Optimized Photodynamic Therapy with Multifunctional Cobalt Magnetic Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
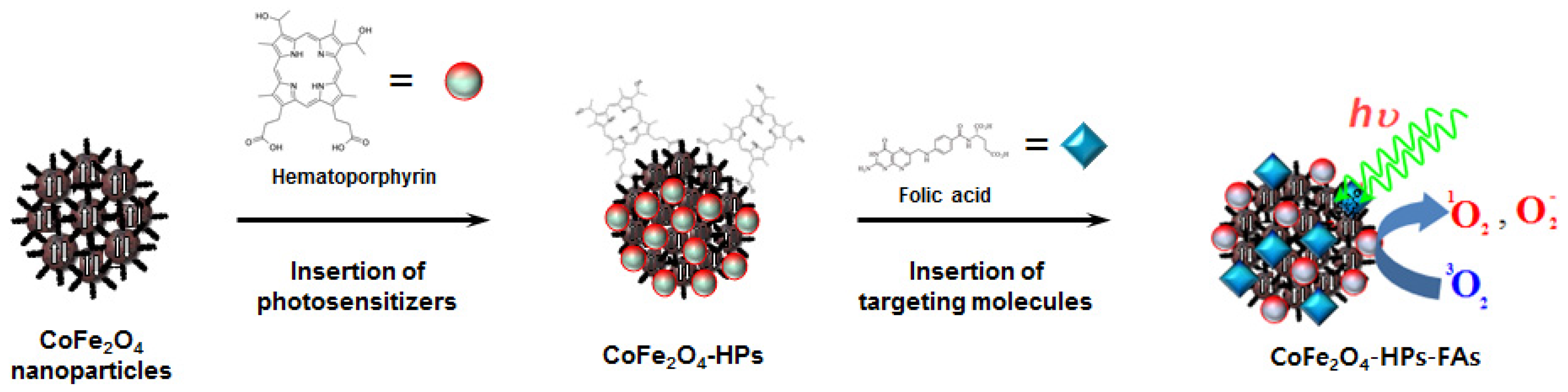
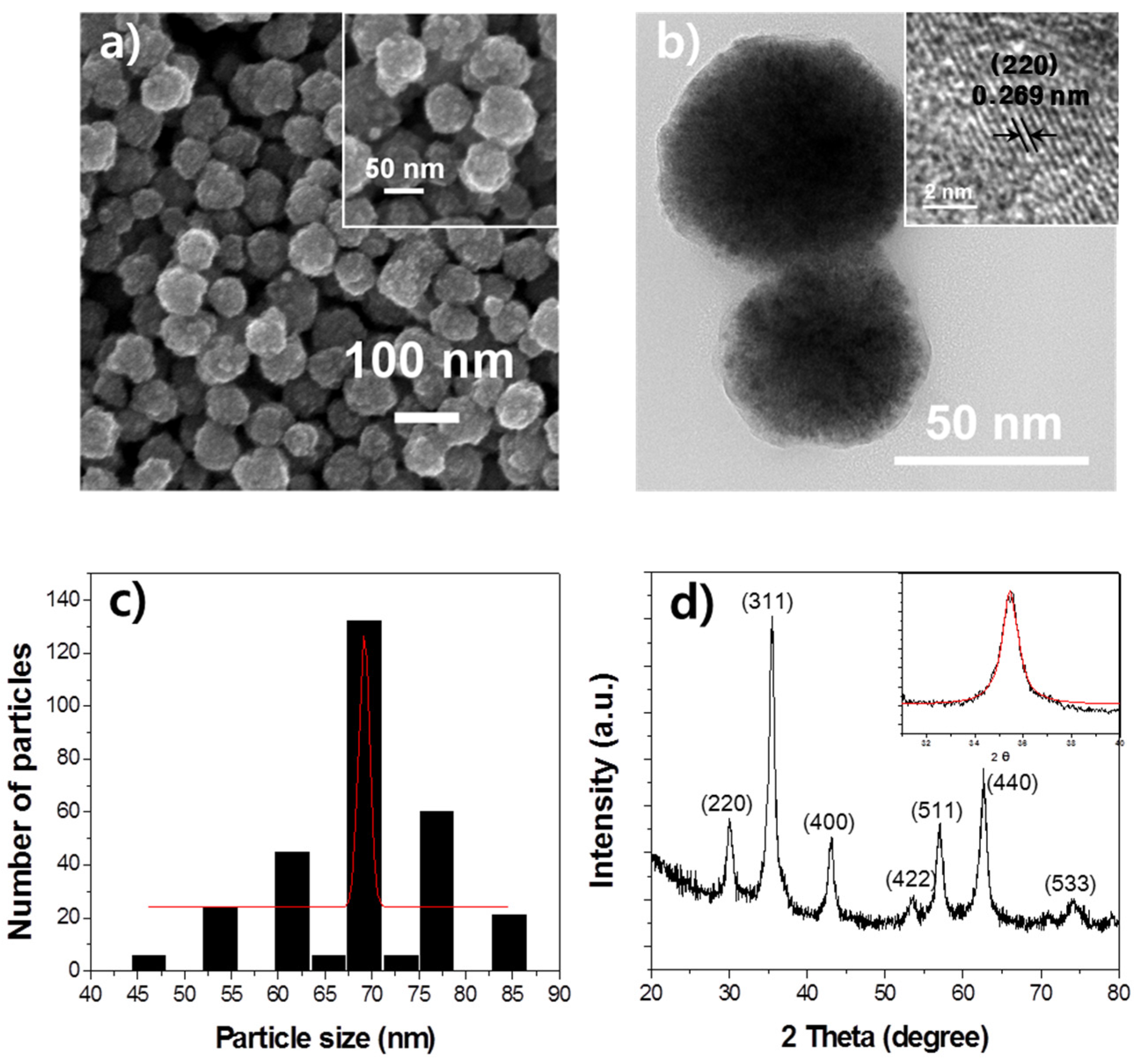
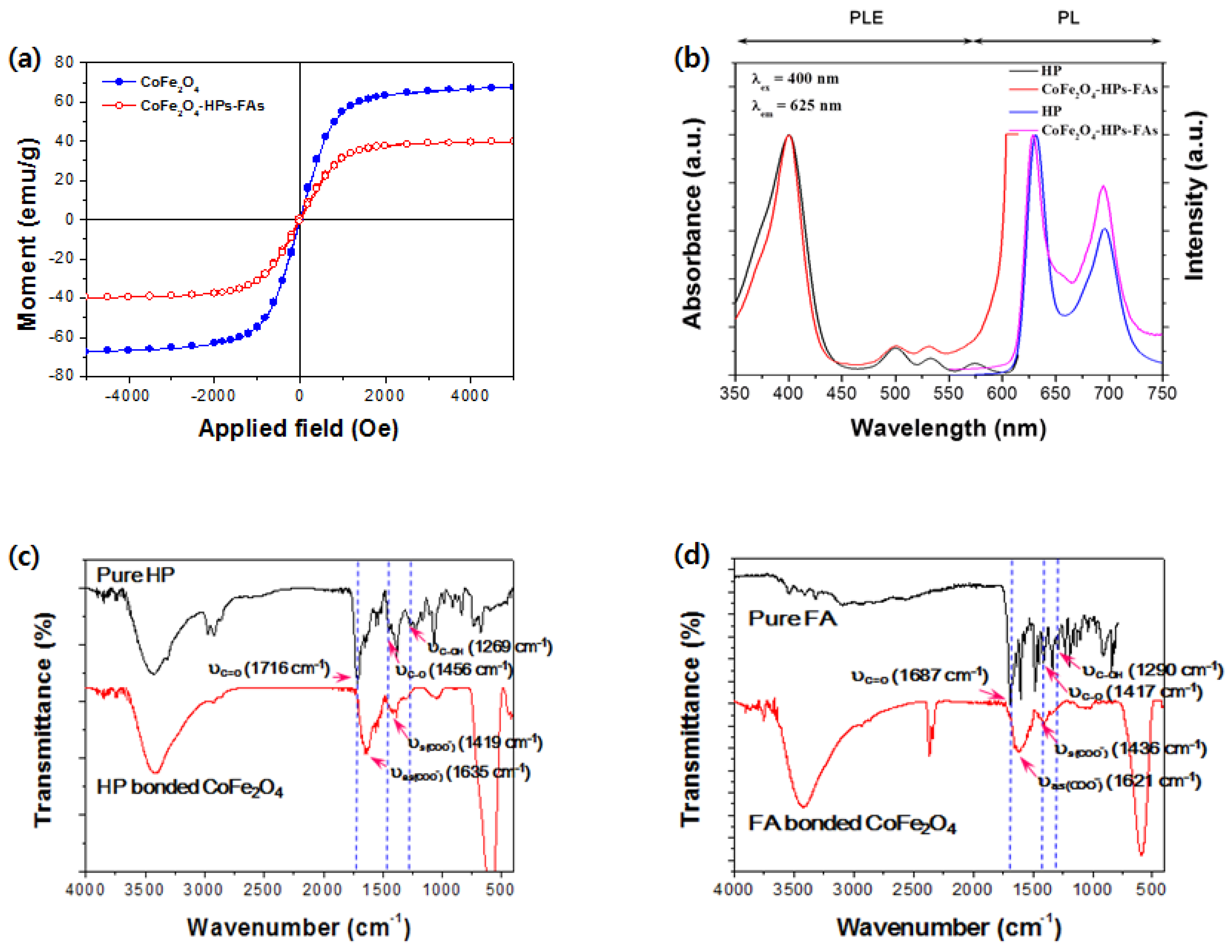
2.1. Characteristics of Multifunctional CoFe2O4-HPs-Fas
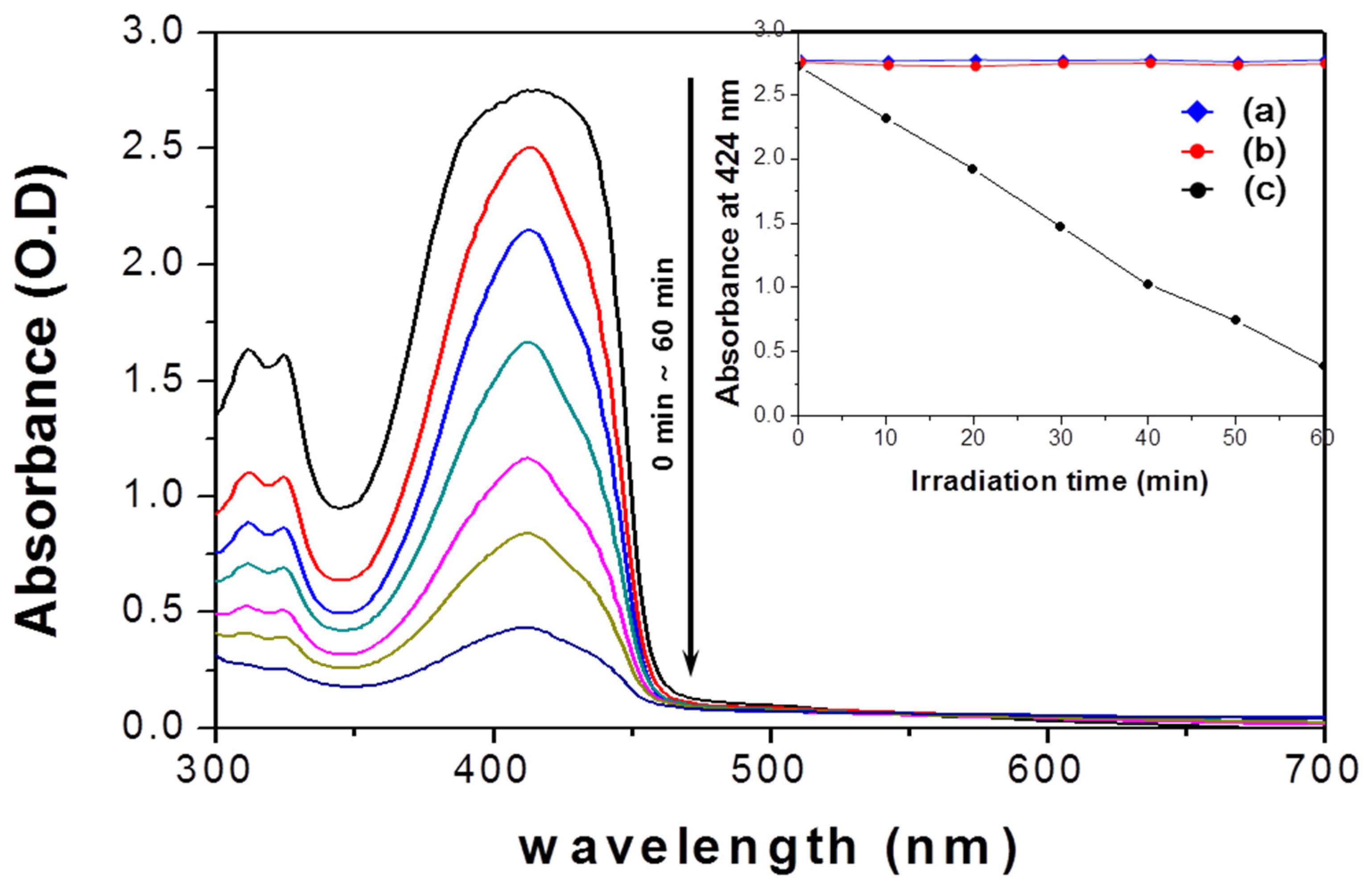
2.2. Singlet Oxygen Generation
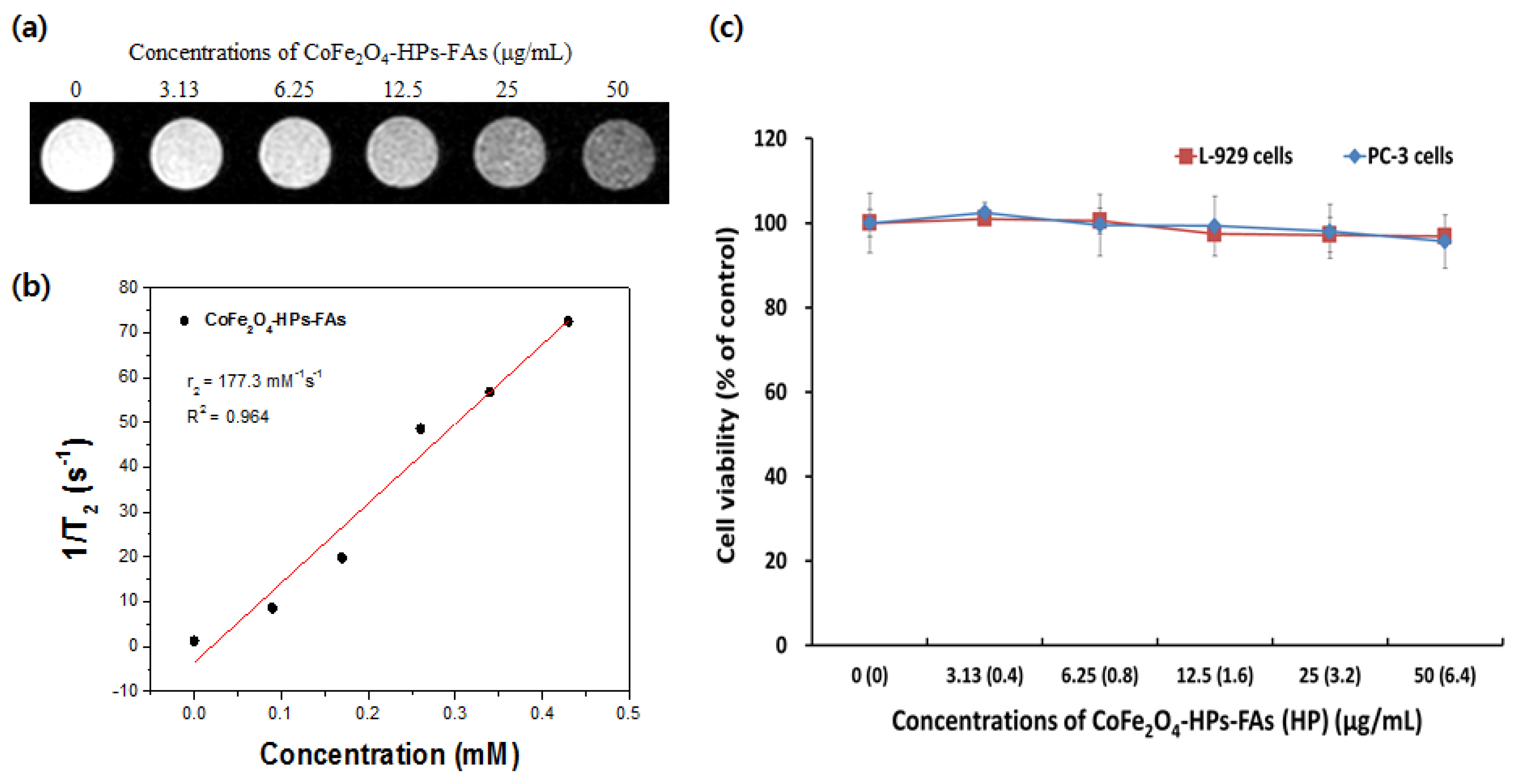
2.3. Biocompatibility of Multifunctional CoFe2O4-HPs-Fas
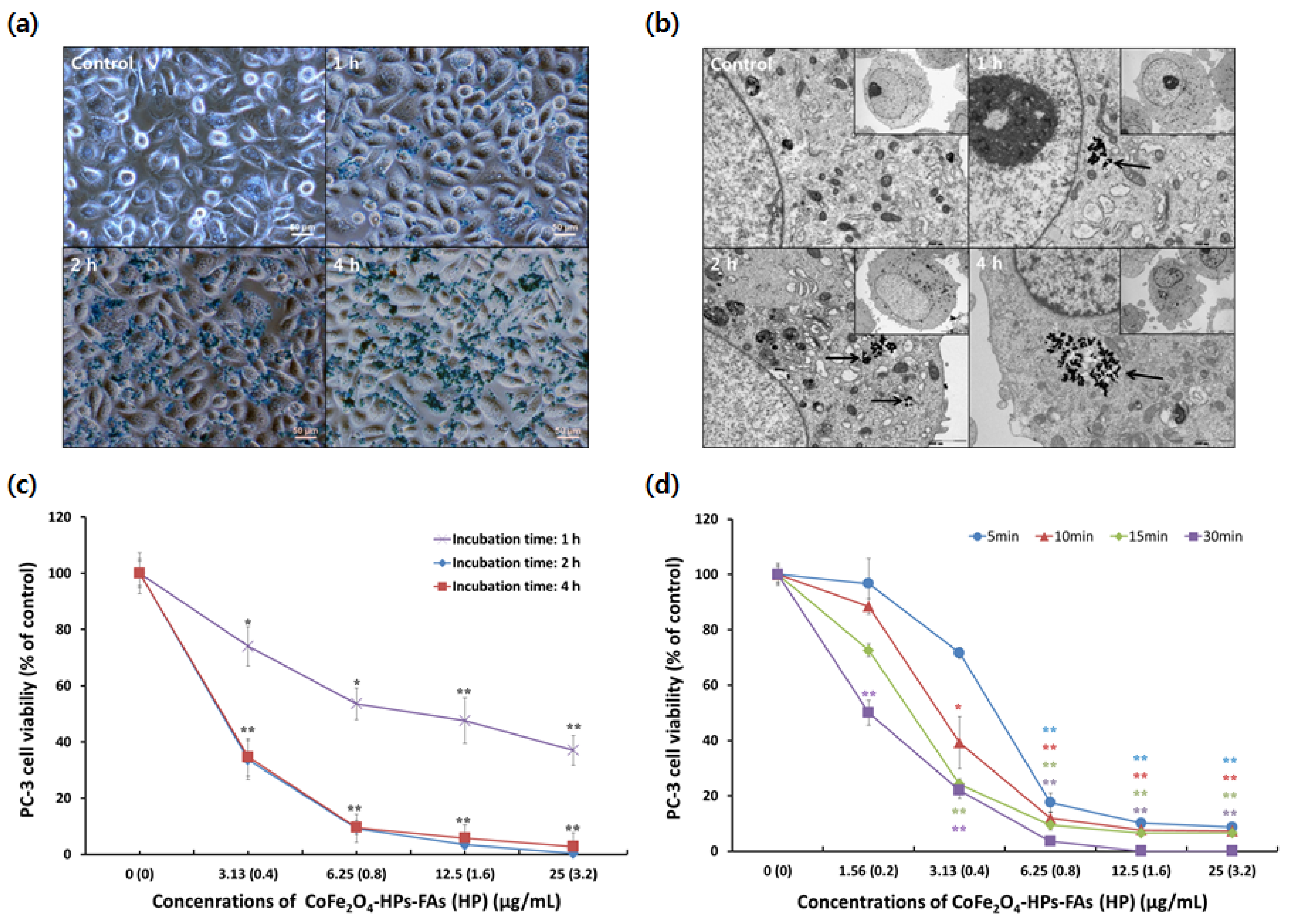
2.4. Optimization of the Cellular Uptake and Light Irradiation Time of CoFe2O4-HPs-Fas
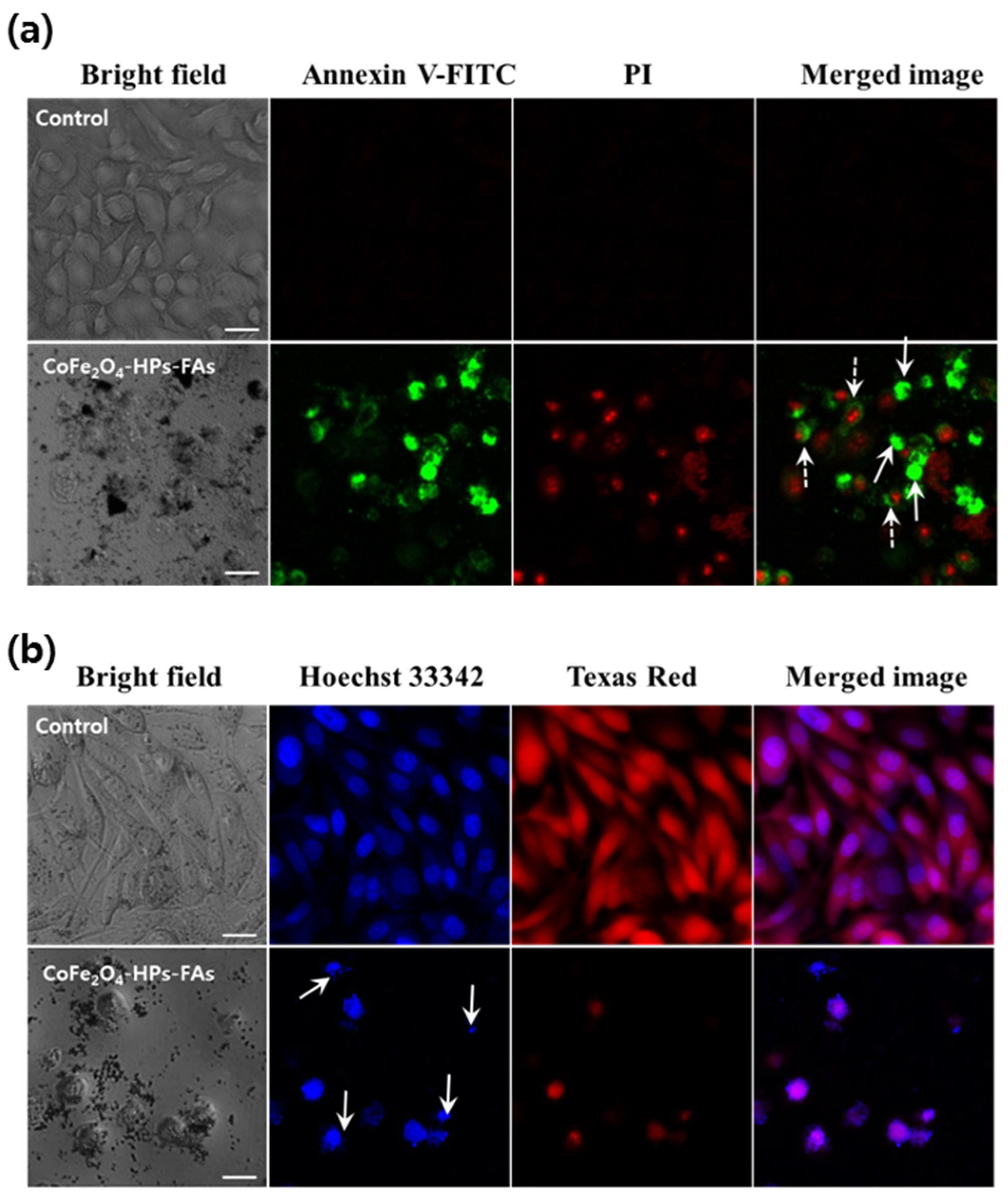
2.5. Anticancer Activity of CoFe2O4-HPs-Fas
3. Materials and Methods
3.1. Synthesis of Multifunctional CoFe2O4-HPs-Fas
3.2. Characterization of Multifunctional CoFe2O4-HPs-Fas
3.3. Detection of Singlet Oxygen
3.4. Magnetic Resonance Imaging (MRI) Analysis In Vitro
3.5. Biocompatibility of Multifunctional CoFe2O4-HPs-Fas
3.6. Optimization of the Cellular Uptake and Light Irradiation Time for Photodynamic Anticancer Activity of Multifunctional CoFe2O4-HPs-Fas
3.7. Morphological Analysis of Apoptotic Cell Death in Prostate Cancer Cells
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xie, J.; Pan, X.; Wang, M.; Yao, L.; Liang, X.; Ma, J.; Fei, Y.; Wang, P.-N.; Mi, L. Targeting and photodynamic killing of cancer cell by nitrogen-doped titanium dioxide coupled with folic acid. Nanomaterials 2016, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Avula, U.M.; Kim, G.; Lee, Y.E.; Morady, F.; Kopelman, R.; Kalifa, J. Cell-specific nanoplatform-enabled photodynamic therapy for cardiac cells. Heart Rhythm 2012, 9, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Sibata, C.H.; Colussi, V.C.; Oleinick, N.L.; Kinsella, T.J. Photodynamic therapy: A new concept in medical treatment. Braz. J. Med. Biol. Res. 2000, 33, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yilmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Bhaumik, J.; Dogutan, D.K.; Aly, Z.; Kamal, Z.; Khalid, L.; Kee, H.L.; Bocian, D.F.; Holten, D.; Lindsey, J.S.; et al. Imidazole metalloporphyrins as photosensitizers for photodynamic therapy: Role of molecular charge, central metal and hydroxyl radical production. Cancer Lett. 2009, 282, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Vrouenraets, M.B.; Visser, G.W.; Snow, G.B.; van Dongen, G.A. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003, 23, 505–522. [Google Scholar] [PubMed]
- Konan, Y.N.; Gurny, R.; Allémann, E. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2002, 66, 89–106. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Elnagheeb, M. Mesoporous silica nanoparticles loaded with cisplatin and phthalocyanine for combination chemotherapy and photodynamic therapy in vitro. Nanomaterials 2015, 5, 2302–2316. [Google Scholar] [CrossRef] [PubMed]
- Hah, H.J.; Kim, G.; Lee, Y.E.; Orringer, D.A.; Sagher, O.; Philbert, M.A.; Kopelman, R. Methylene blue-conjugated hydrogel nanoparticles and tumor-cell targeted photodynamic therapy. Macromol. Biosci. 2011, 11, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, S.; Huang, P.; Wang, Z.; Chen, S.; Niu, G.; Li, W.; He, J.; Cui, D.; Lu, G.; et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano 2013, 7, 5320–5329. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Boca-Farcau, S.; Gabudean, A.M.; Baldeck, P.; Astilean, S. LED-activated Methylene blue-loaded pluronic-nanogold hybrids for in vitro photodynamic therapy. J. Biophotonics 2013, 6, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhang, Y.; Kim, K.S.; Yuan, H.K.; Vo-Dinh, T. Cellular uptake and photodynamic activity of protein nanocages containing Methylene blue photosensitizing drug. Photochem. Photobiol. 2010, 86, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Patterson, M.S. The physics, biophysics and technology of photodynamic therapy. Phys. Med. Biol. 2008, 53, R61–R109. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tian, X.J.; Yang, W.L.; Ehrenberg, B.; Chen, J.Y. The conjugates of gold nanorods and chlorin E6 for enhancing the fluorescence detection and photodynamic therapy of cancers. Phys. Chem. Chem. Phys. 2013, 15, 15727–15733. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Kuo, L.R.; Chang, C.L.; Hwu, Y.K.; Huang, C.K.; Lee, S.Y.; Chen, K.; Sin, S.J.; Huang, J.D.; Chen, Y.Y. In situ real-time investigation of cancer cell photothermolysis mediated by excited gold nanorod surface plasmons. Biomaterials 2010, 31, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shi, S.; Huang, Y.; Tang, S.; Chen, X. Simultaneous photodynamic and photothermal therapy using photosensitizer functionalized Pd nanosheets by single continuous wave laser. ACS Appl. Mater. Interfaces 2014, 6, 8878–8885. [Google Scholar] [CrossRef] [PubMed]
- Vankayala, R.; Lin, C.C.; Kalluru, P.; Chiang, C.S.; Hwang, K.C. Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light. Biomaterials 2014, 35, 5527–5538. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liang, C.; Gong, H.; Chen, Q.; Wang, C.; Liu, Z. Photosensitizer-conjugated albumin-polypyrrole nanoparticles for imaging-guided in vivo photodynamic/photothermal therapy. Small 2015, 11, 3932–3941. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Choi, K.H.; Nam, K.C.; Ali, A.; Min, J.E.; Son, H.; Uhm, H.S.; Kim, H.J.; Jung, J.S.; Choi, E.H. Photodynamic anticancer activities of multifunctional cobalt ferrite nanoparticles in various cancer cells. J. Biomed. Nanotechnol. 2015, 11, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Nam, K.C.; Malkinski, L.; Choi, E.H.; Jung, J.S.; Park, B.J. Size-dependent photodynamic anticancer activity of biocompatible multifunctional magnetic submicron particles in prostate cancer cells. Molecules 2016, 21, 1187. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.A.; Khurana, M.; Moriyama, E.H. Blood-vessel closure using photosensitizers engineered for two-photon excitation. Nat. Photonics 2008, 2, 420–424. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). International Standard ISO 10993-5:2009, Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland.
- Choi, K.H.; Choi, E.W.; Min, J.E.; Son, H.; Uhm, H.S.; Choi, E.H.; Park, B.J.; Jung, J.S. Comparison study on photodynamic anticancer activity of multifunctional magnetic particles by formation of cations. IEEE Trans. Magn. 2014, 50, 5200704. [Google Scholar] [CrossRef]
- Choi, K.H.; Lee, H.J.; Park, B.J.; Wang, K.K.; Shin, E.P.; Park, J.C.; Kim, Y.K.; Oh, M.K.; Kim, Y.R. Photosensitizer and vancomycin-conjugated novel multifunctional magnetic particles as photoinactivation agents for selective killing of pathogenic bacteria. Chem. Commun. 2012, 48, 4591–4593. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Wang, K.K.; Shin, E.P.; Oh, S.L.; Jung, J.S.; Kim, H.K.; Kim, Y.R. Water-soluble magnetic nanoparticles functionalized with photosensitizer for photocatalytic application. J. Phys. Chem. C 2011, 115, 3212–3219. [Google Scholar] [CrossRef]
- Choi, K.H.; Nam, K.C.; Kim, H.J.; Min, J.; Uhm, H.S.; Choi, E.H.; Park, B.J. Synthesis and characterization of photo-functional magnetic nanoparticles (Fe3O4@HP) for applications in photodynamic cancer therapy. J. Korean Phys. Soc. 2014, 65, 1658–1662. [Google Scholar] [CrossRef]
- Park, B.J.; Choi, K.H.; Nam, K.C.; Min, J.; Lee, K.D.; Uhm, H.S.; Choi, E.H.; Kim, H.J.; Jung, J.S. Photodynamic anticancer activity of CoFe2O4 nanoparticles conjugated with hematoporphyrin. J. Nanosci. Nanotechnol. 2015, 15, 7900–7906. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.C.; Choi, K.H.; Lee, K.D.; Kim, J.H.; Jung, J.S.; Park, B.J. Particle size dependent photodynamic anticancer activity of hematophorphyrin-conjugated Fe3O4 particles. J. Nanomater. 2016, 2016, 1278393. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.-H.; Nam, K.C.; Kim, U.-H.; Cho, G.; Jung, J.-S.; Park, B.J. Optimized Photodynamic Therapy with Multifunctional Cobalt Magnetic Nanoparticles. Nanomaterials 2017, 7, 144. https://doi.org/10.3390/nano7060144
Choi K-H, Nam KC, Kim U-H, Cho G, Jung J-S, Park BJ. Optimized Photodynamic Therapy with Multifunctional Cobalt Magnetic Nanoparticles. Nanomaterials. 2017; 7(6):144. https://doi.org/10.3390/nano7060144
Chicago/Turabian StyleChoi, Kyong-Hoon, Ki Chang Nam, Un-Ho Kim, Guangsup Cho, Jin-Seung Jung, and Bong Joo Park. 2017. "Optimized Photodynamic Therapy with Multifunctional Cobalt Magnetic Nanoparticles" Nanomaterials 7, no. 6: 144. https://doi.org/10.3390/nano7060144




