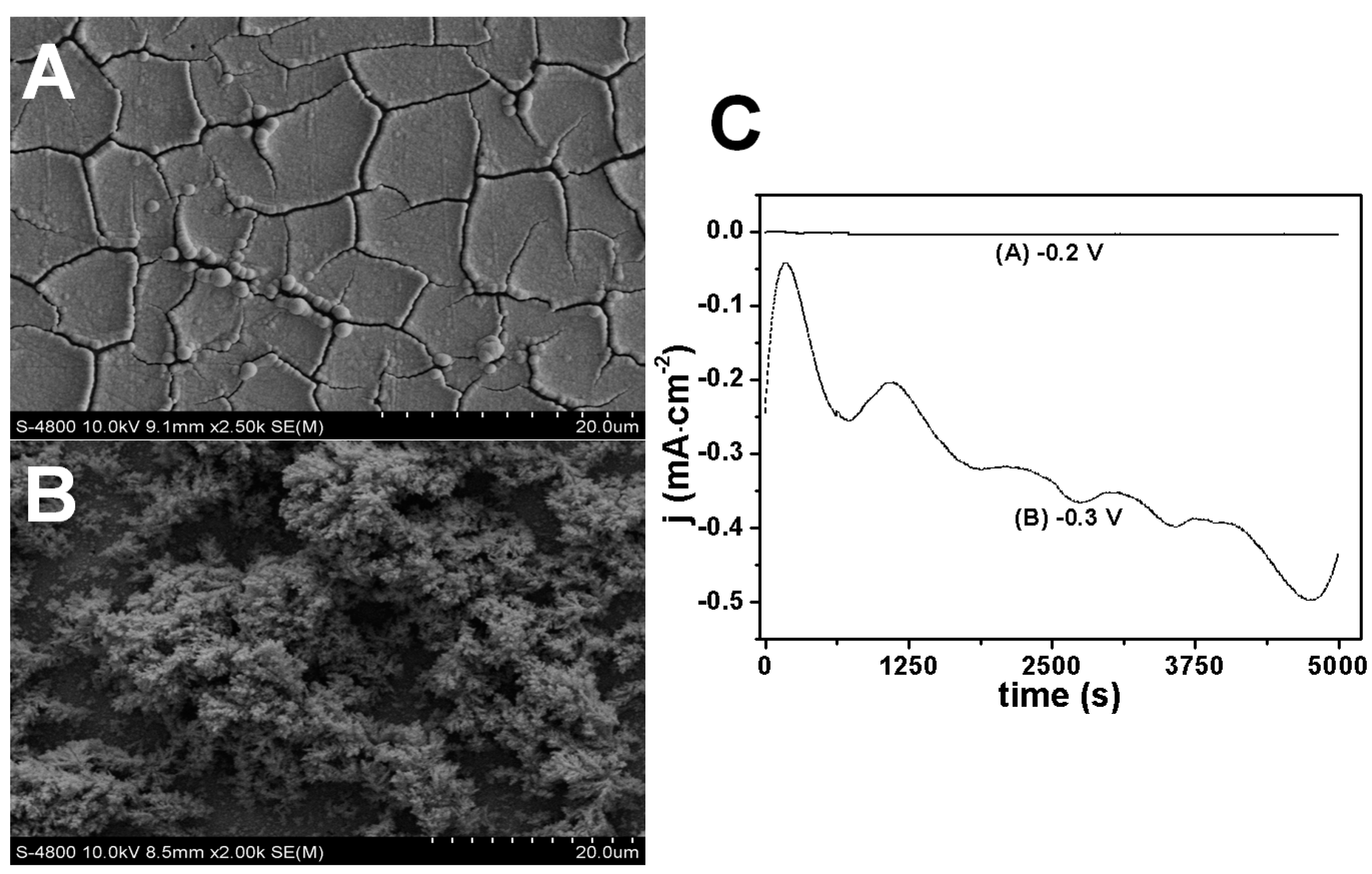
2.2. Influence of Electrode Potential on Morphology of Rh Nanowires
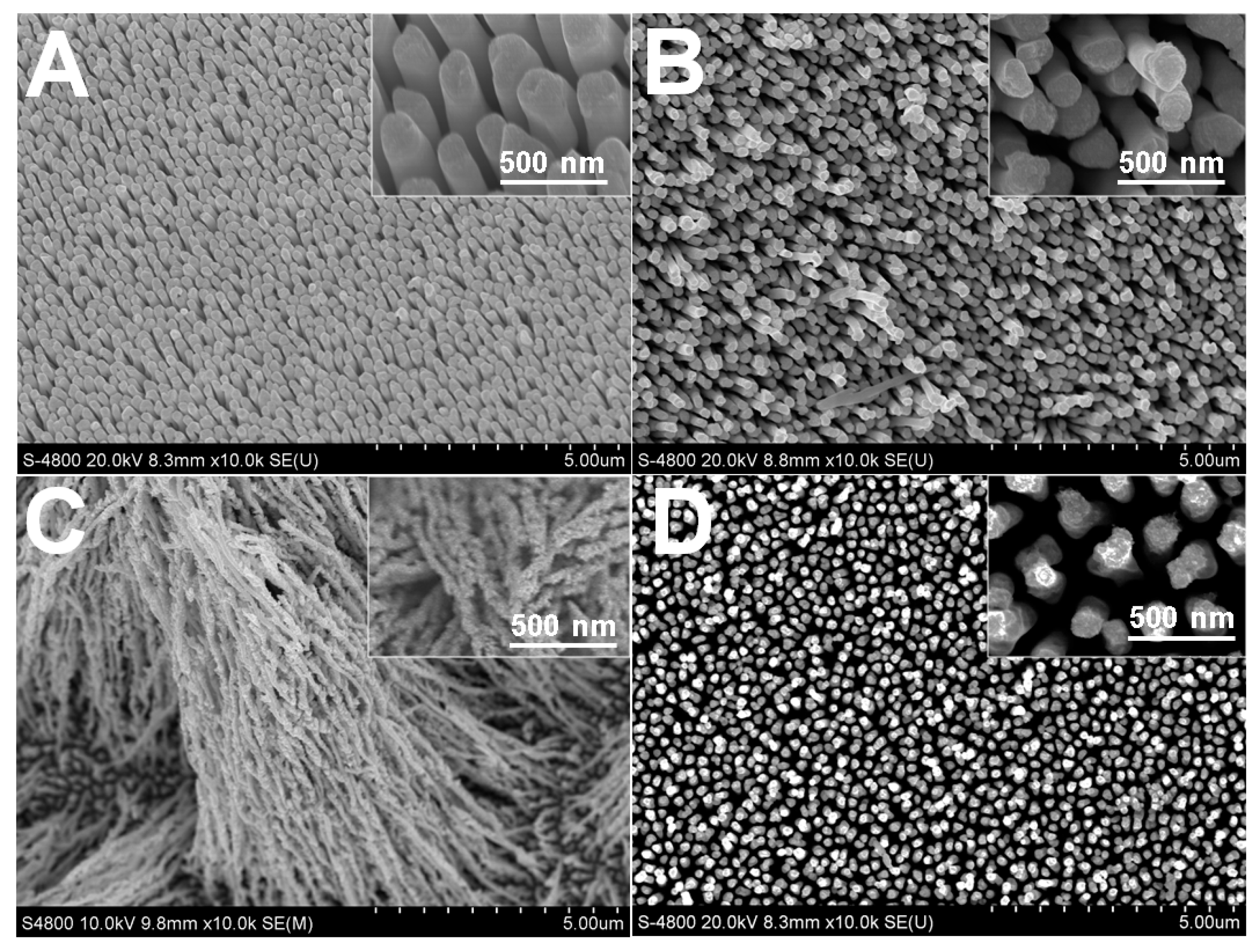
2.3. Concentration Effect of Rh(III)
2.4. pH Influence on the Electrodeposition of Rh Nanowires
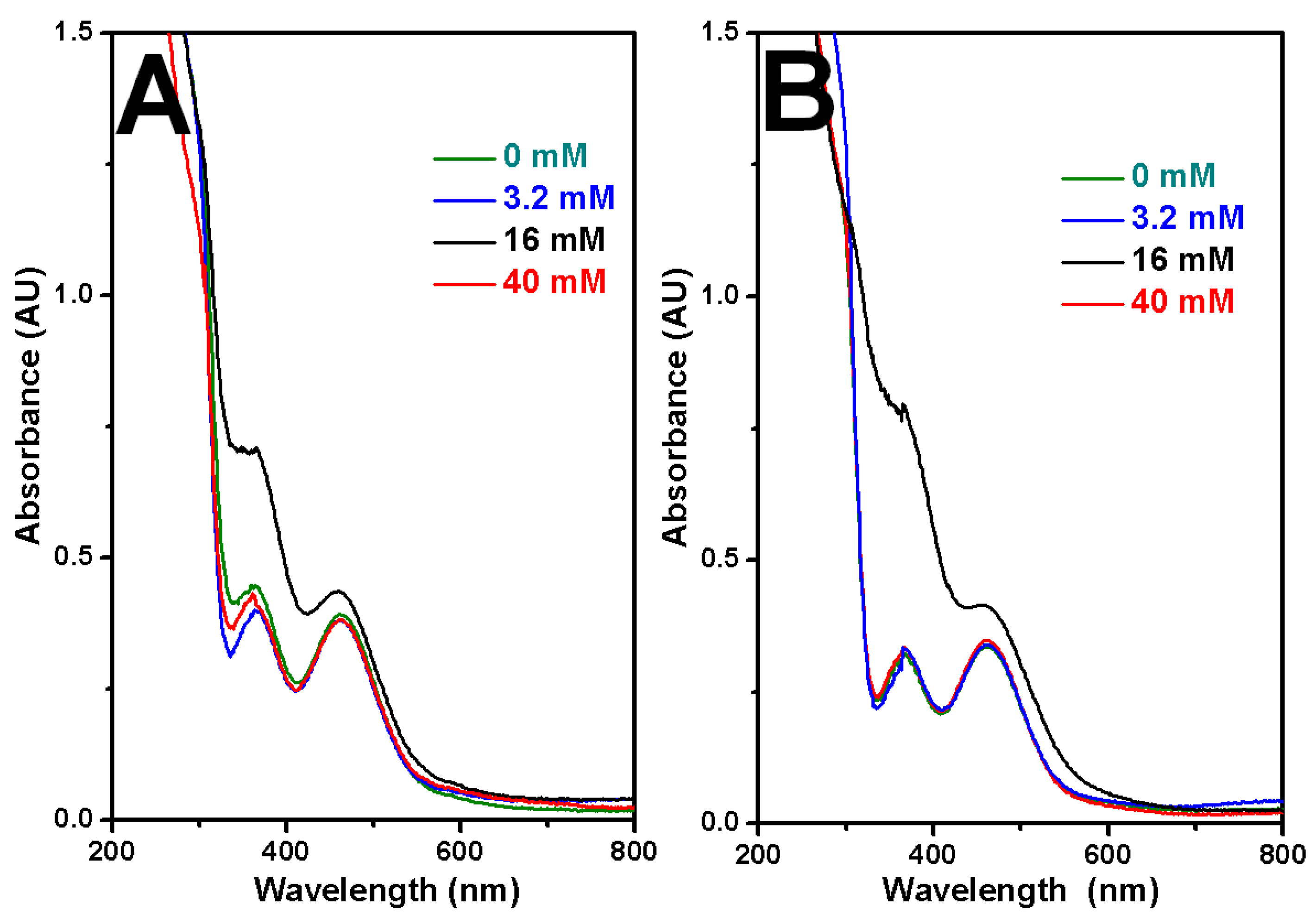
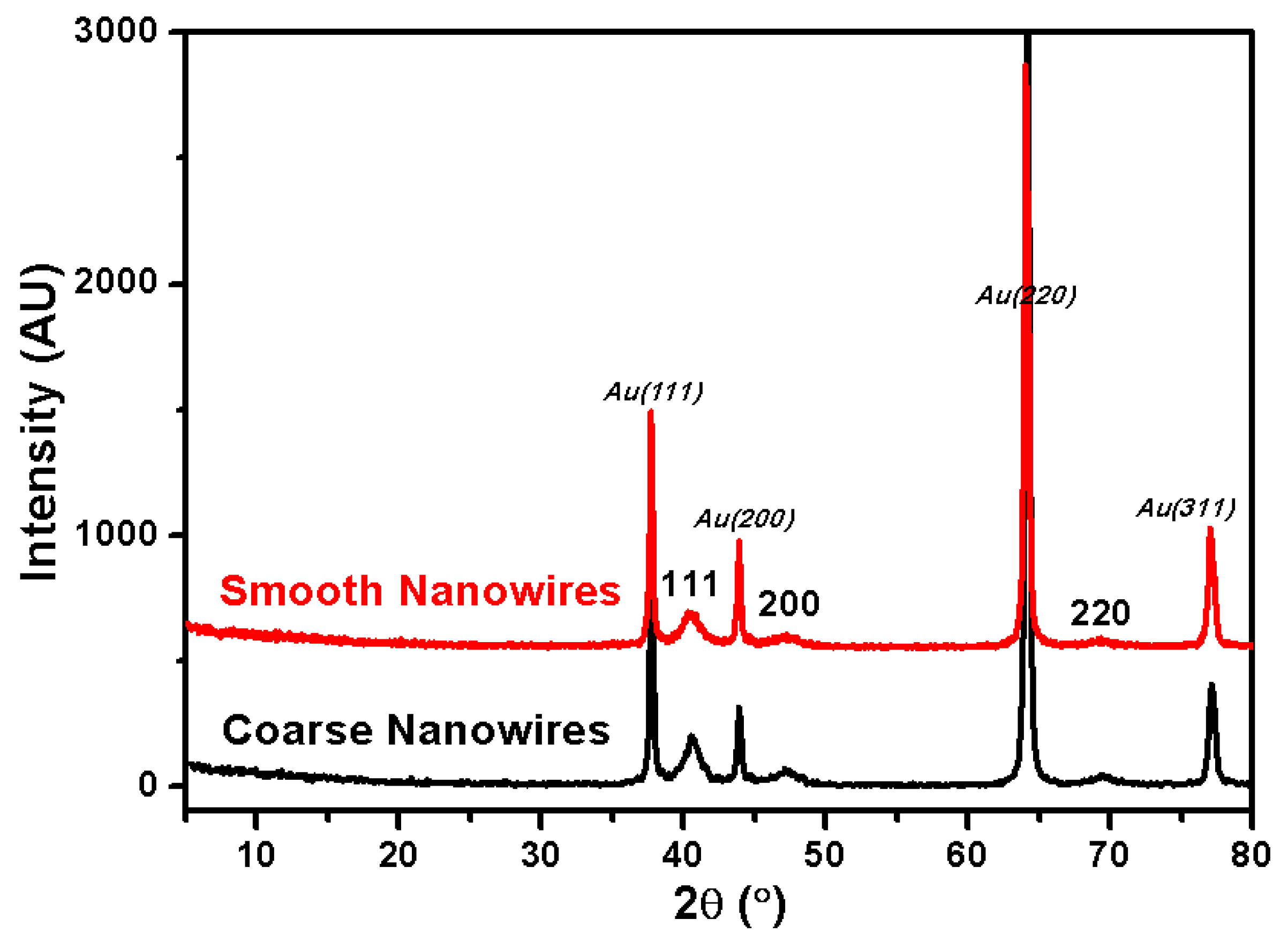
2.5. XRD Characterization for Rh Nanowires
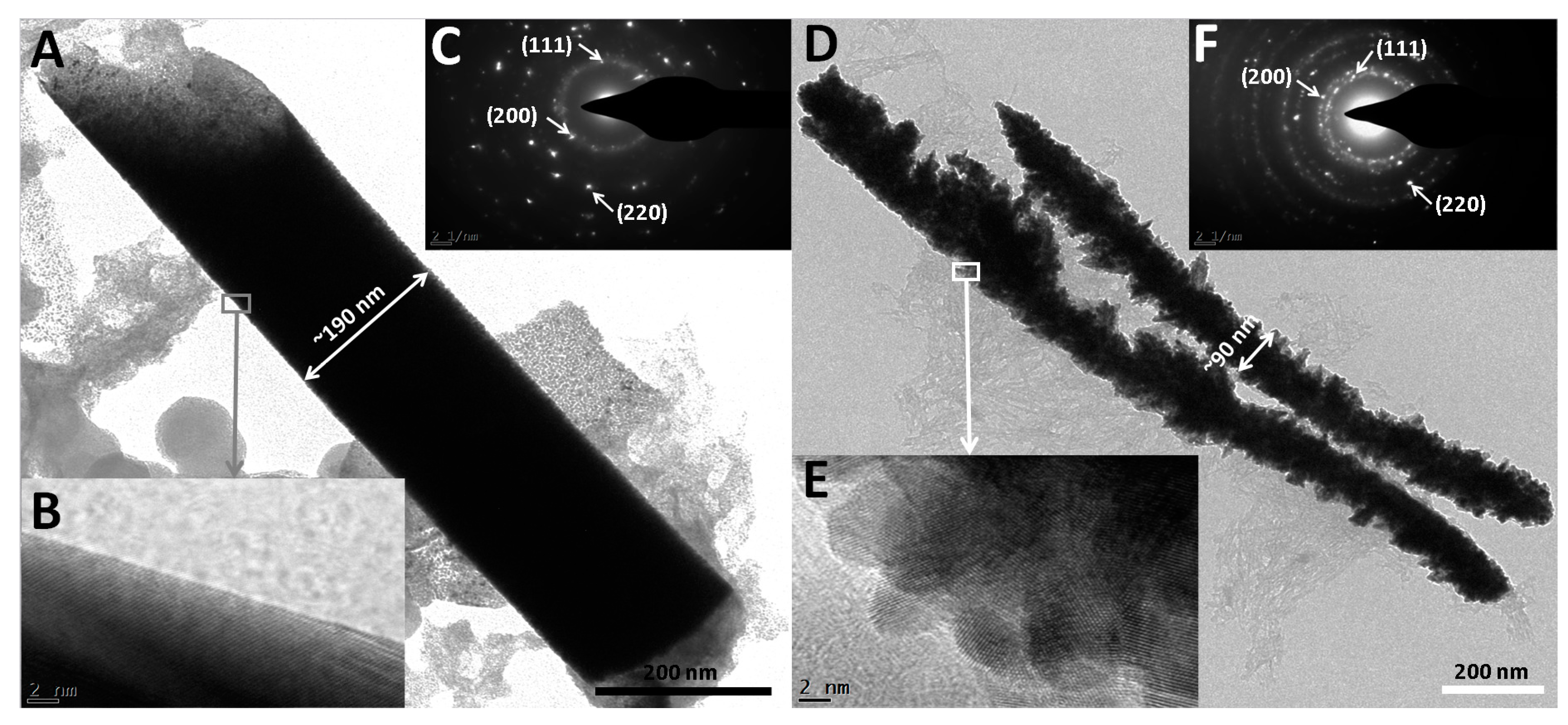
2.6. TEM Characterization for Rh Nanowires
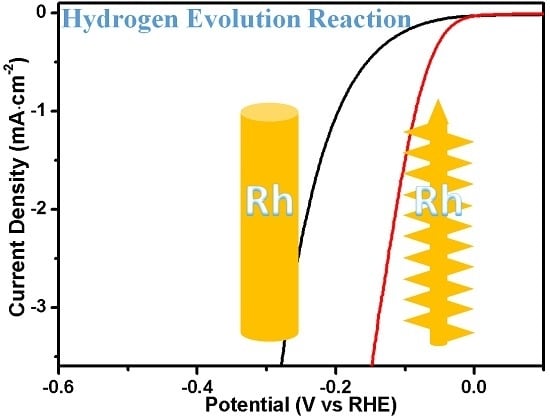
2.7. Morphology-Dependent Hydrogen Evolution Activity
3. Materials and Methods
3.1. Chemicals
3.2. Instruments
3.3. Methods
3.3.1. Conducting Layer Fabrication
3.3.2. Electrodeposition of Rh Nanowires
3.3.3. Characterization
3.3.4. Electrode Fabrication for Dispersed Rh Nanowires
3.3.5 Surface Area Calculation Method
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, X.-F.; Ren, B.; Tian, Z.-Q. Electrochemical and surface-enhanced raman spectroscopic studies on the adsorption and electrooxidation of C1 molecules on a roughened Rh electrode. J. Phy. Chem. B 2004, 108, 981–986. [Google Scholar] [CrossRef]
- Balan, B.K.; Sathe, B.R. Significant enhancement of formic acid oxidation using rhodium nanostructures. J. Nanosci. Nanotechnol. 2012, 12, 8994–8998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, M.; Cai, S.; Cai, Y.; Wang, P.; Bao, S.; Zou, M.; Du, M. Insitu growth of Rh nanoparticles with controlled sizes and dispersions on the cross-linked PVA–PEI nanofibers and their electrocatalytic properties towards H2O2. RSC Adv. 2014, 4, 794–804. [Google Scholar] [CrossRef]
- Leung, L.W.H.; Weaver, M.J. Adsorption and electrooxidation of some simple organic molecules on rhodium (111) as probed by real-time FTIR spectroscopy: Comparisons with platinum (111). J. Phys. Chem. 1989, 93, 7218–7226. [Google Scholar] [CrossRef]
- Kakade, B.A.; Shintri, S.S.; Sathe, B.R.; Halligudi, S.B.; Pillai, V.K. Quantized double-layer charging of rhodium2057 (tridecylamine) 321 clusters using differential pulse and cyclic voltammetry. Adv. Mater. 2007, 19, 272–275. [Google Scholar] [CrossRef]
- Galletti, C.; Specchia, S.; Saracco, G.; Specchia, V. Catalytic performance of rhodium-based catalysts for CO preferential oxidation in H2-rich gases. Ind. Eng. Chem. Res. 2008, 47, 5304–5312. [Google Scholar] [CrossRef]
- Galletti, C.; Fiorot, S.; Specchia, S.; Saracco, G.; Specchia, V. Activity of rhodium-based catalysts for CO preferential oxidation in H2-rich gases. Top. Catal. 2007, 45, 15–19. [Google Scholar] [CrossRef]
- Méndez, E.; Rodríguez, J.L.; Arévalo, M.C.; Pastor, E. Comparative study of ethanol and acetaldehyde reactivities on rhodium electrodes in acidic media. Langmuir 2002, 18, 763–772. [Google Scholar] [CrossRef]
- Zhang, Y.; Grass, M.E.; Habas, S.E.; Tao, F.; Zhang, T.; Yang, P.; Somorjai, G.A. One-step polyol synthesis and langmuir-blodgett monolayer formation of size-tunable monodisperse rhodium nanocrystals with catalytically active (111) surface structures. J. Phys. Chem. C 2007, 111, 12243–12253. [Google Scholar] [CrossRef]
- Zhang, Y.; Grass, M.E.; Kuhn, J.N.; Tao, F.; Habas, S.E.; Huang, W.; Yang, P.; Somorjai, G.A. Highly selective synthesis of catalytically active monodisperse rhodium nanocubes. J. Am. Chem. Soc. 2008, 130, 5868–5869. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, J.; Teramura, K.; Okuoka, S.; Yamazoe, S.; Kato, K.; Shishido, T.; Tanaka, T. Investigation of the formation process of photodeposited Rh nanoparticles on TiO2 by in situ time-resolved energy-dispersive XAFS analysis. Langmuir 2010, 26, 13907–13912. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhou, Z.; Zhuang, J.; Wang, X. Tunable aqueous phase synthesis and shape-dependent electrochemical properties of rhodium nanostructures. Inorg. Chem. 2010, 49, 5515–5521. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Liu, X.Y.; Xia, Y. Shape-Controlled Syntheses of Rhodium Nanocrystals for the Enhancement of Their Catalytic Properties. Nano Res. 2015, 8, 82–96. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Chen, Y.; Chiu, C.-Y.; Ruan, L.; Liu, Y.; Li, M.; Duan, X.; Huang, Y. High Density Catalytic Hot Spots in Ultrafine Wavy Nanowires. Nano Lett. 2014, 14, 3887–3894. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.D.A.; Watson, S.M.D.; Horrocks, B.R.; Houlton, A. Chemical and Electrochemical Routes to DNA-Templated Rhodium Nanowires. J. Mater. Chem. C 2015, 3, 438–446. [Google Scholar] [CrossRef]
- Muench, F.; Neetzel, C.; Kaserer, S.; Brotz, J.; Jaud, J.-C.; Zhao-Karger, Z.; Lauterbach, S.; Kleebe, H.-J.; Roth, C.; Ensinger, W. Fabrication of Porous Rhodium Nanotube Catalysts by Electroless Plating. J. Mater. Chem. 2012, 22, 12784–12791. [Google Scholar] [CrossRef]
- Bi, Y.; Lu, G. Iodide Ions Control Galvanic Replacement Growth of Uniform Rhodiumnanotubes at Room Temperature. Chem. Commun. 2008, 6402–6404. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Goebl, J.; Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2013, 42, 2610–2653. [Google Scholar] [CrossRef] [PubMed]
- Wade, T.L.; Wegrowe, J.-E. Template synthesis of nanomaterials. Eu. Phys. J. Appl. Phys. 2005, 29, 3–22. [Google Scholar] [CrossRef]
- Martin, C.R. Nanomaterials: A membrane-based synthetic approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Liu, D. Template-based synthesis of nanorod, nanowire, and nanotube arrays. Adv. Colloid Interface 2008, 136, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Park, S. Direct formation of thin-walled palladium nanotubes in nanochannels under an electrical potential. Chem. Mater. 2011, 23, 1456–1460. [Google Scholar] [CrossRef]
- Liu, L.; Yoo, S.-H.; Park, S. Synthesis of vertically aligned hollow platinum nanotubes with single crystalline nanoflakes. Chem. Mater. 2010, 22, 2681–2684. [Google Scholar] [CrossRef]
- Kim, S.M.; Liu, L.; Cho, S.H.; Jang, H.Y.; Park, S. Synthesis of bimetallic Pt/Pd nanotubes and their enhanced catalytic activity in methanol electro-oxidation. J. Mater. Chem. A 2013, 1, 15252–15257. [Google Scholar] [CrossRef]
- Liu, L.; Yoo, S.-H.; Lee, S.A.; Park, S. Electrochemical growth of silver nanobelts in cylindrical alumina nanochannels. Cryst. Growth Des. 2011, 11, 3731–3734. [Google Scholar] [CrossRef]
- Liu, L.; Yoo, S.-H.; Lee, S.A.; Park, S. Wet-chemical synthesis of palladium nanosprings. Nano Lett. 2011, 11, 3979–3982. [Google Scholar] [CrossRef] [PubMed]
- Moghimian, N.; Sam, M.; Bhiladvala, R.B. Rhodium Nanowires: Synthesis and Nanostructure Tailoring by Controlling Hydrogen Evolution. Mater. Lett. 2013, 113, 152–155. [Google Scholar] [CrossRef]
- Leontiev, A.P.; Brylev, O.A.; Napolskii, K.S. Arrays of rhodium nanowires based on anodic alumina: Preparation and electrocatalytic activity for nitrate reduction. Electrochim. Acta 2015, 155, 466–473. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size control of gold nanocrystals in citrate reduction: The third role of citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 1973, 241, 20–22. [Google Scholar] [CrossRef]
- González-Peña, O.I.; Chapman, T.W.; Vong, Y.M.; Antaño-López, R. Study of adsorption of citrate on Pt by CV and EQCM. Electrochim. Acta 2008, 53, 5549–5554. [Google Scholar] [CrossRef]
- Kunze, J.; Burgess, I.; Nichols, R.; Buess-Herman, C.; Lipkowski, J. Electrochemical evaluation of citrate adsorption on Au(111) and the stability of citrate-reduced gold colloids. J. Electroanal. Chem. 2007, 599, 147–159. [Google Scholar] [CrossRef]
- Li, Y.; Diao, P.; Jin, T.; Sun, J.; Xu, D. Shape-controlled electrodeposition of standing Rh nanoplates on indium tin oxide substrates and their electrocatalytic activity toward formic acid oxidation. Electrochim. Acta 2012, 83, 146–154. [Google Scholar] [CrossRef]
- Ojani, R.; Hasheminejad, E.; Raoof, J.B. Hydrogen evolution assisted electrodeposition of bimetallic 3D nano/micro-porous PtPd films and their electrocatalytic performance. Int. J. Hydrog. Energy 2014, 39, 8194–8203. [Google Scholar] [CrossRef]
- Zheng, J.; Sheng, W.; Zhuang, Z.; Xu, B.; Yan, Y. Universal dependence of hydrogen oxidation and evolution reaction activity of platinum-group metals on pH and hydrogen binding energy. Sci. Adv. 2016, 2, e1501602. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.; Tilak, B. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, H.; Li, Y.; Liao, F.; Lifshitz, Y.; Sheng, M.; Lee, S.-T.; Shao, M. A rhodium/silicon co-electrocatalyst design concept to surpass platinum hydrogen evolution activity at high overpotentials. Nat. Commun. 2016, 7, 12272. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Xu, Q.; Huang, X. Defect-rich metal nanocrystals in catalysis. ChemCatChem 2016, 8, 480–485. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Liu, L.; Park, S. Nanoparticle films as a conducting layer for anodic aluminum oxide template-assisted nanorod synthesis. J. Colloid Interface Sci. 2009, 339, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.F.; Pippel, E.; Scholz, R.; Gosele, U. Nanoporous Pt-Co alloy nanowires: Fabrication, characterization, and electrocatalytic properties. Nano Lett. 2009, 9, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt Bimetallic Nanodendrites with High Activity for Oxygen Reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, L.; Wang, H.; Shen, H.; Cheng, Q.; Yan, C.; Park, S. Electrodeposition of Rhodium Nanowires Arrays and Their Morphology-Dependent Hydrogen Evolution Activity. Nanomaterials 2017, 7, 103. https://doi.org/10.3390/nano7050103
Zhang L, Liu L, Wang H, Shen H, Cheng Q, Yan C, Park S. Electrodeposition of Rhodium Nanowires Arrays and Their Morphology-Dependent Hydrogen Evolution Activity. Nanomaterials. 2017; 7(5):103. https://doi.org/10.3390/nano7050103
Chicago/Turabian StyleZhang, Liqiu, Lichun Liu, Hongdan Wang, Hongxia Shen, Qiong Cheng, Chao Yan, and Sungho Park. 2017. "Electrodeposition of Rhodium Nanowires Arrays and Their Morphology-Dependent Hydrogen Evolution Activity" Nanomaterials 7, no. 5: 103. https://doi.org/10.3390/nano7050103