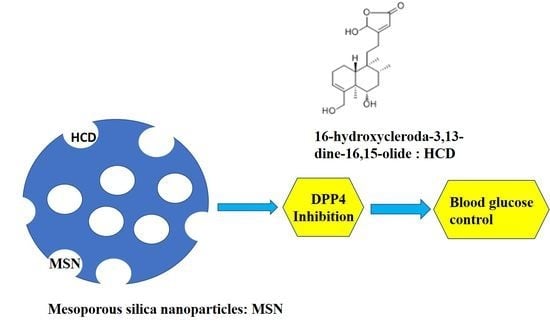
Encapsulation of 16-Hydroxycleroda-3,13-Dine-16,15-Olide in Mesoporous Silica Nanoparticles as a Natural Dipeptidyl Peptidase-4 Inhibitor Potentiated Hypoglycemia in Diabetic Mice
Abstract
:1. Introduction
2. Results
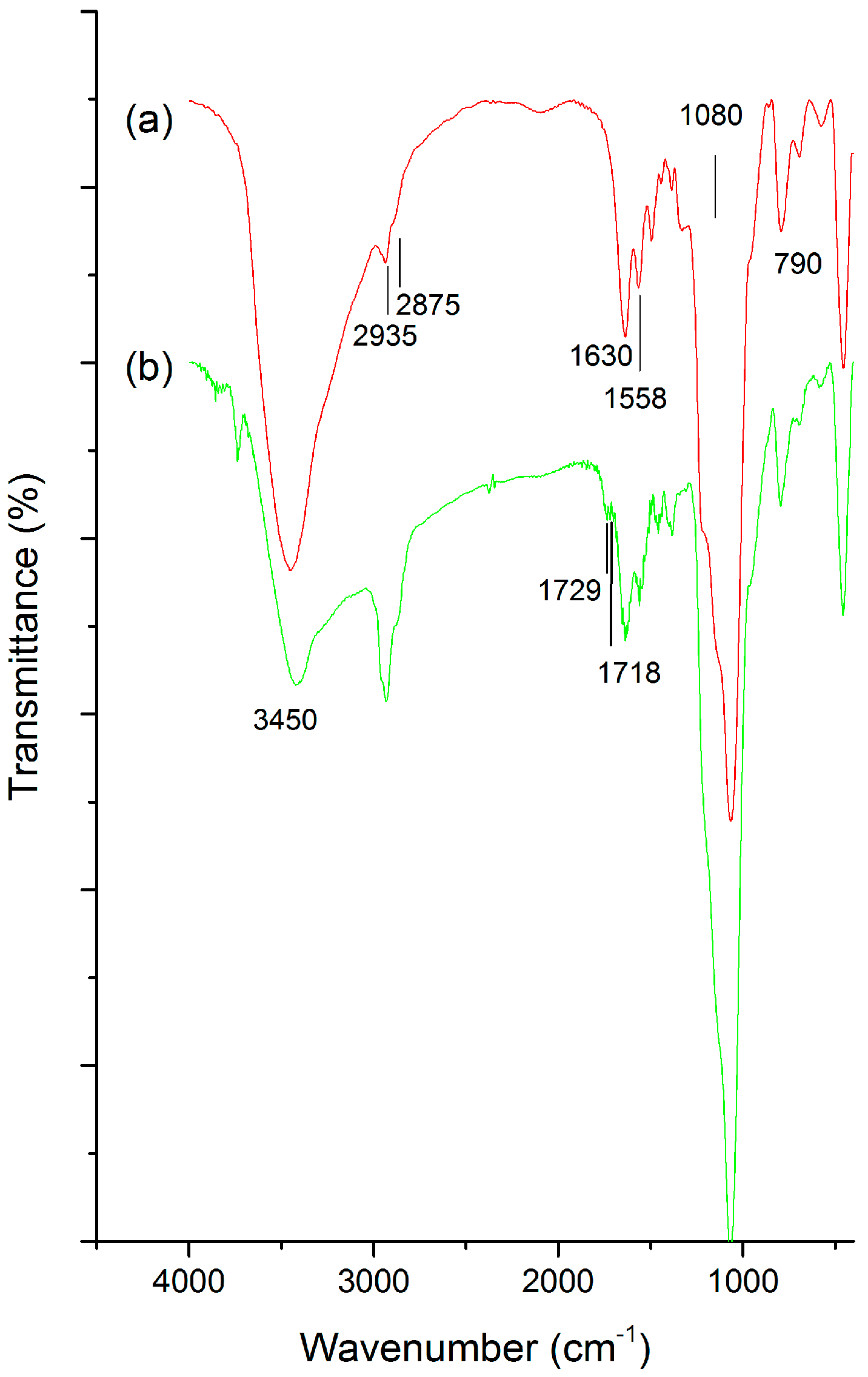
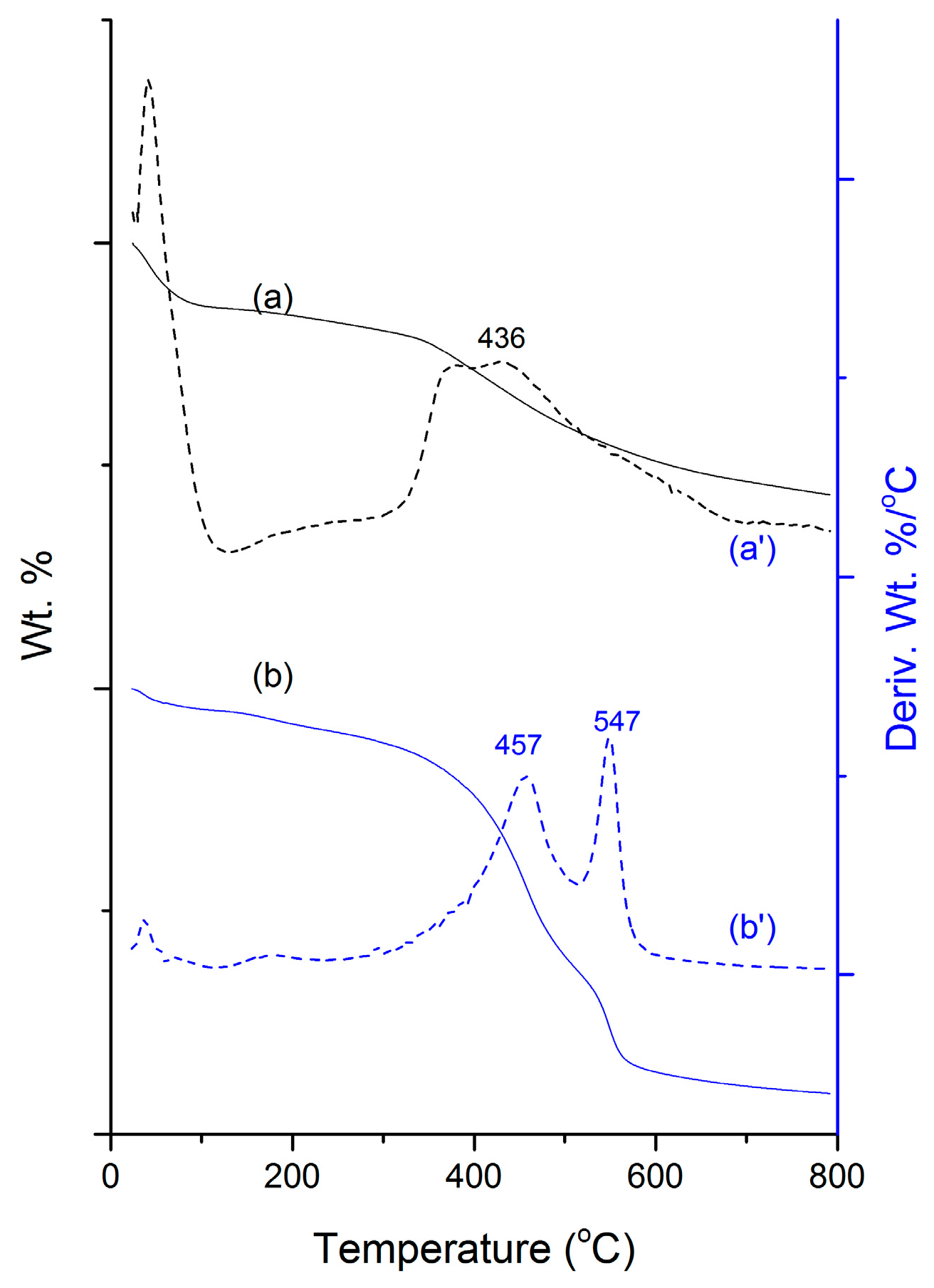
2.1. Physical Characterizations
2.2. In Vitro Assay of MSN-HCD
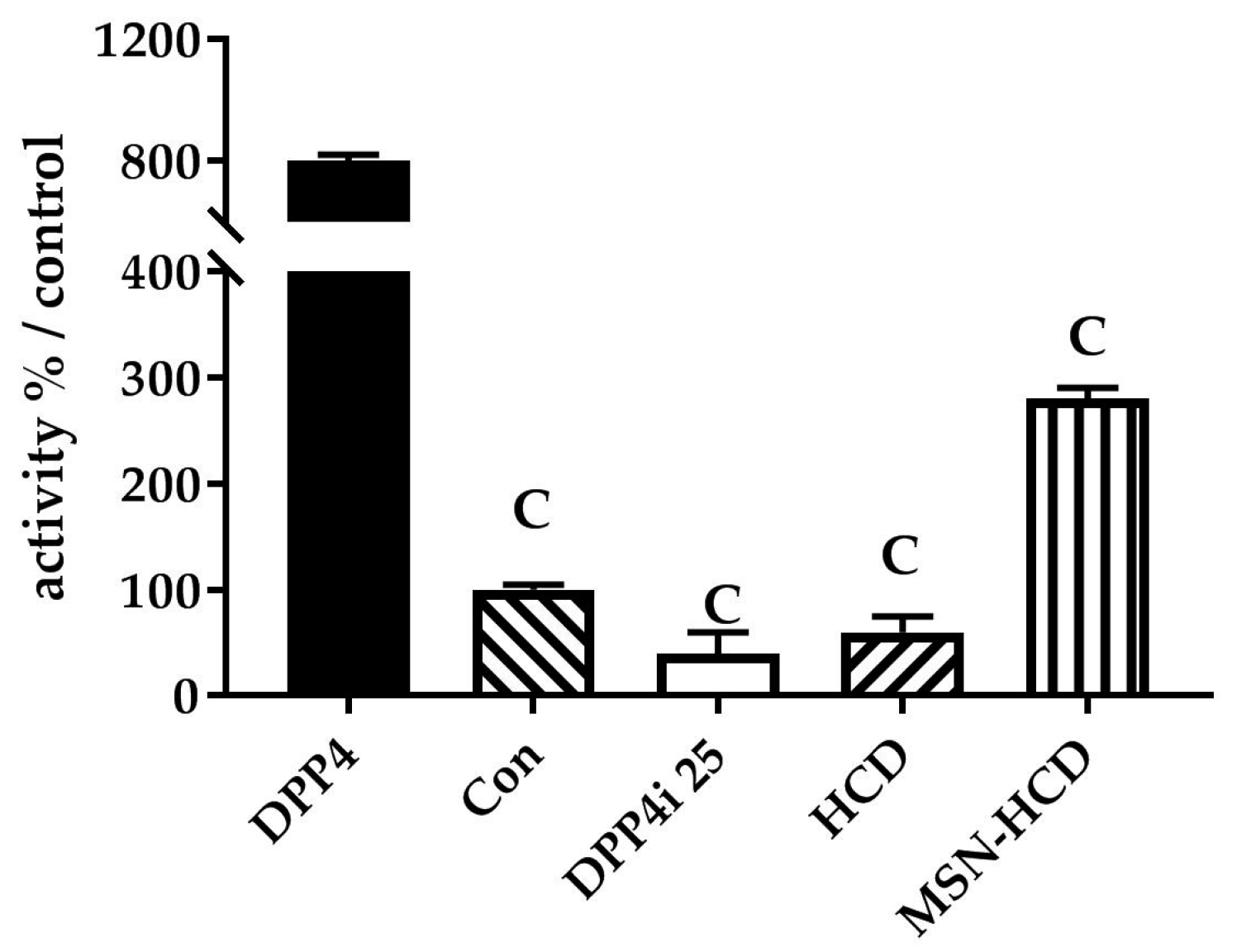
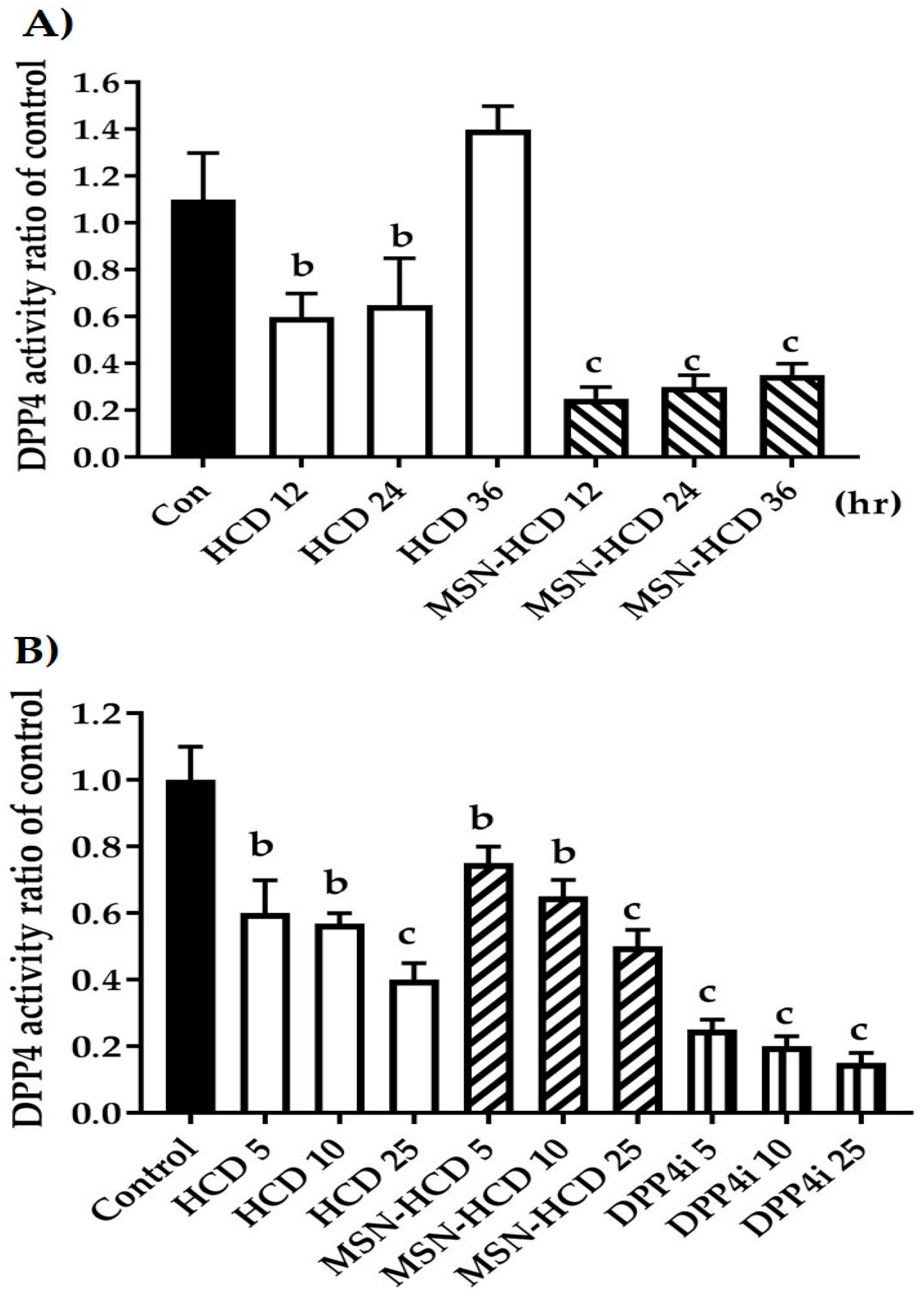
2.2.1. The Inhibition of DPP4 Enzyme Activity
2.2.2. In Vitro Enzyme Activity of DPP4
2.3. In Vivo Test of MSN-HCD
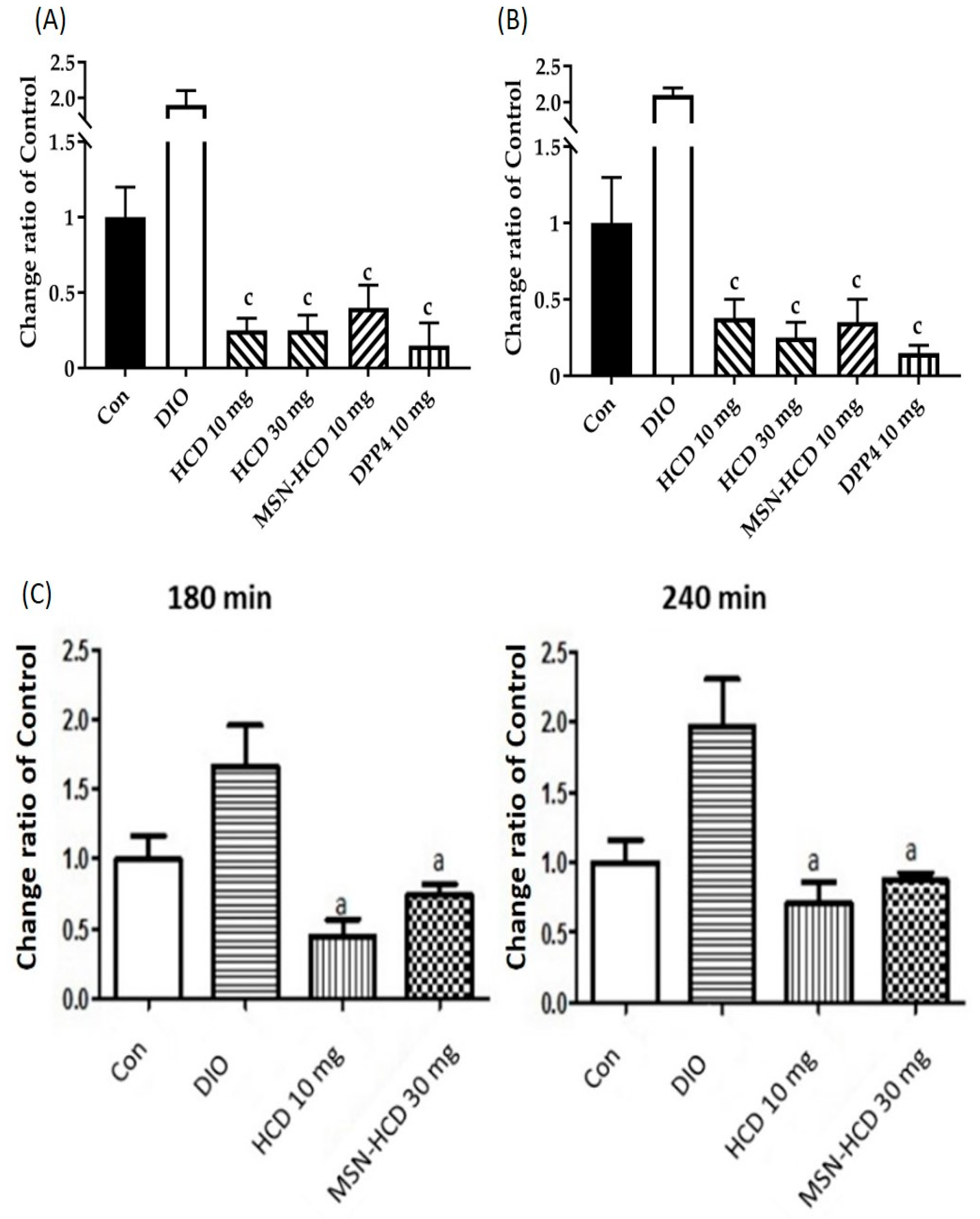
2.3.1. Hypoglycemic Effect via Long Term Administrations
2.3.2. In Vivo Oral Glucose Tolerance Test (OGTT)
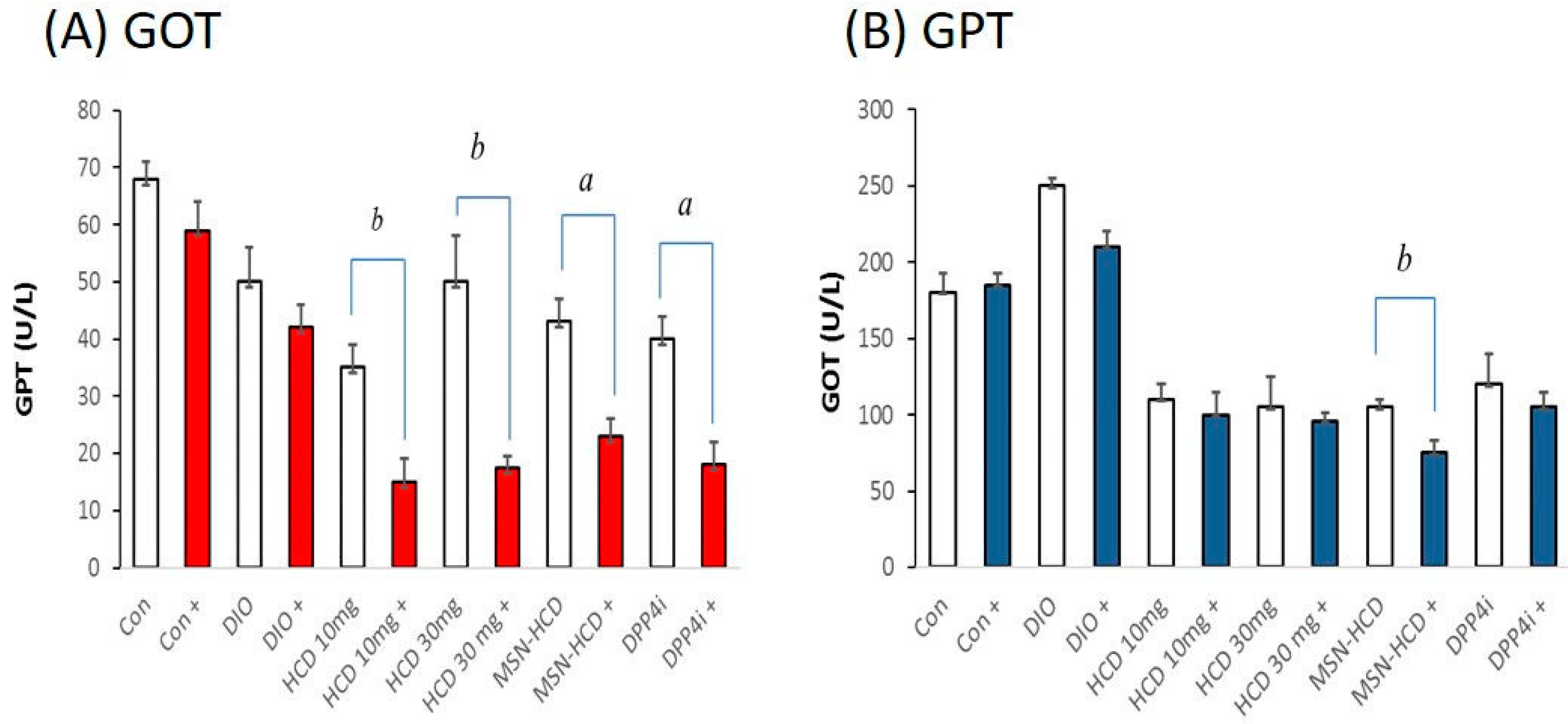
2.3.3. Serum Biochemical Analysis
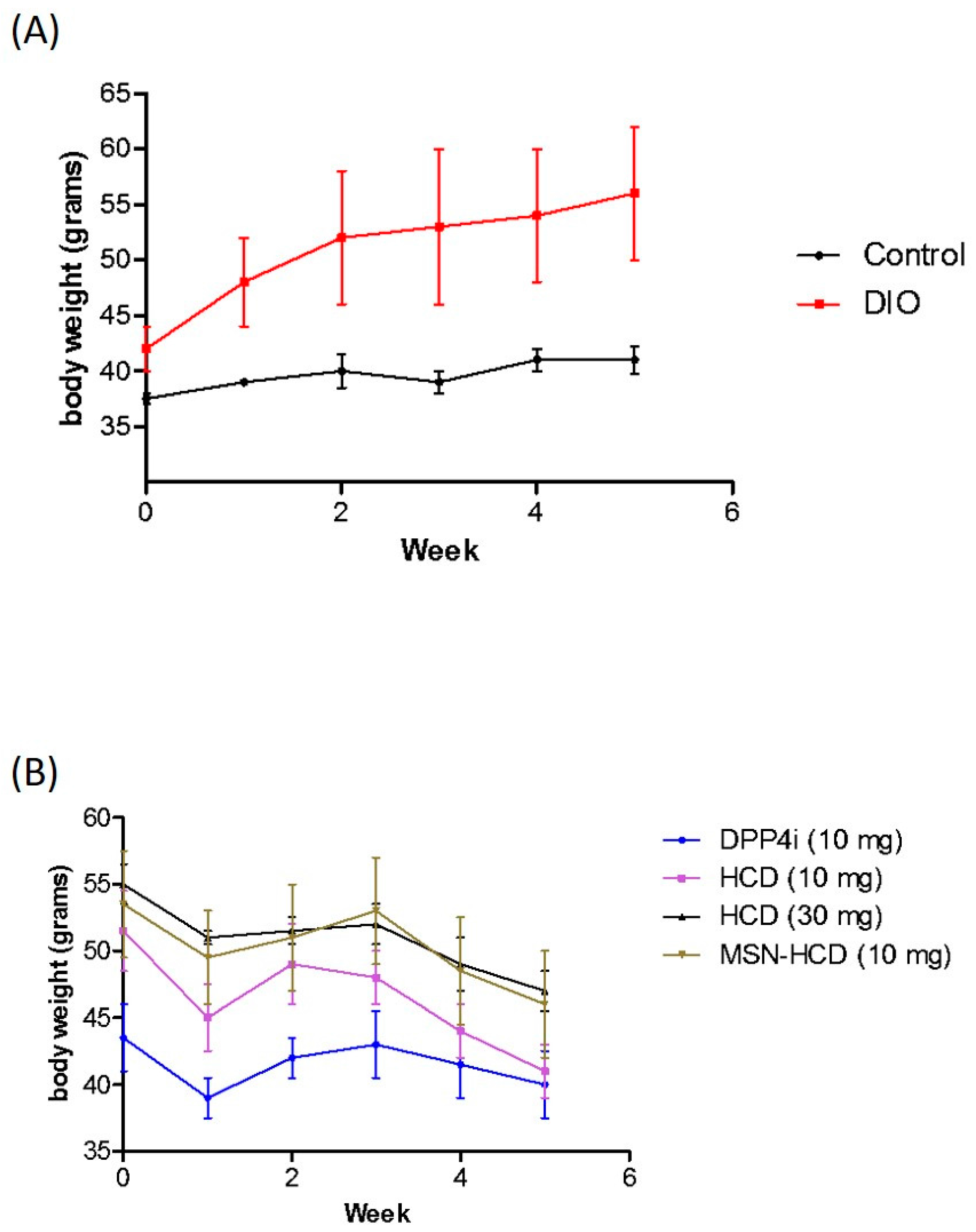
2.3.4. Body Weight Analysis
3. Discussion
3.1. Physical Characterization
3.2. Hypoglycemic Effect of HCD Conjugated MSN
4. Materials and Methods
4.1. Materials
4.2. Preparation of HCD Immobilized MSNs
4.2.1. Preparation of MSNs
4.2.2. Post-Modification of MSNs (MSN-NH2)
4.2.3. Isolation of Natural Compounds
4.2.4. Preparation of HCD Loaded MSN Samples (MSN-HCD)
4.3. In Vitro Test
4.3.1. Cell Culture
4.3.2. DPP4 Enzyme Activity
4.3.3. In Vitro DPP4 Inhibition Assay
4.4. In Vivo Test
4.4.1. Animals
4.4.2. Glucose Intolerance Induced
4.4.3. Diabetes Test
4.4.4. Long Term Administrations
4.4.5. Oral Glucose Tolerance Test (OGTT)
4.4.6. Biochemical Analysis of Blood
4.5. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tai, W.; Mo, R.; Di, J.; Subramanian, V.; Gu, X.; Buse, J.B.; Gu, Z. Bio-inspired synthetic nanovesicles for glucose-responsive release of insulin. Biomacromolecules 2014, 15, 3495–3502. [Google Scholar] [CrossRef] [PubMed]
- Gordijo, C.R.; Koulajian, K.; Shuhendler, A.J.; Bonifacio, L.D.; Huang, H.Y.; Chiang, S.; Ozin, G.A.; Giacca, A.; Wu, X.Y. Nanotechnology-enabled closed loop insulin delivery device: In vitro and in vivo evaluation of glucose-regulated insulin release for diabetes control. Adv. Funct. Mater. 2011, 21, 73–82. [Google Scholar] [CrossRef]
- Mojsov, S.; Weir, G.C.; Habener, J.F. Insulinotropin: Glucagon-like peptide i (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J. Clin. Investig. 1987, 79, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Gallwitz, B.; Schmidt, W.E.; Nauck, M.A. Glucagon-like peptide 1 as a regulator of food intake and body weight: Therapeutic perspectives. Eur. J. Pharmacol. 2002, 440, 269–279. [Google Scholar] [CrossRef]
- Wettergren, A.; Schjoldager, B.; Mortensen, P.E.; Myhre, J.; Christiansen, J.; Holst, J.J. Truncated-1 (proglucagon 78–107-amide) inhibits gastric and pancreatic functions in man. Dig. Dis. Sci. 1993, 38, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Naslund, E. Glucagon-like peptide-1, satiety and appetite control. Br. J. Nutr. 1999, 81, 259–260. [Google Scholar] [PubMed]
- Vilsboll, T.; Knop, F.K. Long-acting GLP-1 analogs for the treatment of type 2 diabetes mellitus. BioDrugs 2008, 22, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Gromada, J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E199–E206. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, B.; Walker, M.; Gravholt, C.H.; Shearing, P.A.; Sturis, J.; Alberti, K.G.; Holst, J.J.; Schmitz, O. Twenty-four-hour insulin secretion rates, circulating concentrations of fuel substrates and gut incretin hormones in healthy offspring of type ii (non-insulin-dependent) diabetic parents: Evidence of several aberrations. Diabetologia 1999, 42, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Toft-Nielsen, M.B.; Damholt, M.B.; Madsbad, S.; Hilsted, L.M.; Hughes, T.E.; Michelsen, B.K.; Holst, J.J. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2001, 86, 3717–3723. [Google Scholar] [CrossRef] [PubMed]
- Del Prato, S.; Marchetti, P. Beta- and alpha-cell dysfunction in type 2 diabetes. Horm. Metab. Res. 2004, 36, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.H.; Liu, T.L.; Li, L.L.; Liu, H.Y.; Chen, D.; Tang, F.Q. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 2013, 34, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lin, T.-S.; Mou, C.-Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Huang, I.P.; Sun, S.-P.; Cheng, S.-H.; Lee, C.-H.; Wu, C.-Y.; Yang, C.-S.; Lo, L.-W.; Lai, Y.-K. Enhanced chemotherapy of cancer using ph-sensitive mesoporous silica nanoparticles to antagonize P-glycoprotein-mediated drug resistance. Mol. Cancer Ther. 2011, 10, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Cheng, S.-H.; Huang, I.P.; Souris, J.S.; Yang, C.-S.; Mou, C.-Y.; Lo, L.-W. Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew. Chem. Int. Ed. 2010, 49, 8214–8219. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Kuthati, Y.; Liu, C.-L.; Mou, C.-Y.; Lee, C.-H. Killing cancer cells by delivering a nanoreactor for inhibition of catalase and catalytically enhancing intracellular levels of ros. RSC Adv. 2015, 5, 86072–86081. [Google Scholar] [CrossRef]
- Lin, C.-H.; Cheng, S.-H.; Liao, W.-N.; Wei, P.-R.; Sung, P.-J.; Weng, C.-F.; Lee, C.-H. Mesoporous silica nanoparticles for the improved anticancer efficacy of cis-platin. Int. J. Pharm. 2012, 429, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Hung, B.-Y.; Kuthati, Y.; Kankala, R.; Kankala, S.; Deng, J.-P.; Liu, C.-L.; Lee, C.-H. Utilization of enzyme-immobilized mesoporous silica nanocontainers (IBN-4) in prodrug-activated cancer theranostics. Nanomaterials 2015, 5, 2169–2191. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, X.; Zhang, H.; Yang, C.; Liu, Y.; Yang, W.; Guo, C.; Wang, C. Controlled release hydrogen sulfide delivery system based on mesoporous silica nanoparticles protects graft endothelium from ischemia-reperfusion injury. Int. J. Nanomed. 2016, 11, 3255–3263. [Google Scholar] [CrossRef] [PubMed]
- Summerlin, N.; Qu, Z.; Pujara, N.; Sheng, Y.; Jambhrunkar, S.; McGuckin, M.; Popat, A. Colloidal mesoporous silica nanoparticles enhance the biological activity of resveratrol. Colloids Surf. B Biointerfaces 2016, 144, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Pochert, A.; Lindén, M.; Davoudi, M.; Schmidtchen, A.; Nordström, R.; Malmsten, M. Membrane interactions of mesoporous silica nanoparticles as carriers of antimicrobial peptides. J. Colloid Interface Sci. 2016, 475, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, X.; Xiong, L.; Zhang, X.; Kleitz, F.; Qiao, S.Z. Mesoporous silica nanoparticles with organo-bridged silsesquioxane framework as innovative platforms for bioimaging and therapeutic agent delivery. Biomaterials 2016, 91, 90–127. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R. Nano dot technology enters clinical trials. J. Natl. Cancer Inst. 2011, 103, 1428–1429. [Google Scholar] [CrossRef] [PubMed]
- Xiaojian, W.; Wei, L. Biodegradable mesoporous bioactive glass nanospheres for drug delivery and bone tissue regeneration. Nanotechnology 2016, 27, 225102. [Google Scholar]
- Vivero-Escoto, J.L.; Chiang, Y.D.; Wu, K.C.W.; Yamauchi, Y. Recent progress in mesoporous titania materials: Adjusting morphology for innovative applications. Sci. Technol. Adv. Mater. 2012, 13, 013003. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.W.; Yamauchi, Y.; Hong, C.Y.; Yang, Y.H.; Liang, Y.H.; Funatsu, T.; Tsunoda, M. Biocompatible, surface functionalized mesoporous titania nanoparticles for intracellular imaging and anticancer drug delivery. Chem. Commun. 2011, 47, 5232–5234. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Tsai, P.Y.; Kuthati, Y.; Wei, P.R.; Liu, C.L.; Lee, C.H. Overcoming multidrug resistance through co-delivery of ros-generating nano-machinery in cancer therapeutics. J. Mater. Chem. 2017, 5, 1507–1517. [Google Scholar] [CrossRef]
- Lian, H.Y.; Hu, M.; Liu, C.H.; Yamauchi, Y.; Wu, K.C.W. Highly biocompatible, hollow coordination polymer nanoparticles as cisplatin carriers for efficient intracellular drug delivery. Chem. Commun. 2012, 48, 5151–5153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Liu, C.H.; Liang, Y.H.; Lin, F.H.; Wu, K.C.W. Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with enhanced drug loading and pH-responsive release properties for intracellular drug delivery. J. Mater. Chem. 2013, 1, 2447–2450. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Hsu, Y.C.; Liao, S.H.; Wu, K.C.W.; Inoue, M.; Yusa, S.; Nakashima, K.; Yamauchi, Y. Inorganic-organic hybrid nanoparticles with biocompatible calcium phosphate thin shells for fluorescence enhancement. Chem. Asian J. 2013, 8, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Torad, N.L.; Li, Y.; Ishihara, S.; Ariga, K.; Kamachi, Y.; Lian, H.-Y.; Hamoudi, H.; Sakka, Y.; Chaikittisilp, W.; Wu, K.C.-W.; et al. Mof-derived nanoporous carbon as intracellular drug delivery carriers. Chem. Lett. 2014, 43, 717–719. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Zheng, C.; Wu, Z.; Li, C. A pH gated, glucose-sensitive nanoparticle based on worm-like mesoporous silica for controlled insulin release. J. Phys. Chem. B 2013, 117, 3852–3860. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; He, D.; Cai, L.; He, X.; Wang, K.; Yang, X.; Li, L.; Li, S.; Su, X. Alizarin complexone functionalized mesoporous silica nanoparticles: A smart system integrating glucose-responsive double-drugs release and real-time monitoring capabilities. ACS Appl. Mater. Interfaces 2016, 8, 8358–8366. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chang, F.R.; Shih, Y.C.; Hsieh, T.J.; Chia, Y.C.; Tseng, H.Y.; Chen, H.C.; Chen, S.J.; Hsu, M.C.; Wu, Y.C. Cytotoxic constituents of polyalthia longifolia var. Pendula. J. Nat. Prod. 2000, 63, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.R.; Hwang, T.L.; Yang, Y.L.; Li, C.E.; Wu, C.C.; Issa, H.H.; Hsieh, W.B.; Wu, Y.C. Anti-inflammatory and cytotoxic diterpenes from formosan Polyalthia longifolia var. Pendula. Planta Med. 2006, 72, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lee, C.C.; Chan, W.L.; Chang, W.H.; Wu, Y.C.; Chang, J.G. 16-hydroxycleroda-3,13-dien-15,16-olide deregulates PI3K and aurora B activities that involve in cancer cell apoptosis. Toxicology 2011, 285, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lee, C.C.; Chang, F.R.; Chang, W.H.; Wu, Y.C.; Chang, J.G. 16-hydroxycleroda-3,13-dien-15,16-olide regulates the expression of histone-modifying enzymes PRC2 complex and induces apoptosis in CML k562 cells. Life Sci. 2011, 89, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.M.; Liversidge, G.G. Drug nanoparticles: Formulating poorly water-soluble compounds. Toxicol. Pathol. 2008, 36, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, V.; Lin, S.X.; Lee, C.H.; Weng, C.F. A focal adhesion kinase inhibitor 16-hydroxy-cleroda-3,13-dien-16,15-olide incorporated into enteric-coated nanoparticles for controlled anti-glioma drug delivery. Colloids Surf. B Biointerfaces 2016, 141, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lo, L.-W.; Mou, C.-Y.; Yang, C.-S. Synthesis and characterization of positive-charge functionalized mesoporous silica nanoparticles for oral drug delivery of an anti-inflammatory drug. Adv. Funct. Mater. 2008, 18, 3283–3292. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Nam, J.S.; Doss, G.P.C.; Lee, S.S.; Chakraborty, C. Nanoparticle based insulin delivery system: The next generation efficient therapy for type 1 diabetes. J. Nanobiotechnol. 2015, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Thipkaew, C.; Wattanathorn, J.; Muchimapura, S. Electrospun nanofibers loaded with quercetin promote the recovery of focal entrapment neuropathy in a rat model of streptozotocin-induced diabetes. BioMed Res. Int. 2017, 2017, 2017493. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Nogueira, T.; Gehm, C.; Ferreira, D.; Sarmento, B. Chitosan-coated solid lipid nanoparticles enhance the oral absorption of insulin. Drug Deliv. Transl. Res. 2011, 1, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.P.; Liang, N.; Gong, X.F.; An, W.W.; Kawashima, Y.; Cui, F.D.; Yan, P.F. Multifunctional composite microcapsules for oral delivery of insulin. Int. J. Mol. Sci. 2016, 18, 54. Available online: https://www.mdpi.com/1422-0067/18/1/54/htm (accessed on 28 December 2016). [CrossRef] [PubMed]
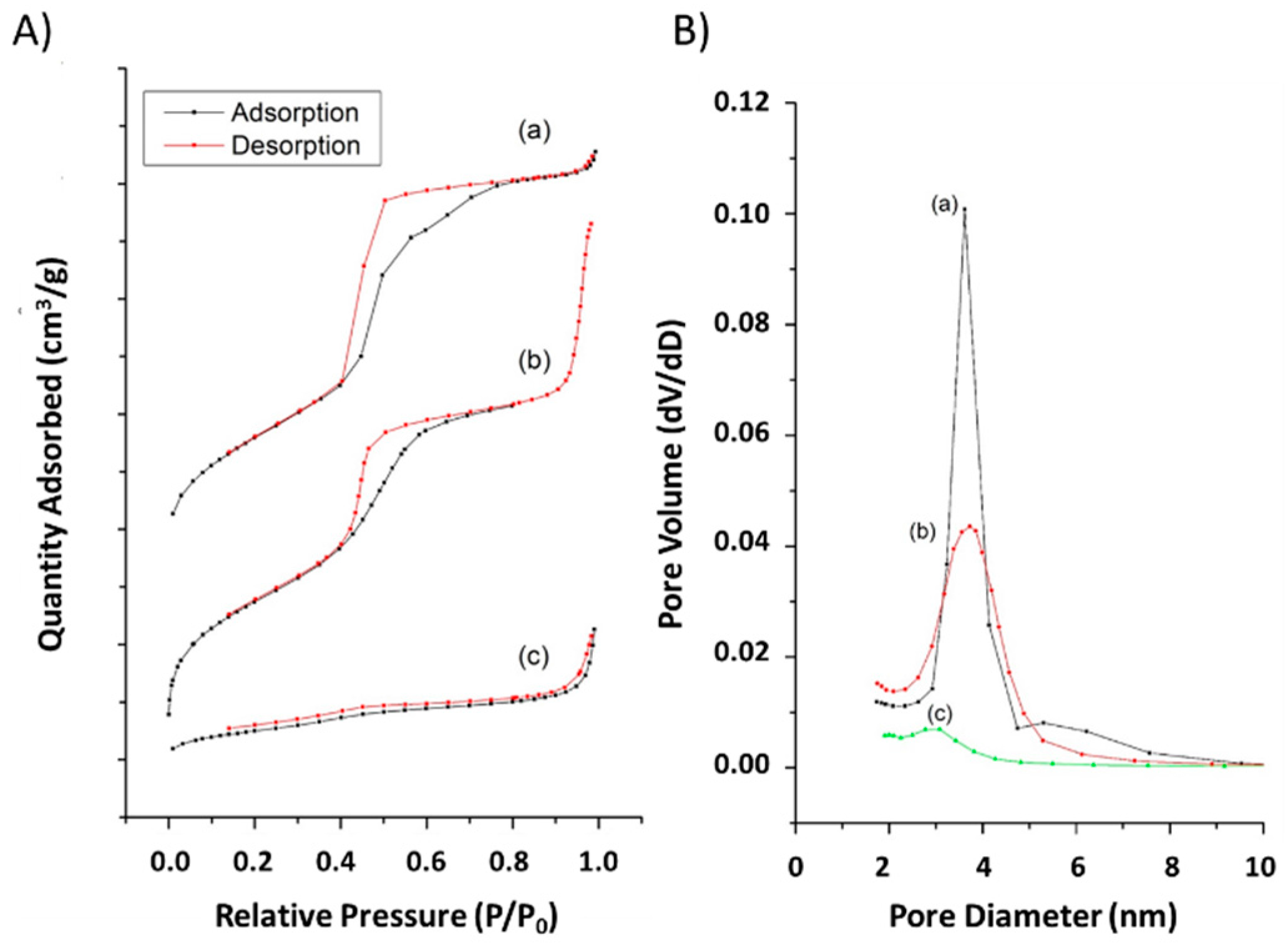

| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Particle Size (nm) b | Zeta Potential (mV) b |
|---|---|---|---|---|---|
| MSN-NH2 | 855 | 1.3 | 3.9 | 168 ± 4.5 | 16 ± 0.4 |
| MSN-HCD | 194 | 0.4 | N.D. a | 258 ± 5.7 | 4 ± 0.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-K.; Lin, S.-X.; Tsai, M.-J.; Leong, M.K.; Lin, S.-R.; Kankala, R.K.; Lee, C.-H.; Weng, C.-F. Encapsulation of 16-Hydroxycleroda-3,13-Dine-16,15-Olide in Mesoporous Silica Nanoparticles as a Natural Dipeptidyl Peptidase-4 Inhibitor Potentiated Hypoglycemia in Diabetic Mice. Nanomaterials 2017, 7, 112. https://doi.org/10.3390/nano7050112
Huang P-K, Lin S-X, Tsai M-J, Leong MK, Lin S-R, Kankala RK, Lee C-H, Weng C-F. Encapsulation of 16-Hydroxycleroda-3,13-Dine-16,15-Olide in Mesoporous Silica Nanoparticles as a Natural Dipeptidyl Peptidase-4 Inhibitor Potentiated Hypoglycemia in Diabetic Mice. Nanomaterials. 2017; 7(5):112. https://doi.org/10.3390/nano7050112
Chicago/Turabian StyleHuang, Po-Kai, Shi-Xiang Lin, May-Jywan Tsai, Max K. Leong, Shian-Ren Lin, Ranjith Kumar Kankala, Chia-Hung Lee, and Ching-Feng Weng. 2017. "Encapsulation of 16-Hydroxycleroda-3,13-Dine-16,15-Olide in Mesoporous Silica Nanoparticles as a Natural Dipeptidyl Peptidase-4 Inhibitor Potentiated Hypoglycemia in Diabetic Mice" Nanomaterials 7, no. 5: 112. https://doi.org/10.3390/nano7050112