Structural Inheritance and Redox Performance of Nanoporous Electrodes from Nanocrystalline Fe85.2B10-14P0-4Cu0.8 Alloys
Abstract
:1. Introduction
2. Materials and Experimental Procedure
3. Results and Discussion
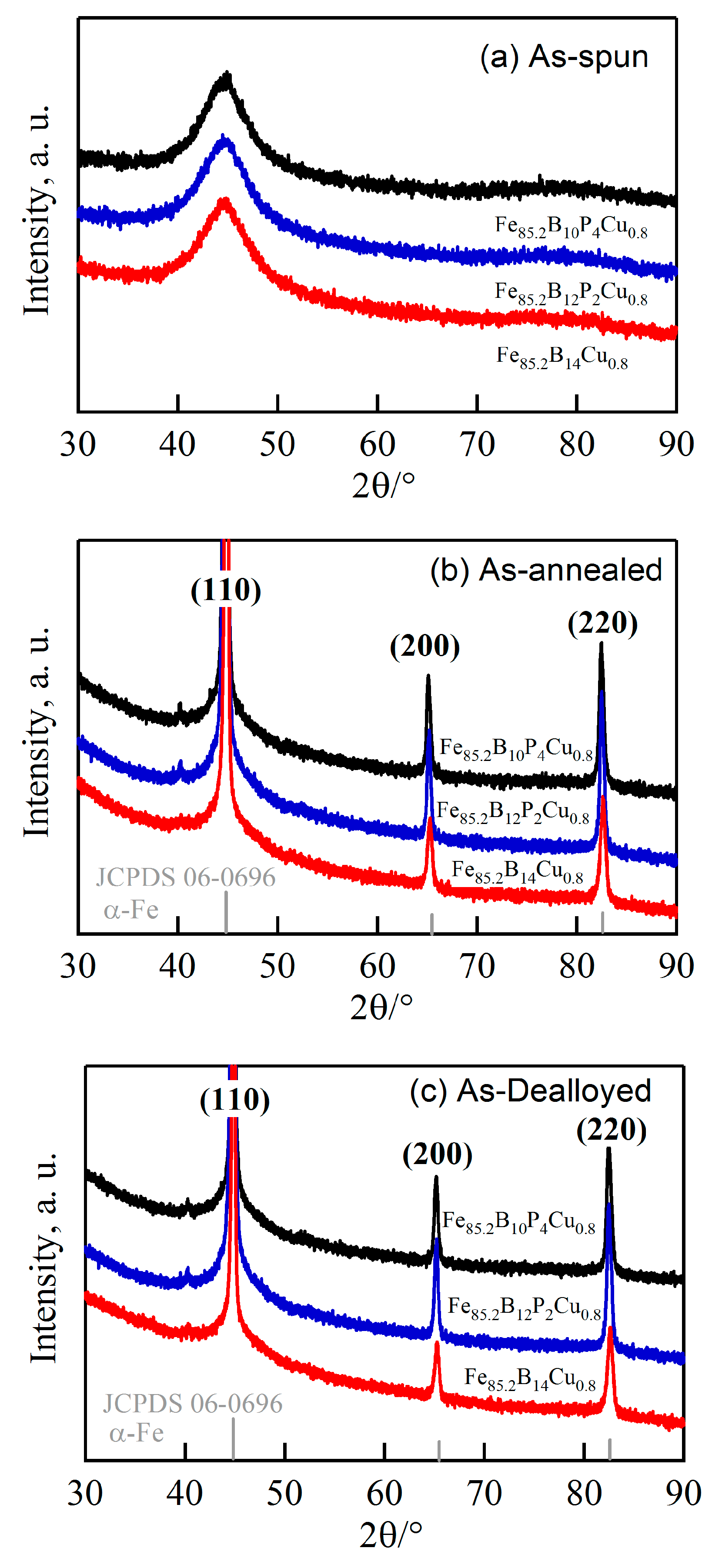
3.1. Structural Characteristics of Amorphous and Nanocrystalline Fe85.2B14−xPxCu0.8 Precursor Alloys
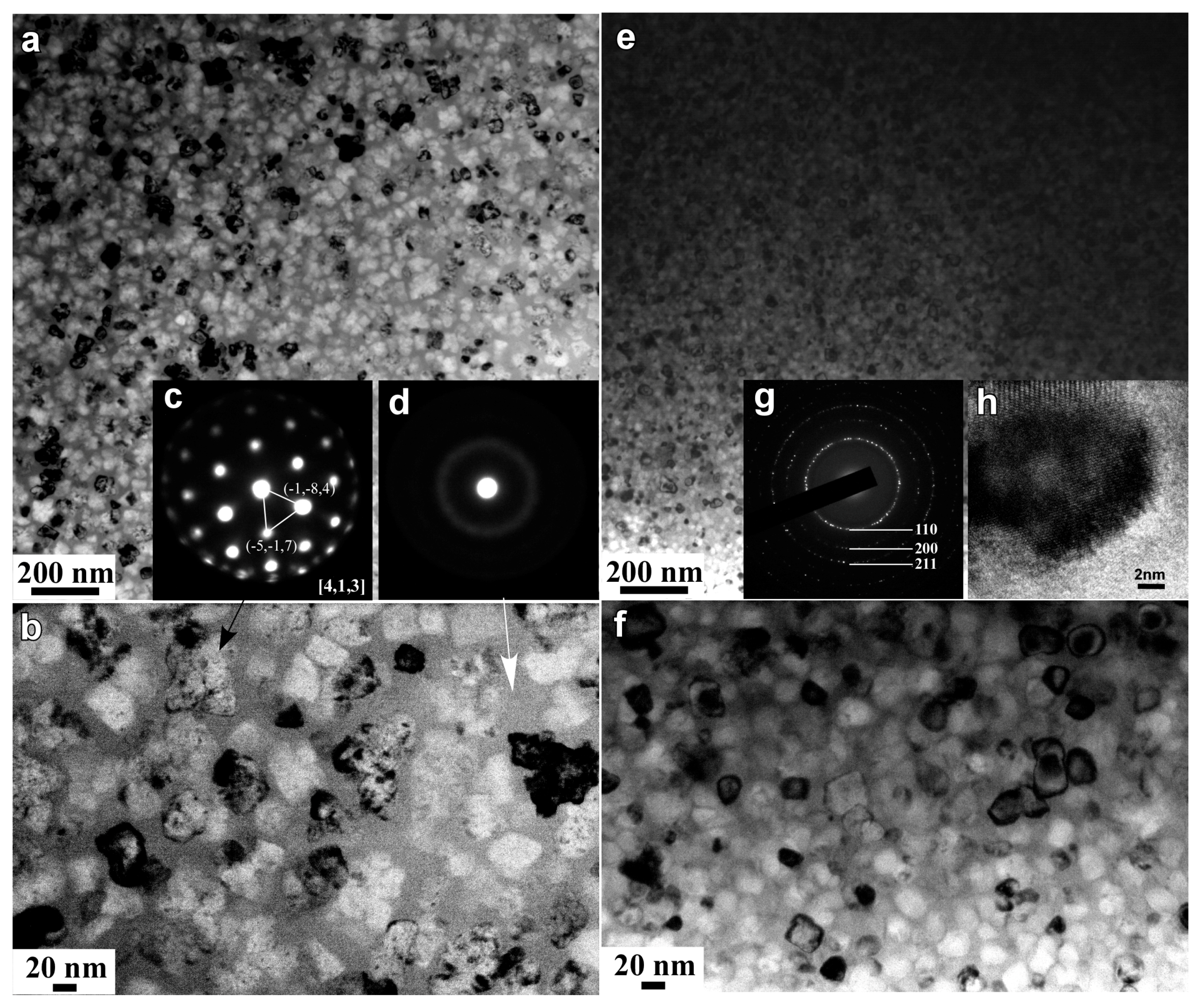
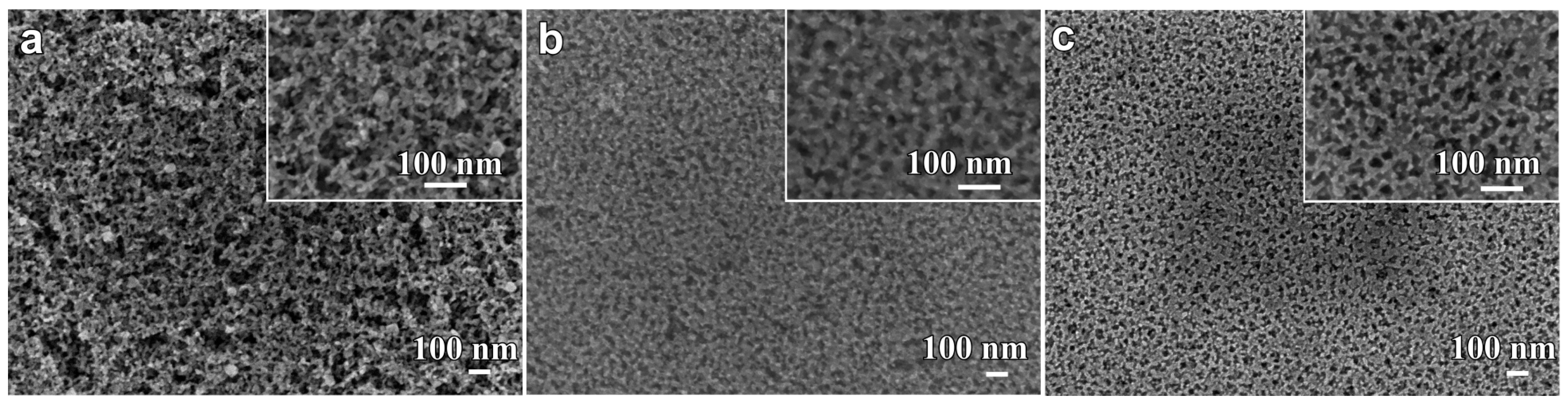
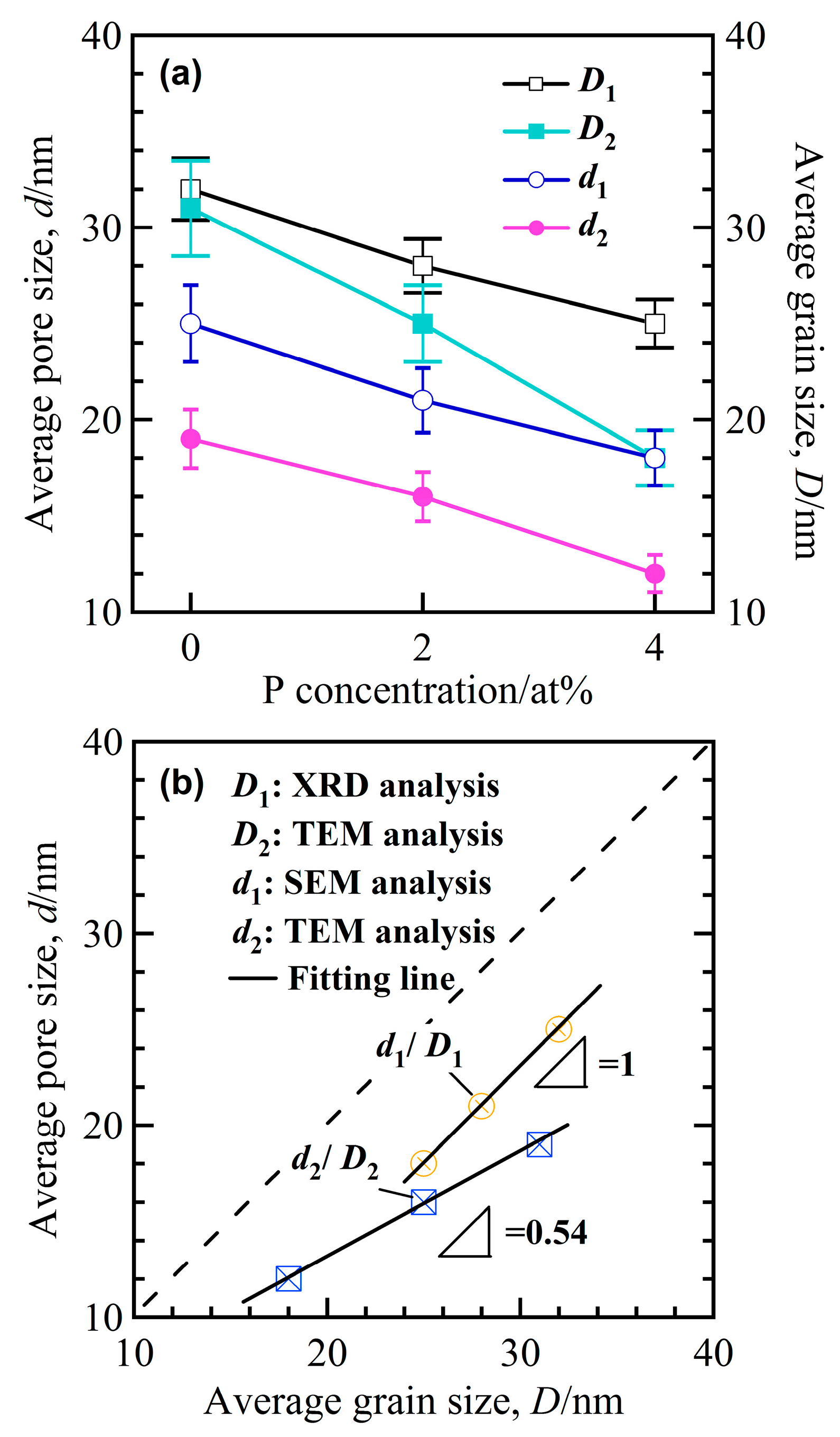
3.2. Structural Characteristics of Nanoporous Alloys
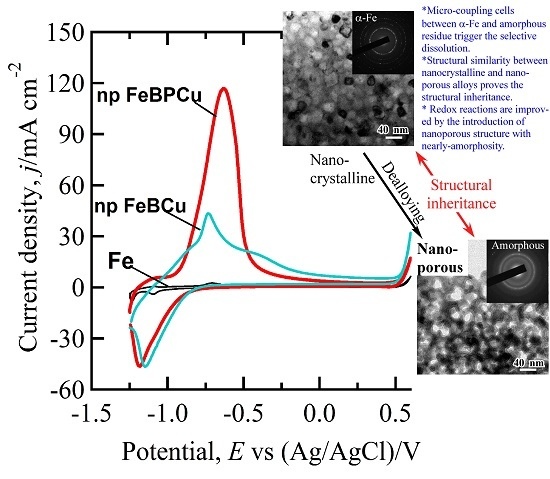
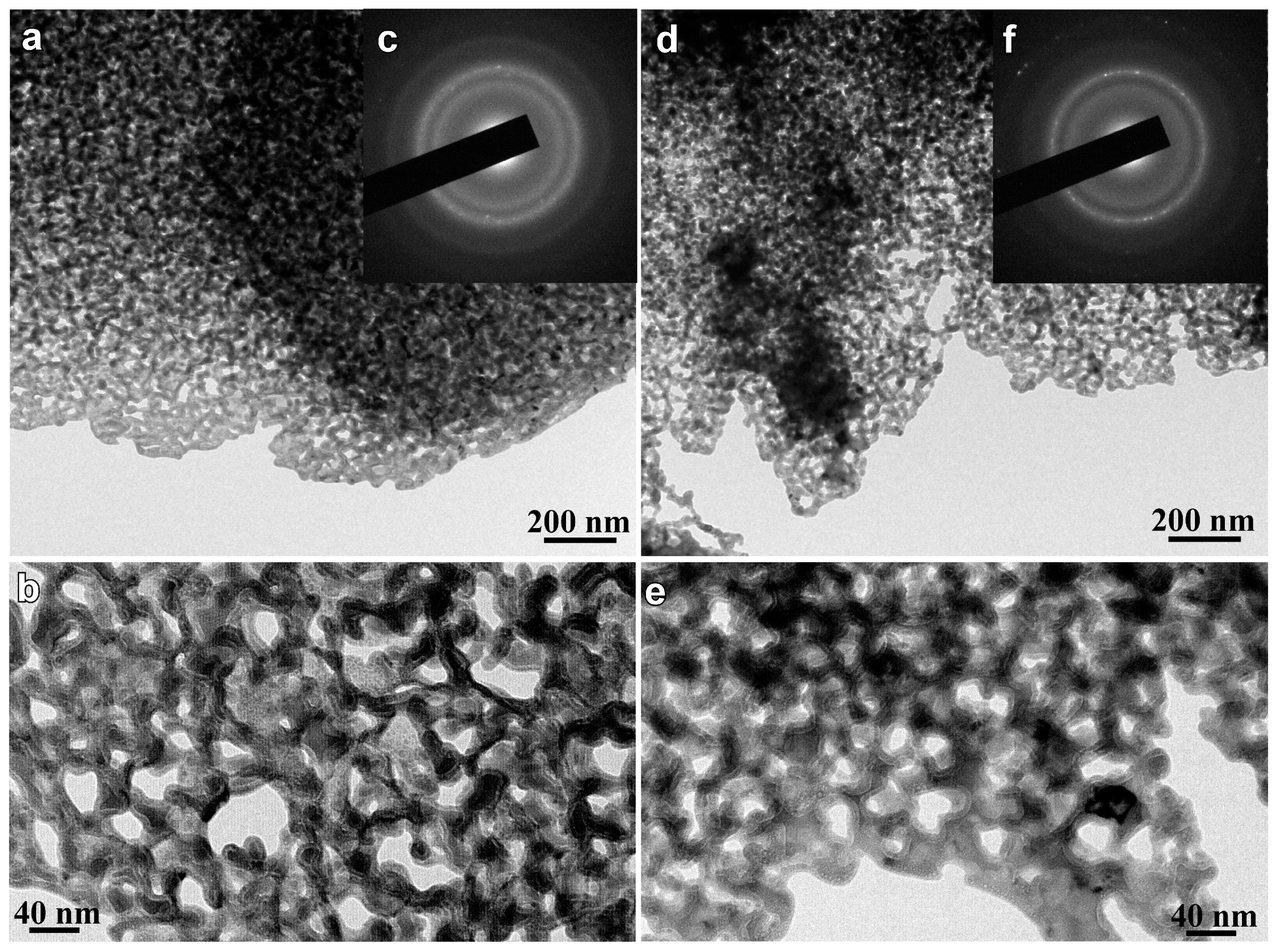
3.3. Structural Inheritance of Nanoporousalloys
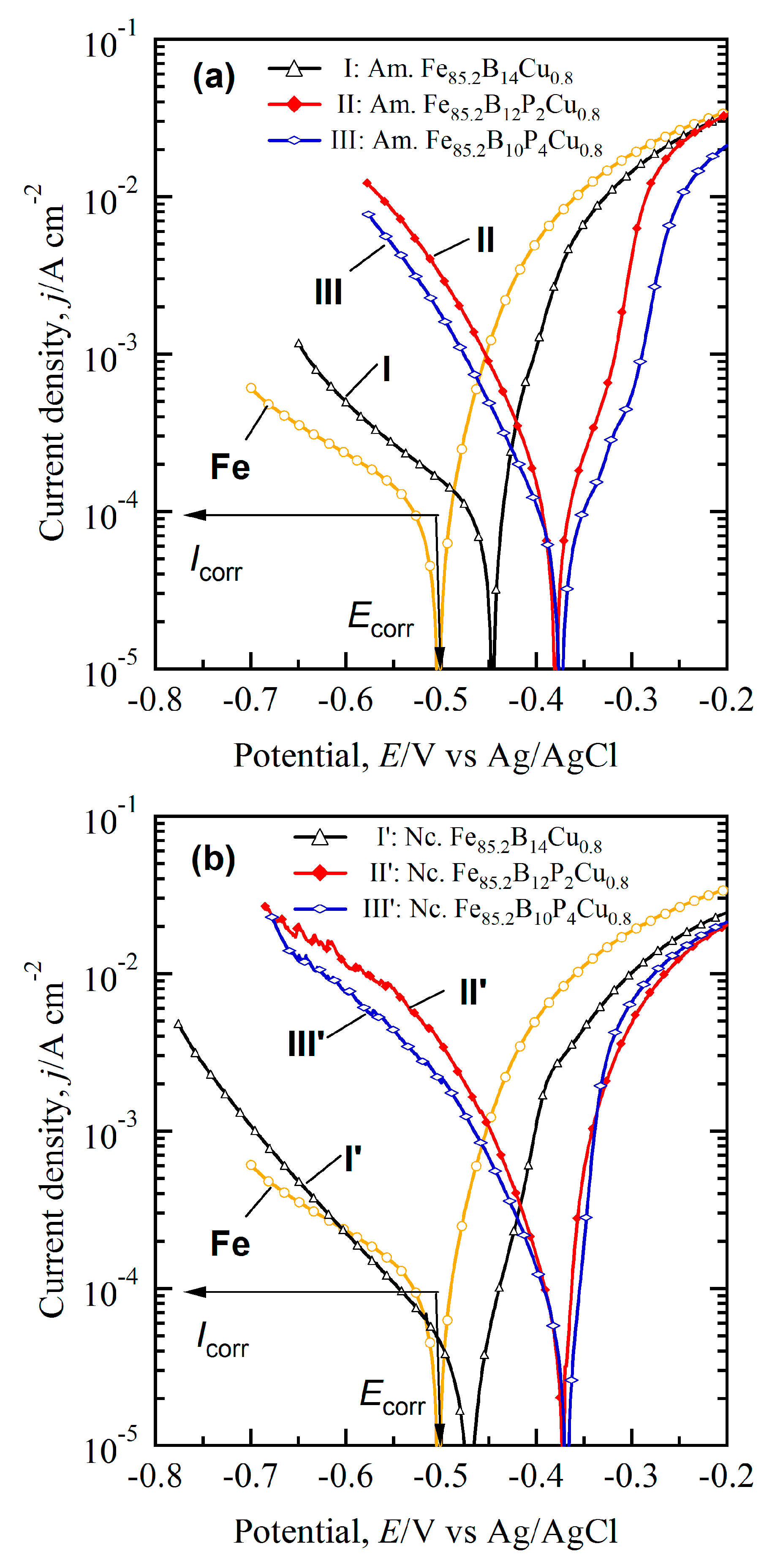
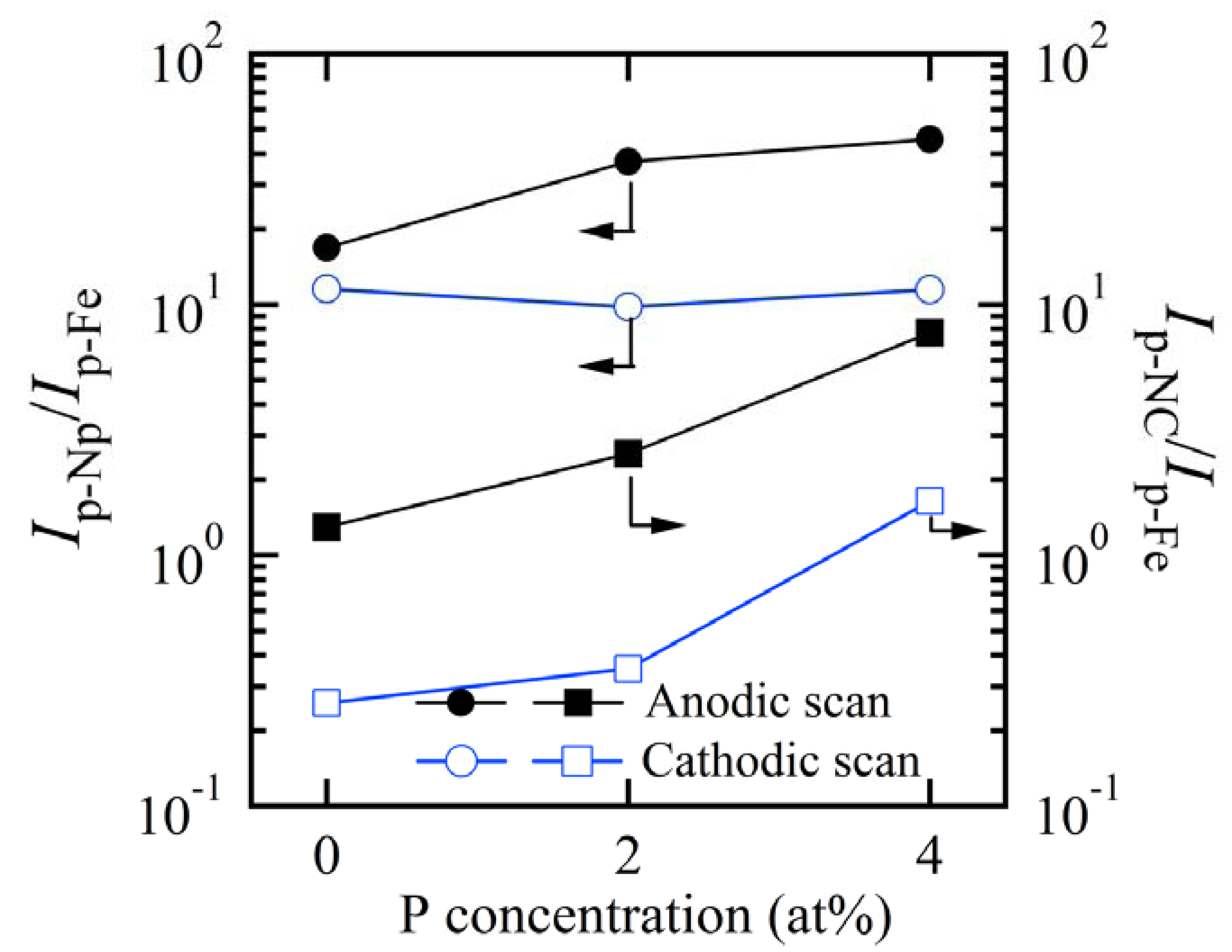
3.4. Enhanced Redox Performance of Nanocrystalline and Nanoporousalloys
3.5. Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Z.H.; Wang, Y.; Qi, Z.; Zhang, W.H.; Qin, J.Y.; Frenzel, J. Generalized Fabrication of Nanoporous Metals (Au, Pd, Pt, Ag, and Cu) through Chemical Dealloying. J. Phys. Chem. C 2009, 113, 12629–12636. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of Nanoporosity in Dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.C.; Thompson, D.T. Catalysis by Gold. Catal. Rev. 1999, 41, 319–388. [Google Scholar] [CrossRef]
- Weissmueller, J.R.; Viswanath, N.; Kramer, D.; Zimmer, P.; Wuerschum, R.; Gleiter, H. Charge-induced Reversible Strain in a Metal. Science 2003, 300, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H.; Choi, S.J.; Kwa, K.J.; Liu, Z.; Terasaki, O.; Ryoo, R. Ordered Nanoporous Arrays of Carbon Supporting High Dispersions of Platinum Nanoparticles. Nature 2001, 412, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, J. An Atomistic Description of Dealloying: Porosity Evolution, the Critical Potential, and Rate-limiting Behavior. J. Electrochem. Soc. 2004, 151, C614–C626. [Google Scholar] [CrossRef]
- Jayaraj, J.; Park, B.J.; Kim, D.H.; Kim, W.T.; Fleury, E. Nanometer-sized Porous Ti-based Metallic Glass. Scr. Mater. 2006, 55, 1063–1066. [Google Scholar] [CrossRef]
- Gebert, A.; Kündig, A.A.; Schultz, L.; Hono, K. Selective Electrochemical Dissolution in Two-phase La–Zr–Al–Cu–Ni Metallic Glass. Scr. Mater. 2004, 51, 961–965. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Sugawara, Y.; Muto, I.; Hara, N. Dealloying Behaviours of an Equiatomic TiCu Alloy. Mater. Trans. 2013, 54, 1120–1125. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Akaki, T.; Koizumi, Y.; Minamino, Y.; Fujimoto, S. Selective Pore Growth on Lamellar Ti–41at %Al Alloy. Electrochem. Commun. 2013, 26, 117–120. [Google Scholar] [CrossRef]
- Mihailov, L.; Redzheb, M.; Spassov, T. Selective Dissolution of Amorphous and Nanocrystalline Zr2Ni. Corros. Sci. 2013, 74, 308–313. [Google Scholar] [CrossRef]
- Rösler, J.; Näth, O.; Jäger, S.; Schmitz, F.; Mukherji, D. Fabrication of Nanoporous Ni-based SuperalloyMembranes. Acta Mater. 2005, 53, 1397–1406. [Google Scholar] [CrossRef]
- Makino, A.; Men, H.; Kubota, T.; Yubuta, K.; Makabe, M.; Inoue, A. New Excellent Soft Magnetic FeSiBPCu Nanocrystallized Alloys with High Bs of 1.9 T from Nanohetero-Amorphous Phase. IEEE Trans. Magn. 2009, 45, 4302–4305. [Google Scholar] [CrossRef]
- Makino, A.; Kubota, T.; Yubuta, K.; Inoue, A.; Urata, A.; Matsumoto, H.; Yoshida, S. Low Core Losses and Magnetic Properties of Fe85–86Si1–2B8P4Cu1Nanocrystalline Alloys with High B for Power Applications. J. Appl. Phys. 2011, 109, 07A302. [Google Scholar] [CrossRef]
- Makino, A.; Men, H.; Yubuta, K.; Kubota, T. New Fe-metalloids Based Nanocrystalline Alloys with High Bs of 1.9 T and Excellent Magnetic Softness. J. Appl. Phys. 2009, 105, 07A308. [Google Scholar] [CrossRef]
- Lee, M.H.; Sordelet, D.J. Nanoporous Metallic Glass with High Surface Area. Scr. Mater. 2006, 55, 947–950. [Google Scholar] [CrossRef]
- Li, X.; Kato, H.; Yubuta, K.; Makino, A.; Inoue, A. Effect of Cu on nanocrystallization and plastic properties of FeSiBPCu bulk metallic glasses. Mater. Sci. Eng. A 2010, 527, 2598–2602. [Google Scholar] [CrossRef]
- Sharma, P.; Zhang, X.; Zhang, Y.; Makino, A. Competition Driven Nanocrystallization in High Bs and Low Coreloss Fe-Si-B-P-Cu Soft Magnetic Alloys. Scr. Mater. 2015, 95, 3–6. [Google Scholar] [CrossRef]
- Makino, A.; Kubota, T.; Chang, C.T.; Makabe, M.; Inoue, A. FeSiBP Bulk Metallic Glasses with High Magnetization and Excellent Magnetic Softness. J. Magn. Magn. Mater. 2008, 320, 2499–2503. [Google Scholar] [CrossRef]
- Dan, Z.H.; Makino, A.; Hara, N. Effects of P Addition on Corrosion Properties of Soft Magnetic FeSiB Alloys. Mater. Trans. 2013, 54, 1691–1696. [Google Scholar] [CrossRef]
- Leofantia, G.; Padovanb, M.; Tozzolac, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xu, D.; Xu, J.J.; Zhang, X.B. Oxygen Electrocatalysts in Metal–air Batteries: From Aqueous to Nonaqueous Electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef] [PubMed]
- Manohar, A.K.; Malkhandi, S.; Yang, B.; Yang, C.G.; Prakash, G.S.K.; Narayanan, S.R. High-performance rechargeable iron electrode for large-scale battery-based energy storage. J. Electrochem. Soc. 2012, 159, A1209–A1214. [Google Scholar] [CrossRef]
- Ojefors, L.; Carlsson, L. An Iron-air Vehicle Battery. J. Power Sources 1978, 2, 287–296. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, R.S. Elements of X-ray Diffractions; Addition-Wesley: Reading, MA, USA, 1978. [Google Scholar]
- Inoue, A. Stabilization of Metallic Supercooled Liquid and Bulk Amorphous Alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry: Principles, Methods and Application; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Pickering, H.W. Characteristic Features of Alloy Polarization Curves. Corros. Sci. 1983, 23, 1107–1120. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Zhang, Y.; Makino, A.; Chang, H.; Hara, N. Mechanism of Active Dissolution of Nanocrystalline Fe-Si-B-P-Cu Soft Magnetic Alloys. Mater. Charact. 2016, 121, 9–16. [Google Scholar] [CrossRef]
- Ojefors, L. Self-discharge of the Alkaline Iron Electrode. Electrochim. Acta 1976, 21, 263–266. [Google Scholar] [CrossRef]
- Beck, F.; Kaus, R.; Oberst, M. Transpassive Dissolution of Iron to Ferrate (VI) in Concentrated Alkali Hydroxide Solutions. Electrochim. Acta 1985, 30, 173–183. [Google Scholar] [CrossRef]
- Synder, J.; Livi, K.; Erlebacher, J. Dealloying Silver/gold Alloys in Neutral Silver Nitrate Solution: Porosity Evolution, Surface Composition, and Surface Oxides. J. Electrochem. Soc. 2008, 155, C464–C473. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Xie, G.Q.; Louzguine-Luzgin, D.V.; Inoue, A.; Perepezko, J.H. Rapid Degradation of Azo dye by Fe-based Metallic Glass Powder. Adv. Funct. Mater. 2012, 22, 2567–2571. [Google Scholar] [CrossRef]
- Hu, Y.C.; Wang, Y.Z.; Su, R.; Cao, C.R.; Li, F.; Sun, C.W.; Yang, Y.; Guan, P.F.; Ding, D.W.; Wang, Z.L.; et al. A Highly Efficient and Self-stabilizing Metallic-glass Catalyst for Electrochemical Hydrogen Generation. Adv. Mater. 2016, 28, 10293–10297. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Xu, L.; Dan, Z.; Makino, A.; Hara, N.; Qin, F.; Chang, H. Structural Inheritance and Redox Performance of Nanoporous Electrodes from Nanocrystalline Fe85.2B10-14P0-4Cu0.8 Alloys. Nanomaterials 2017, 7, 141. https://doi.org/10.3390/nano7060141
Fu C, Xu L, Dan Z, Makino A, Hara N, Qin F, Chang H. Structural Inheritance and Redox Performance of Nanoporous Electrodes from Nanocrystalline Fe85.2B10-14P0-4Cu0.8 Alloys. Nanomaterials. 2017; 7(6):141. https://doi.org/10.3390/nano7060141
Chicago/Turabian StyleFu, Chaoqun, Lijun Xu, Zhenhua Dan, Akihiro Makino, Nobuyoshi Hara, Fengxiang Qin, and Hui Chang. 2017. "Structural Inheritance and Redox Performance of Nanoporous Electrodes from Nanocrystalline Fe85.2B10-14P0-4Cu0.8 Alloys" Nanomaterials 7, no. 6: 141. https://doi.org/10.3390/nano7060141