Characterisation of the Chemical Composition and Structural Features of Novel Antimicrobial Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
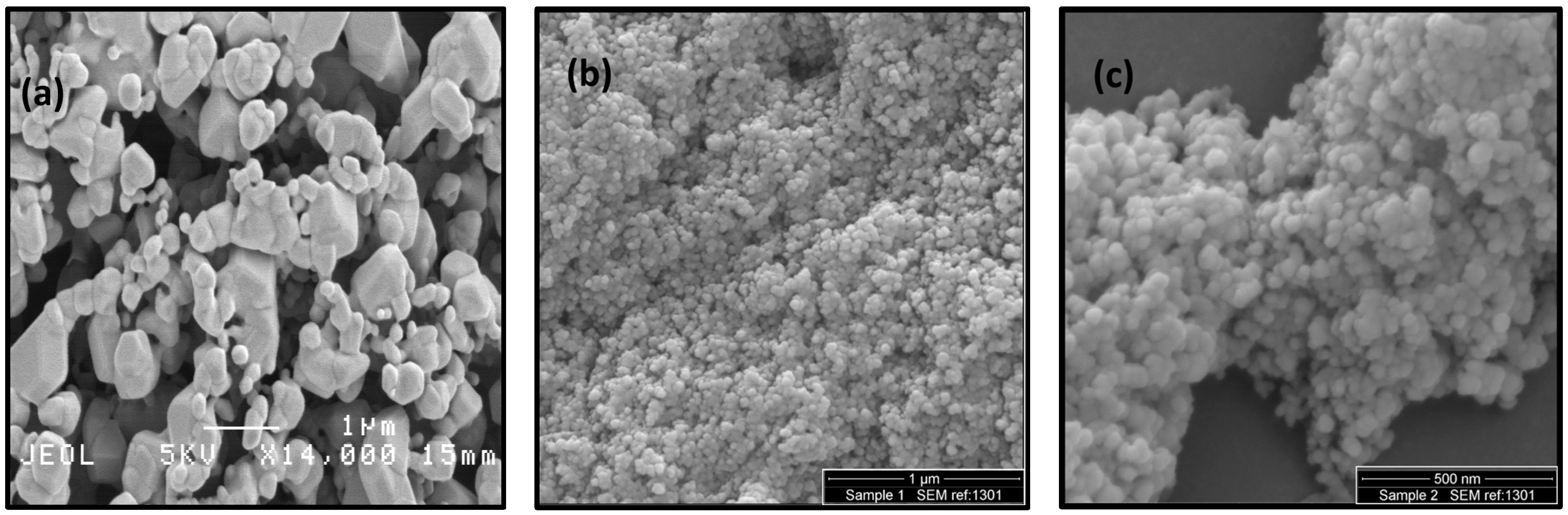
2.1. Particle Characteristics
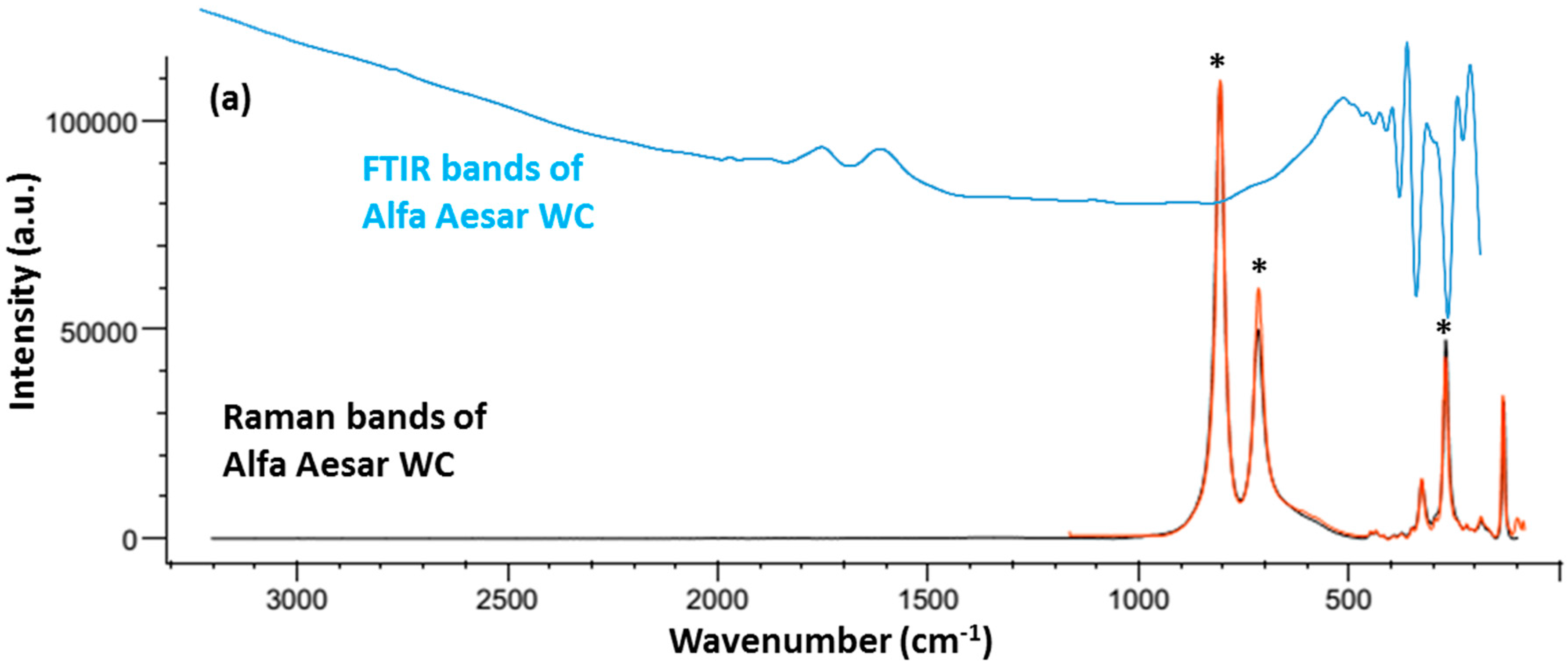
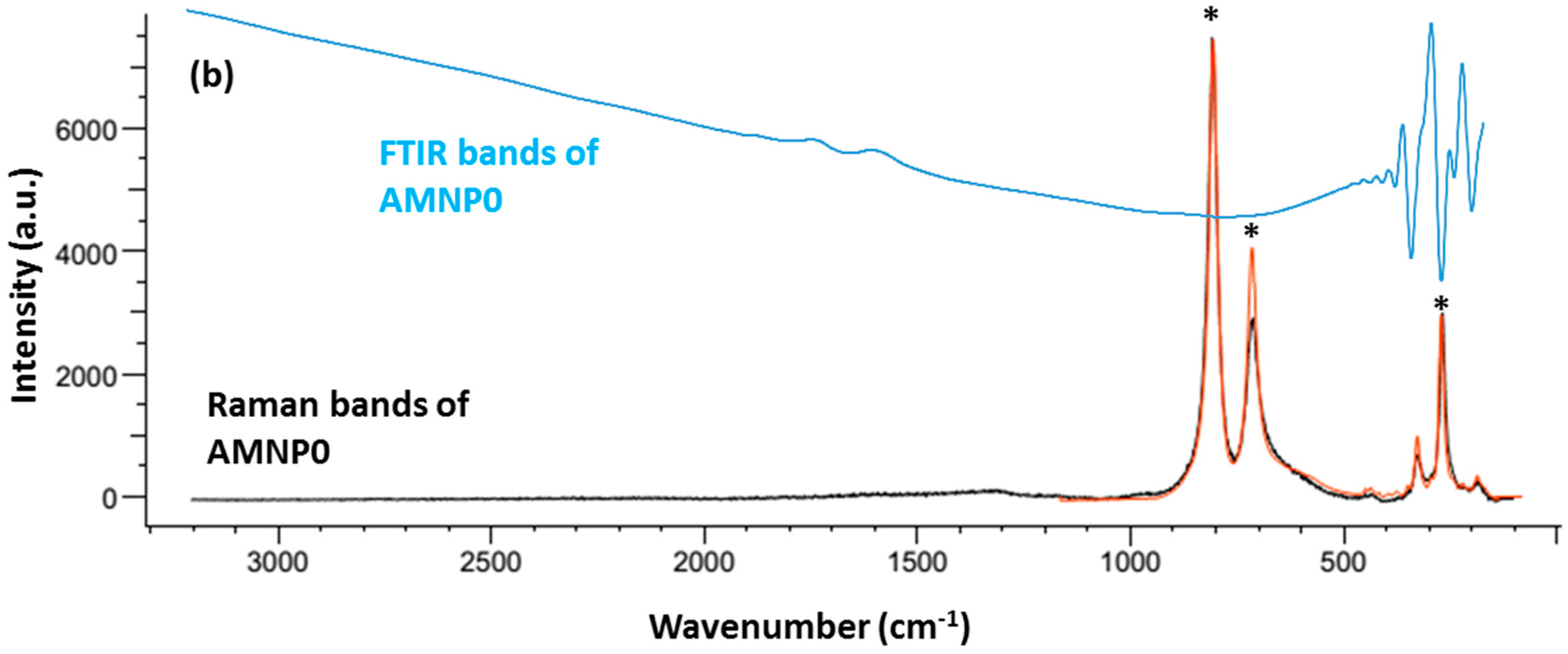
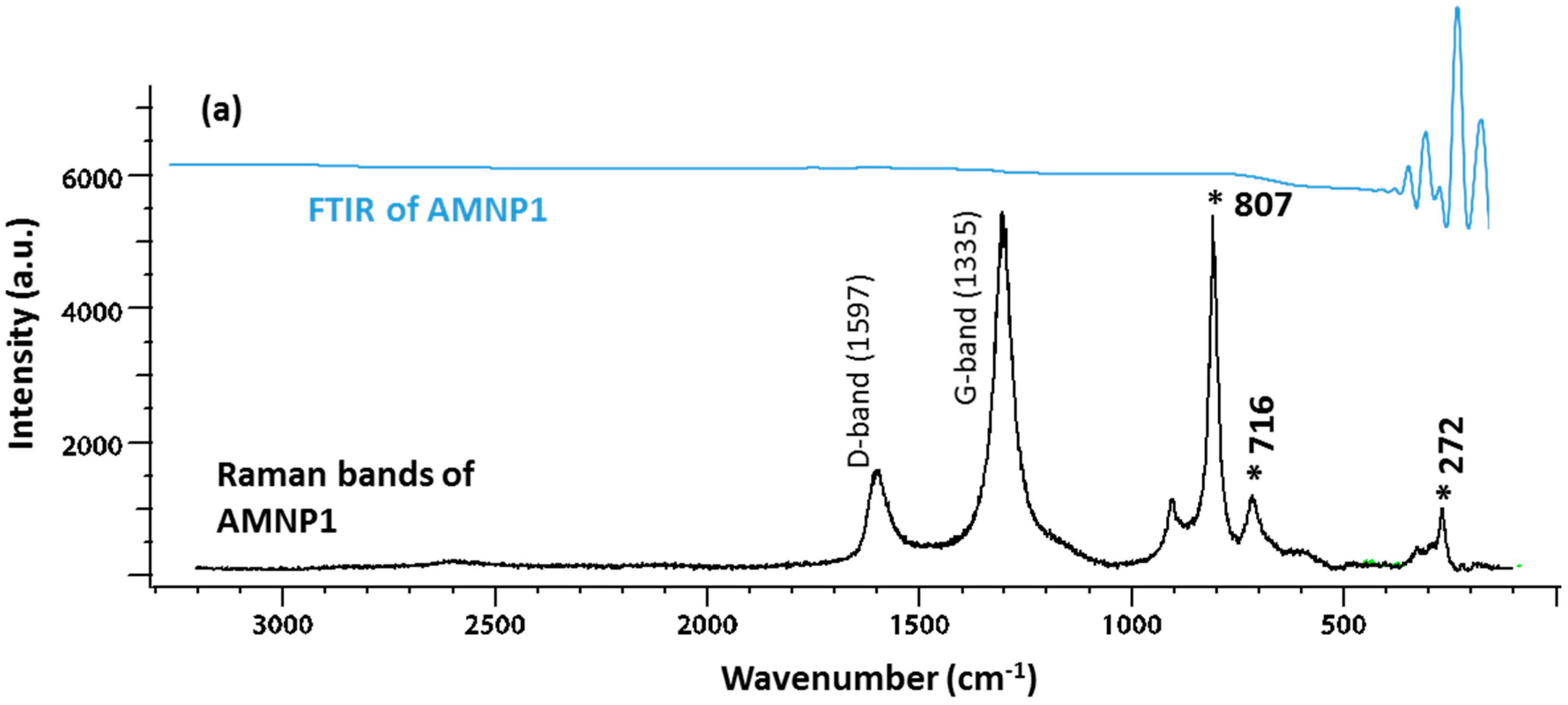
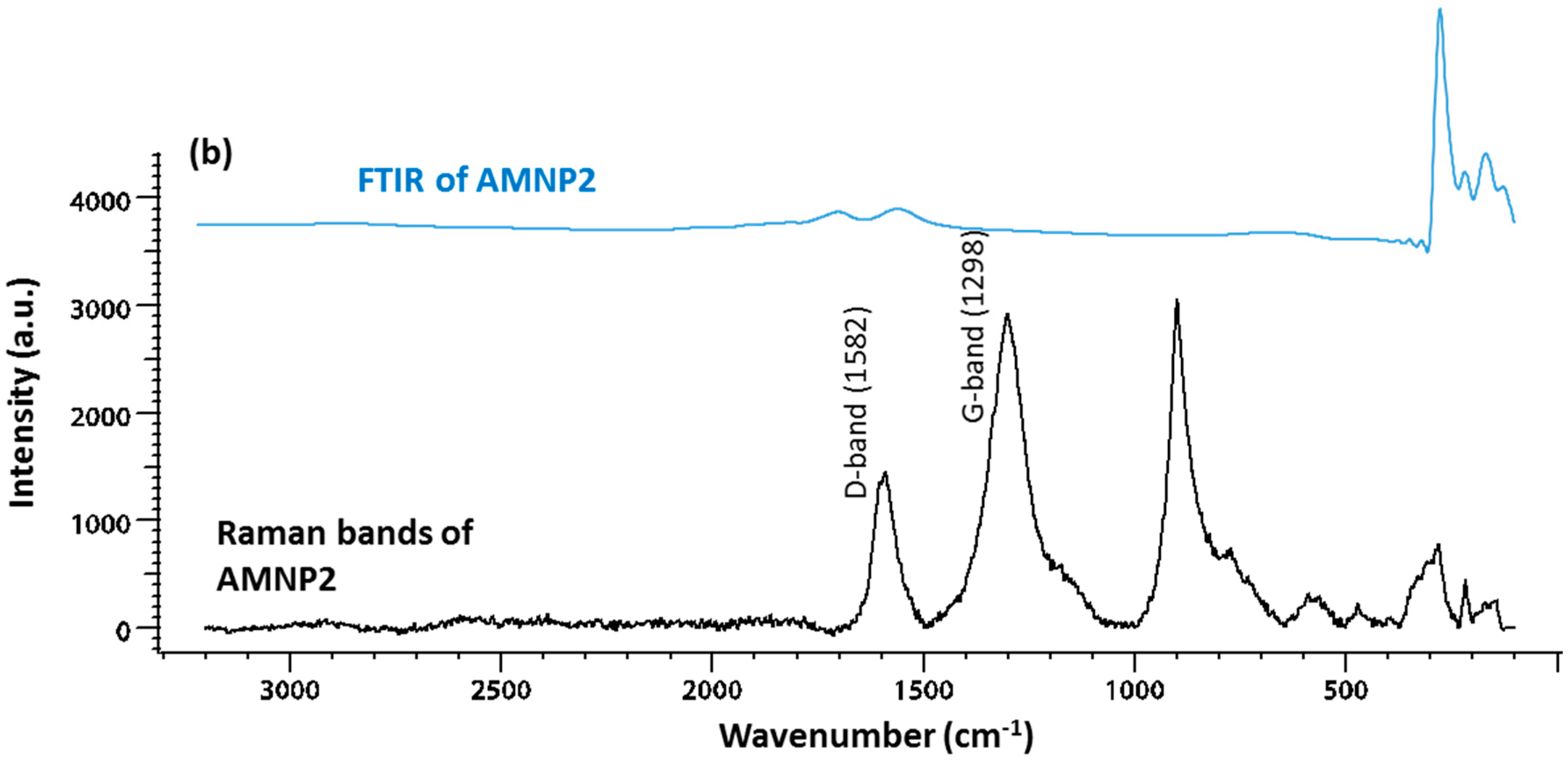
2.2. Chemical Characteristics
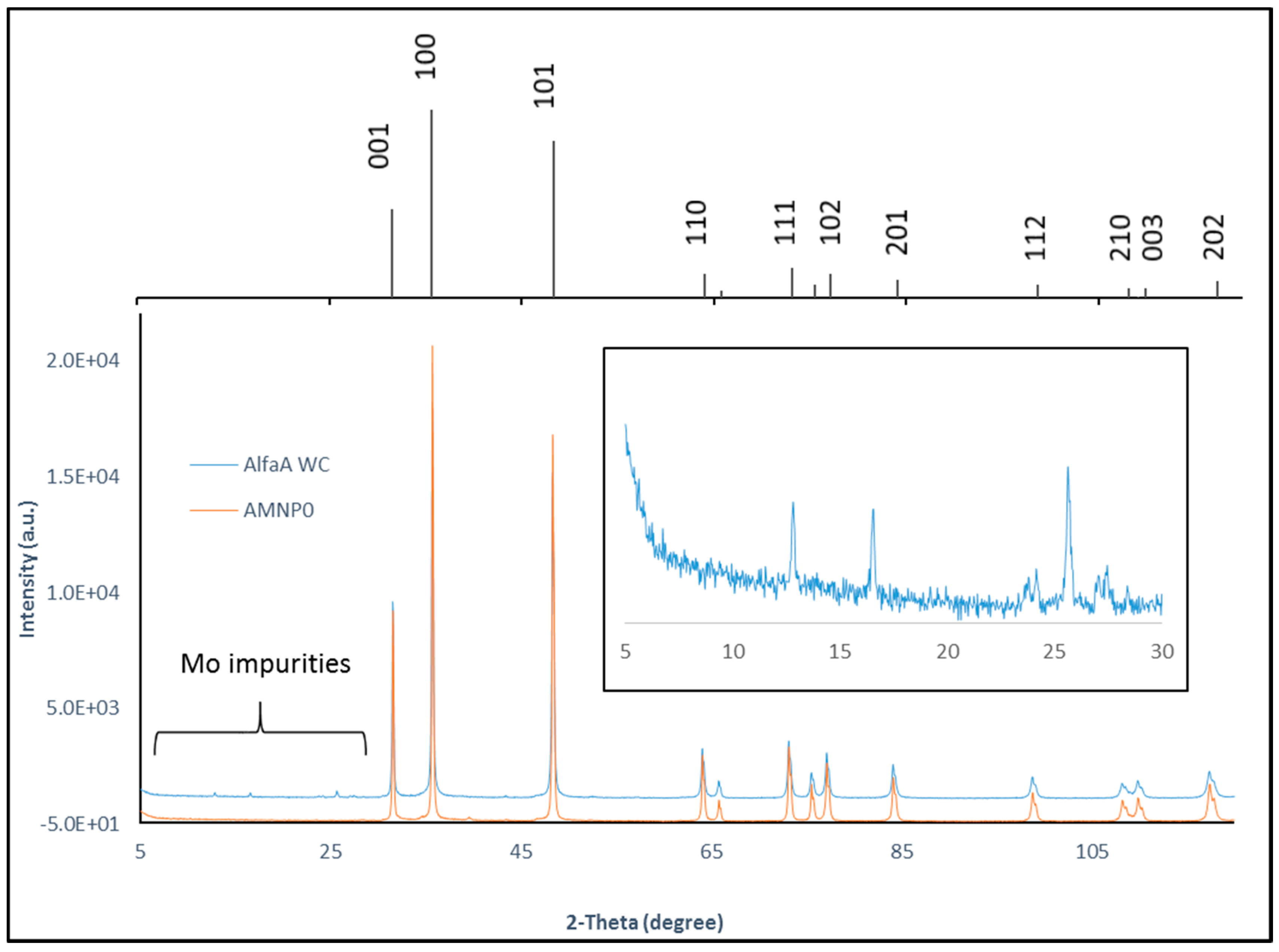
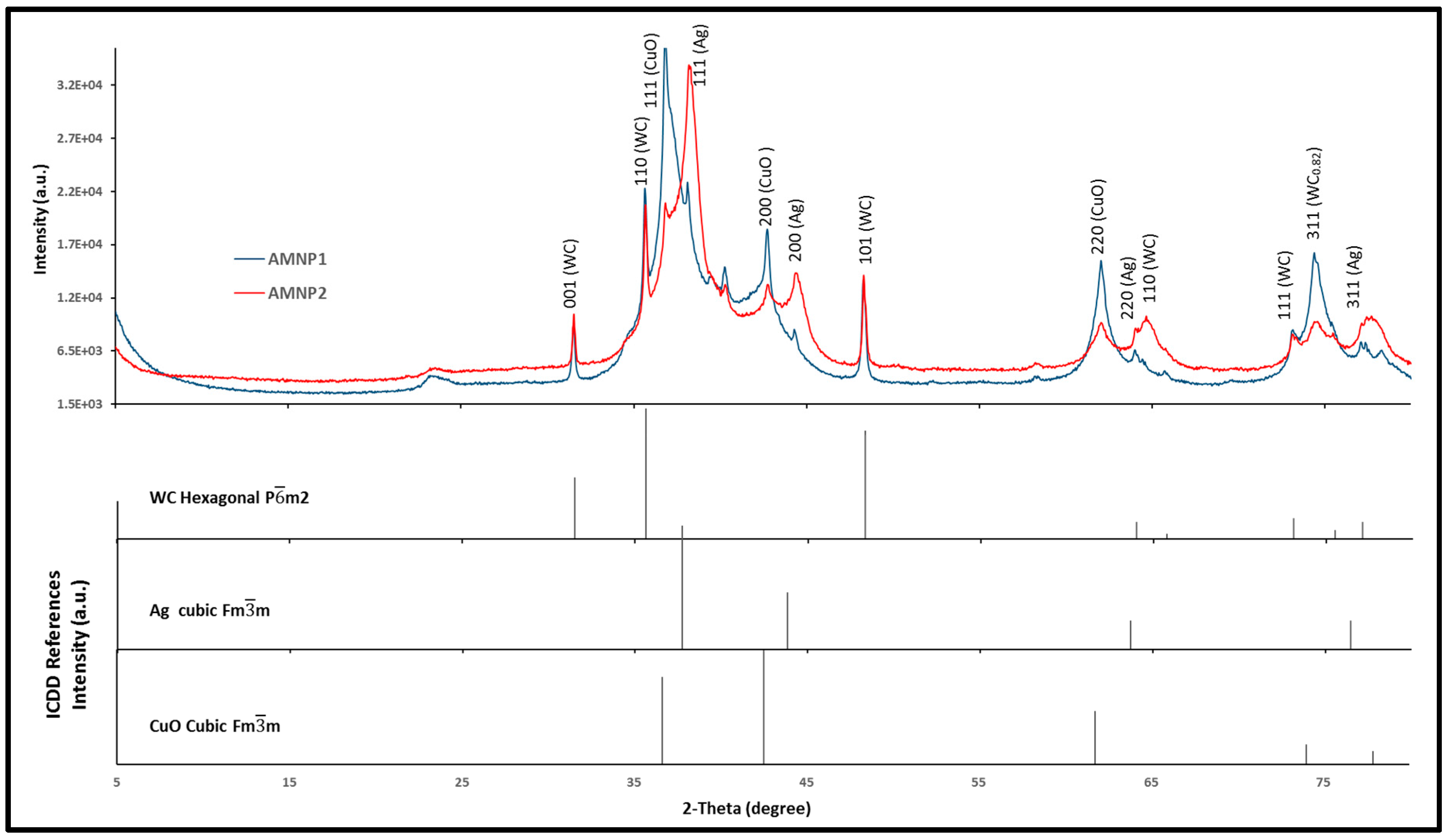
2.3. Phase Identification
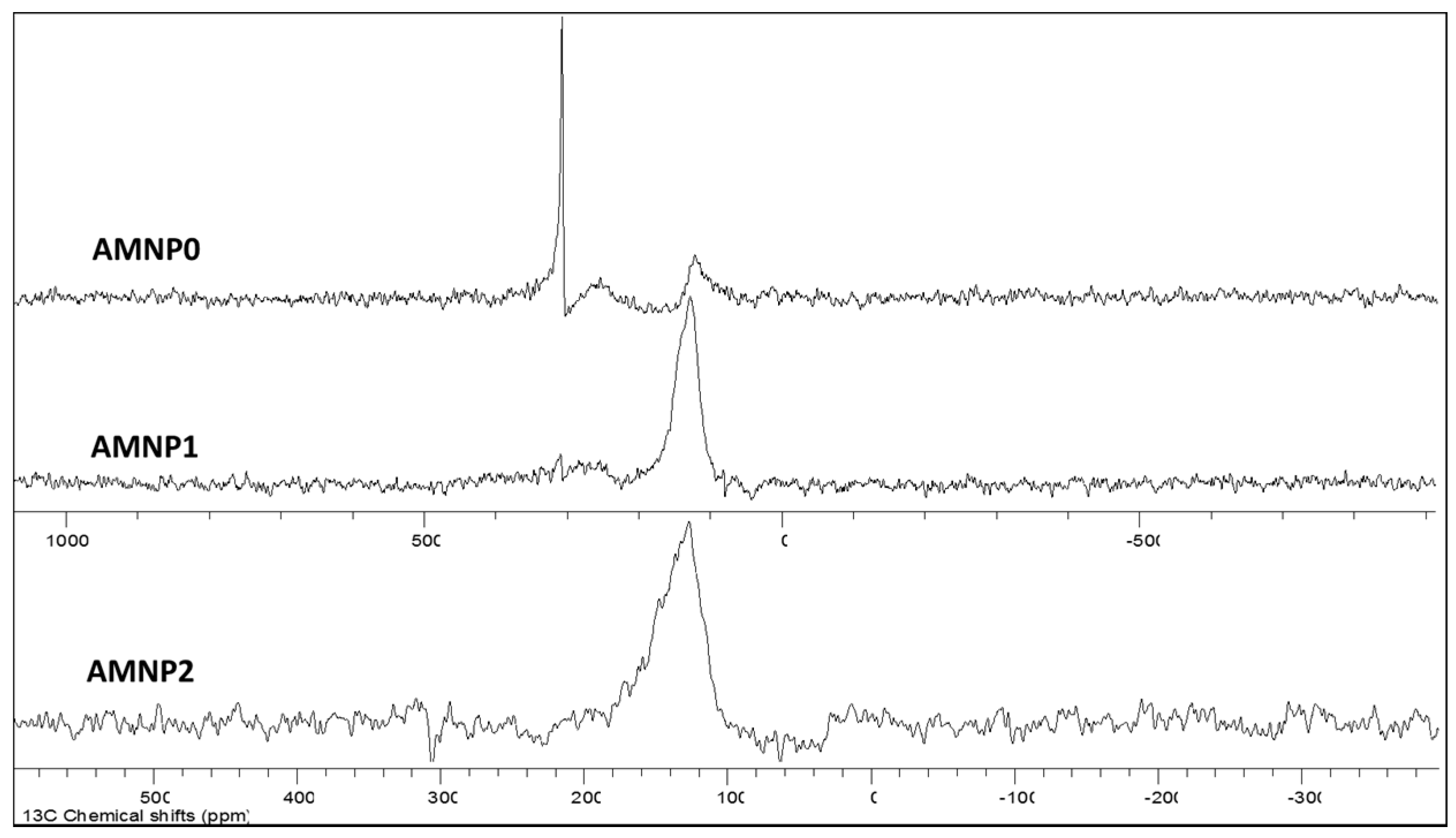
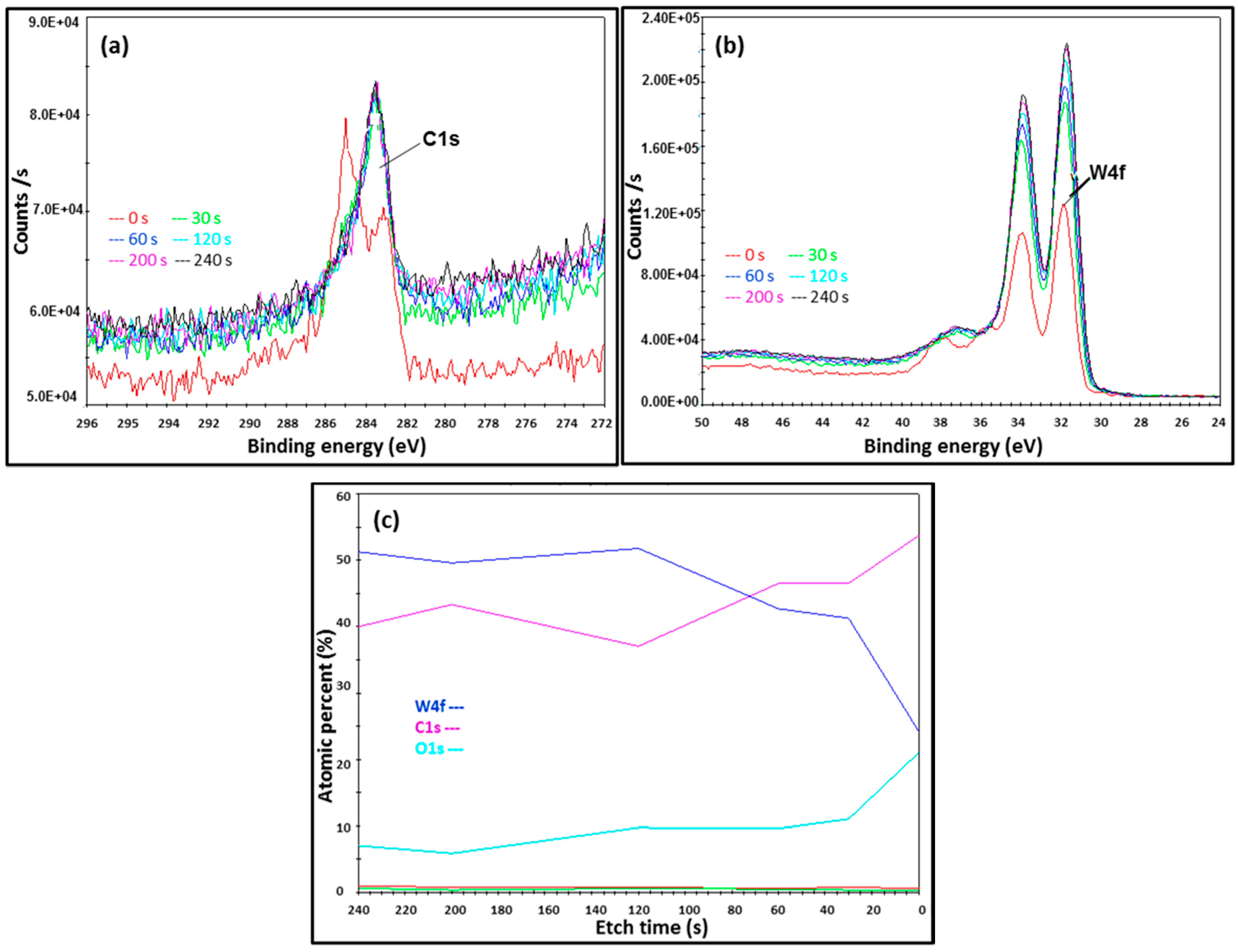
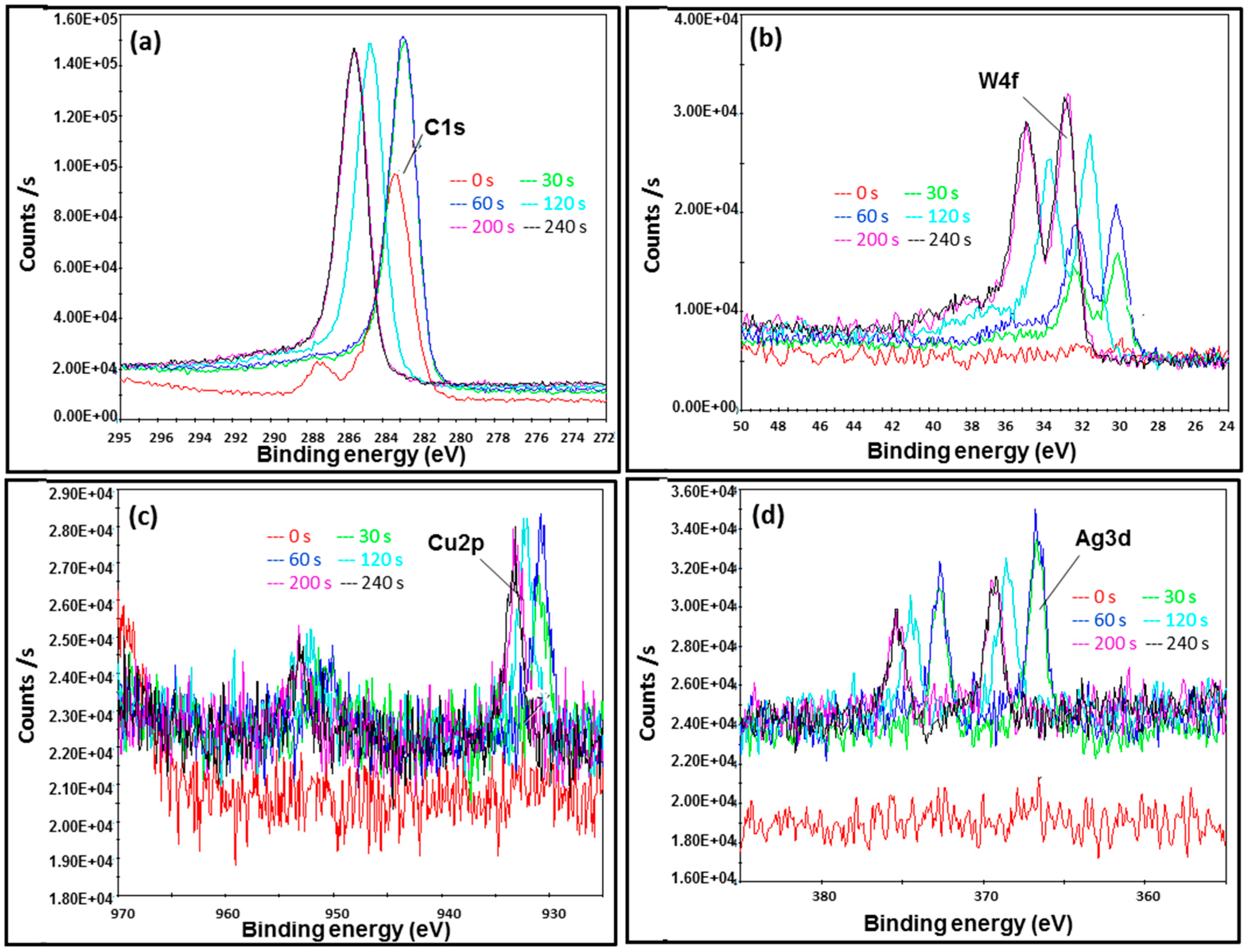
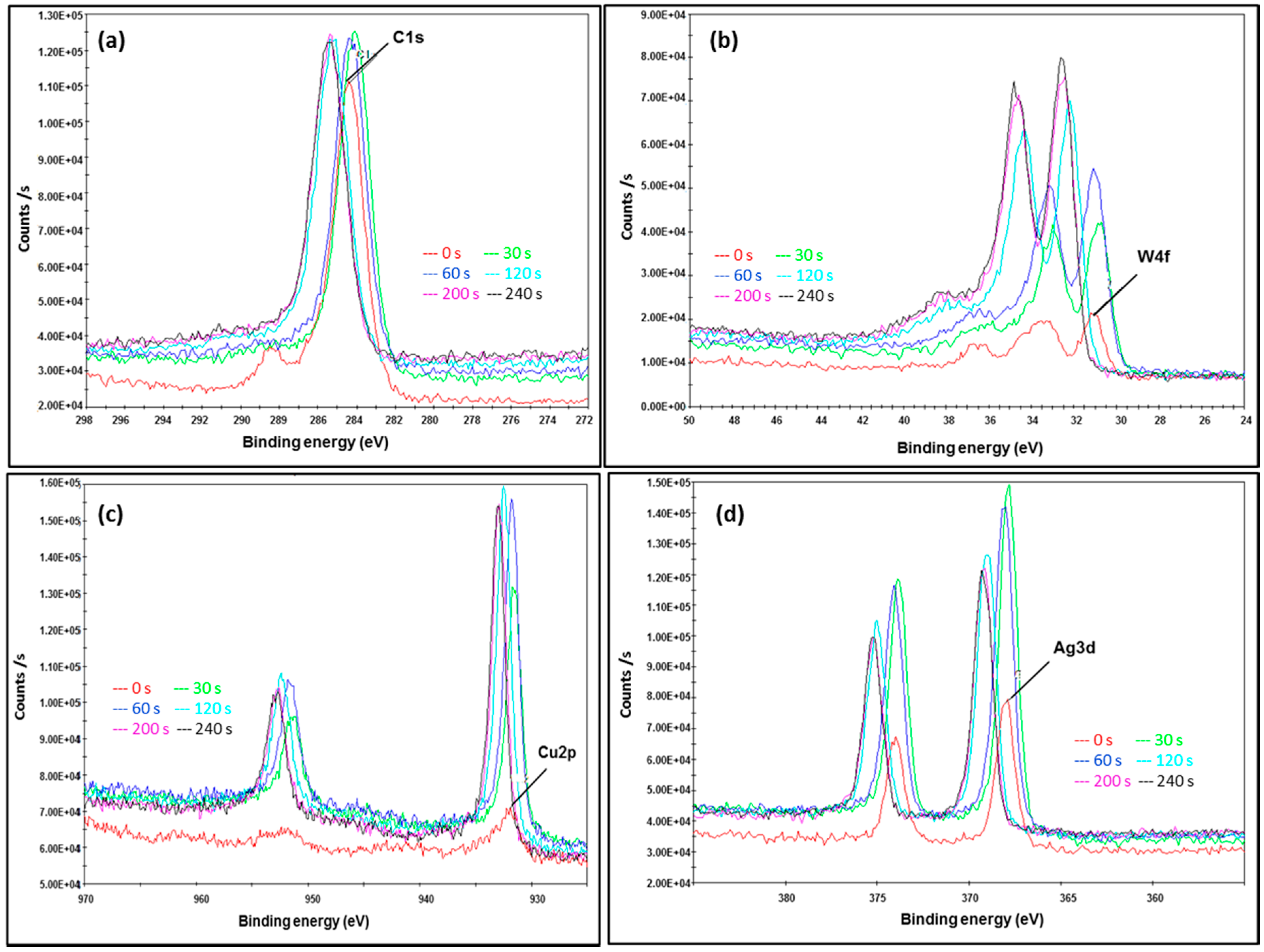
2.4. Atomic and Chemical State Analyses
3. Materials and Methods
3.1. Materials
3.2. Scanning Electron Microscopy
3.3. Fourier Transform Infrared and Raman Spectroscopy
3.4. Solid State Nuclear Magnetic Resonance Spectroscopy
3.5. X-ray Powder Diffraction
3.6. Energy Dispersive X-ray Spectroscopy
3.7. X-ray Photoelectron Spectroscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.K.; Jha, P.K.; Chaudhury, K.; Rana, S.V.; Guha, S.K. An emerging interface between life science and nanotechnology: Present status and prospects of reproductive healthcare aided by nano-biotechnology. Nano Rev. 2014, 5, 22762. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Bellare, J.R. Nanotechnology and nanomedicine for healthcare: Challenges in translating innovations from bench to bedside. J. Biomed. Nanotechnol. 2011, 7, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.M.; Huang, C.M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [PubMed]
- Raffa, V.; Vittorio, O.; Riggio, C.; Cuschieri, A. Progress in nanotechnology for healthcare. Minim. Invasive Ther. Allied Technol. 2010, 19, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Munir, A.B.; Yasin, S.H. Nanotechnology in healthcare: Are existing laws adequate? Eur. J. Health Law. 2007, 14, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.R.; Ramakrishna, S. Nanotechnology: 21st century revolution in restorative healthcare. Nanomedicine 2016, 11, 1511–1513. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Barick, K.C.; Bahadur, D. Surface engineered magnetic nanoparticles for removal of toxic metal ions and bacterial pathogens. J. Hazard. Mater. 2011, 192, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chen, C.-Y.; Lu, Y.-Y.; Lin, Y.-S. Recent Advances in Antimicrobial Polymers: A Mini-Review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Xu, D.; Yang, C.; Shahzad, M.B.; Sun, Z.; Xia, J.; Zhao, J.; Gu, T.; Yang, K.; Wang, G. An investigation of the antibacterial ability and cytotoxicity of a novel cu-bearing 317L stainless steel. Sci. Rep. 2016, 6, 29244. [Google Scholar] [CrossRef] [PubMed]
- Fatoba, O.S.; Esezobor, D.E.; Akanji, O.L.; Fatoba, A.J.; Macgregor, D.; Etubor, J. The Study of the Antimicrobial Properties of Selected Engineering Materials’ Surfaces. J. Miner. Mater. Charact. Eng. 2014, 2, 78–87. [Google Scholar] [CrossRef]
- Sun, D.; Shahzad, M.B.; Li, M.; Wang, G.; Xu, D. Antimicrobial materials with medical applications. Mater.Technol. Adv. Biomater. 2015, 30, B90–B95. [Google Scholar] [CrossRef]
- Devasconcellos, P.; Bose, S.; Beyenal, H.; Bandyopadhyay, A.; Zirkle, L.G. Antimicrobial Particulate Silver Coatings on Stainless Steel Implants for Fracture Management. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Chaudhry, M.Q.; Boxall, A.B.; Hull, M. Manufacture and use of nanomaterials: Current status in the UK and global trends. Occup. Med. (Lond.) 2006, 56, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Cloutman-Green, E.; Canales, M.; Zhou, Q.; Ciric, L.; Hartley, J.C.; McDonnell, G. Biochemical and microbial contamination of surgical devices: A quantitative analysis. Am. J. Infect. Control 2015, 43, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Ciric, L.; Brouwer, M.S.; Mullany, P.; Roberts, A.P. Minocycline resistance in an oral Streptococcus infantis isolate is encoded by tet(S) on a novel small, low copy number plasmid. FEMS Microbiol. Lett. 2014, 353, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative Antimicrobial Approach: Nano-Antimicrobial Materials. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Reus, M.A.; Memarzadeh, K.; Huang, J.; Ren, G.G.; Allaker, R.P. Antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. Int. J. Antimicrob. Agents 2012, 40, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, W.; Ren, G.; Yang, Z. The possible mechanism of silver nanoparticle impact on hippocampal synaptic plasticity and spatial cognition in rats. Toxicol. Lett. 2012, 209, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, J.; Li, Z.; Ren, G.; Yang, Z. In vitro toxicity of multi walled carbon nanotubes in C6 rat glioma cells. NeuroToxicol. 2012, 33, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Z.W.; Allaker, R.P.; Reip, P.; Oxford, J.; Ahmad, Z.; Ren, G. A review of nanoparticle functionality and toxicity on the central nervous system. J. R. Soc. Interface 2010, 7 (Suppl. 4), S411–S422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, S.; Ren, G.; Zhang, T.; Yang, Z. Nano-CuO inhibited voltage-gated sodium current of hippocampal CA1 neurons via reactive oxygen species but independent from G-proteins pathway. J. Appl. Toxicol. 2011, 31, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, G.; Zhang, T.; Yang, Z. The inhibitory effects of nano-Ag on voltage-gated potassium currents of hippocampal CA1 neurons. Environ. Toxicol. 2011, 26, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, L.; Zhang, T.; Ren, G.; Yang, Z. Influences of nanoparticle zinc oxide on acutely isolated rat hippocampal CA3 pyramidal neurons. Neurotoxicology 2009, 30, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yang, J.; Ren, G.; Yang, Z.; Zhang, T. Multi-walled carbon nanotube increases the excitability of hippocampal CA1 neurons through inhibition of potassium channels in rat's brain slices. Toxicol. Lett. 2013, 217, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yang, J.; Zhang, H.; Ren, G.; Yang, Z. Multi-walled carbon nanotube inhibits CA1 glutamatergic synaptic transmission in rat’s hippocampal slices. Toxicol. Lett. 2014, 229, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ren, L.; Xu, K.; Yang, F.; Li, Y.; Cheng, T.; Kang, X.; Xu, C.; Shi, Q. Supercritical fluid extraction with carbon nanotubes as a solid collection trap for the analysis of polycyclic aromatic hydrocarbons and their derivatives. J. Chromatogr. A 2015, 1395, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P.; Ren, G. Potential impact of nanotechnology on the control of infectious diseases. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Oxford, J.S.; Reip, P.W.; Lambkin-Williams, R. Anti-Viral Formulations Nanomaterials and Nanoparticles. U.S. Patent 2013/0091611 A1, 18 April 2013. [Google Scholar]
- Mahalingam, S.; Edirisinghe, M. Forming of polymer nanofibers by a pressurised gyration process. Macromol. Rapid Commun. 2013, 34, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Illangakoon, U.E.; Mahalingam, S.; Wang, K.; Cheong, Y.K.; Canales, E.; Ren, G.G.; Cloutman-Green, E.; Edirisinghe, M.; Ciric, L. Gyrospun antimicrobial nanoparticle loaded fibrous polymeric filters. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liang, Z.; Hu, Z. Bacterial response to a continuous long-term exposure of silver nanoparticles at sub-ppm silver concentrations in a membrane bioreactor activated sludge system. Water Res. 2014, 50, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Matejka, V.; Tokarsky, J. Photocatalytical nanocomposites: A review. J. Nanosci. Nanotechnol. 2014, 14, 1597–1616. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Yudovin-Farber, I.; Perez-Davidi, M.; Domb, A.J.; Weiss, E.I. Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 22038–22043. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.R.; Allen, J.J.; Bott, S.; Jebb, M.; Jiang, C.; Kienast, W. Physical Methods in Chemistry and Nano Science; CONNEXIONS Rice University: Houston, TX, USA, 2012. [Google Scholar]
- Yan, Y.; Xia, B.; Qi, X.; Wang, H.; Xu, R.; Wang, J.-Y.; Zhang, H.; Wang, X. Nano-tungsten carbide decorated graphene as co-catalysts for enhanced hydrogen evolution on molybdenum disulfide. Chem. Commun. 2013, 49, 4884–4886. [Google Scholar] [CrossRef] [PubMed]
- Krasovskii, P.V.; Malinovskaya, O.S.; Samokhin, A.V.; Blagoveshchenskiy, Y.V.; Kazakov, V.A.; Ashmarin, A.A. XPS study of surface chemistry of tungsten carbides nanopowders produced through DC thermal plasma/hydrogen annealing process. Appl. Surf. Sci. 2015, 339, 46–54. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, D.; Zhou, J.; Xie, Z.; Xia, Y. Synthesis and characterization of tungsten carbide and application to electrocatalytic hydrogen evolution. RSC Adv. 2016, 6, 76307–76311. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Nakajima, K.; Wakahara, T.; Tsuchiya, T.; Ishitsuka, M.O.; Maeda, Y.; Akasaka, T.; Waelchli, M.; Mizorogi, N.; Nagase, S. Observation of 13C NMR chemical shifts of metal carbides encapsulated in fullerenes: Sc2C2@C82, Sc2C2@C84, and Sc3C2@C80. Angew. Chem. Int. Ed. Engl. 2008, 47, 7905–7908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fuhrer, T.; Fu, W.; Ge, J.; Bearden, D.W.; Dallas, J.; Duchamp, J.; Walker, K.; Champion, H.; Azurmendi, H.; et al. Nanoscale Fullerene Compression of an Yttrium Carbide Cluster. J. Am. Chem. Soc. 2012, 134, 8487–8493. [Google Scholar] [CrossRef] [PubMed]
- Buss, J.A.; Agapie, T. Four-electron deoxygenative reductive coupling of carbon monoxide at a single metal site. Nature 2015, 529, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Kurlov, A.S.; Gusev, A.I. Oxidation of tungsten carbide powders in air. Int. J. Refract. Met. Hard Mater. 2013, 41, 300–307. [Google Scholar] [CrossRef]
- Kalikhman, V.L. Inorg. Mater. (Engl. Transl.) 1983, 19, 957. A Primary Reference Source Provided by the International Centre for Diffraction Data (ICDD 04-004-57230). Available online: https://link.springer.com/journal/10789 (accessed on 21 June 2017).
- Lindqvist, I. The Crystal structure of the yellow Molybdic acid Mo3.2H2O. On the existence of the H4O2+ ion. Acta Chem. Scand. 1950, 4, 650–657. [Google Scholar] [CrossRef]
- Metcalfe, A.G. J. Inst. Met. 1947, 73, 591. A Primary Reference Source Provided by the International Centre for Diffraction Data (ICDD 00-051-0939). Available online: https://catalog.hathitrust.org/Record/008893915 (accessed on 21 June 2017).
- Ren, X.; Peng, Z.; Peng, Y.; Wang, C.; Fu, Z.; Qi, L.; Miao, H. Ultrafine binderless WC-based cemented carbides with varied amounts of AlN nano-powder fabricated by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2013, 41, 308–314. [Google Scholar] [CrossRef]
- Sara, R.V. Phase Equilibria in the System Tungsten—Carbon. J. Am. Ceram. Soc. 1965, 48, 251–257. [Google Scholar] [CrossRef]
- Katagiri, A.; Suzuki, M.; Takehara, Z. Electrodeposition of Tungsten in ZnBr2-NaBr and ZnCl2-NaCl Melts. J. Electrochem. Soc. 1991, 138, 767–773. [Google Scholar] [CrossRef]
- Predel, B.; Bankstahl, H. Metastabile Phasen im System Silber-Wismut. Z. Metallkd. 1976, 67, 793–799. [Google Scholar]
- Schmahl, N.G.; Eikerling, G.F.Z. Über Kryptomodifikationen des Cu(II)-Oxids. Phys. Chem. Neue Folge. (Wiesbaden) 1968, 62, 268–279. [Google Scholar] [CrossRef]
- Briggs, D. Handbook of X-ray Photoelectron Spectroscopy C.D. Wanger, W.M. Riggs, L.E. Davis, J.F. Moulder and G.E.Muilenberg Perkin-Elmer Corp., Physical Electronics Division, Eden Prairie, Minnesota, USA, 1979. 190 pp. 195. Surf. Interface Anal. 1981, 3. [Google Scholar] [CrossRef]
- Susi, T.; Kaukonen, M.; Havu, P.; Ljungberg, M.P.; Ayala, P.; Kauppinen, E.I. Core level binding energies of functionalized and defective graphene. Beilstein J. Nanotechnol. 2014, 5, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Susi, T.; Pichler, T.; Ayala, P. X-ray photoelectron spectroscopy of graphitic carbon nanomaterials doped with heteroatoms. Beilstein J. Nanotechnol. 2015, 6, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.A.; Babar, S.; Bhatti, A.S.; Bokhari, H. Antibacterial Effects of Silver Nanoparticles on the Bacterial Strains Isolated from Catheterized Urinary Tract Infection Cases. J. Biomed. Nanotechnol. 2009, 5, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.; Ren, G.; Wang, D.; Yang, K. Antibacterial Performance of Cu-Bearing Stainless Steel against Staphylococcus aureus and Pseudomonas aeruginosa in Whole Milk. J. Mater. Sci. Technol. 2016, 32, 445–451. [Google Scholar] [CrossRef]
- Xu, Z.; Mahalingam, S.; Rohn, J.L.; Ren, G.; Edirisinghe, M. Physio-chemical and antibacterial characteristics of pressure spun nylon nanofibres embedded with functional silver nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 195–204. [Google Scholar] [CrossRef] [PubMed]
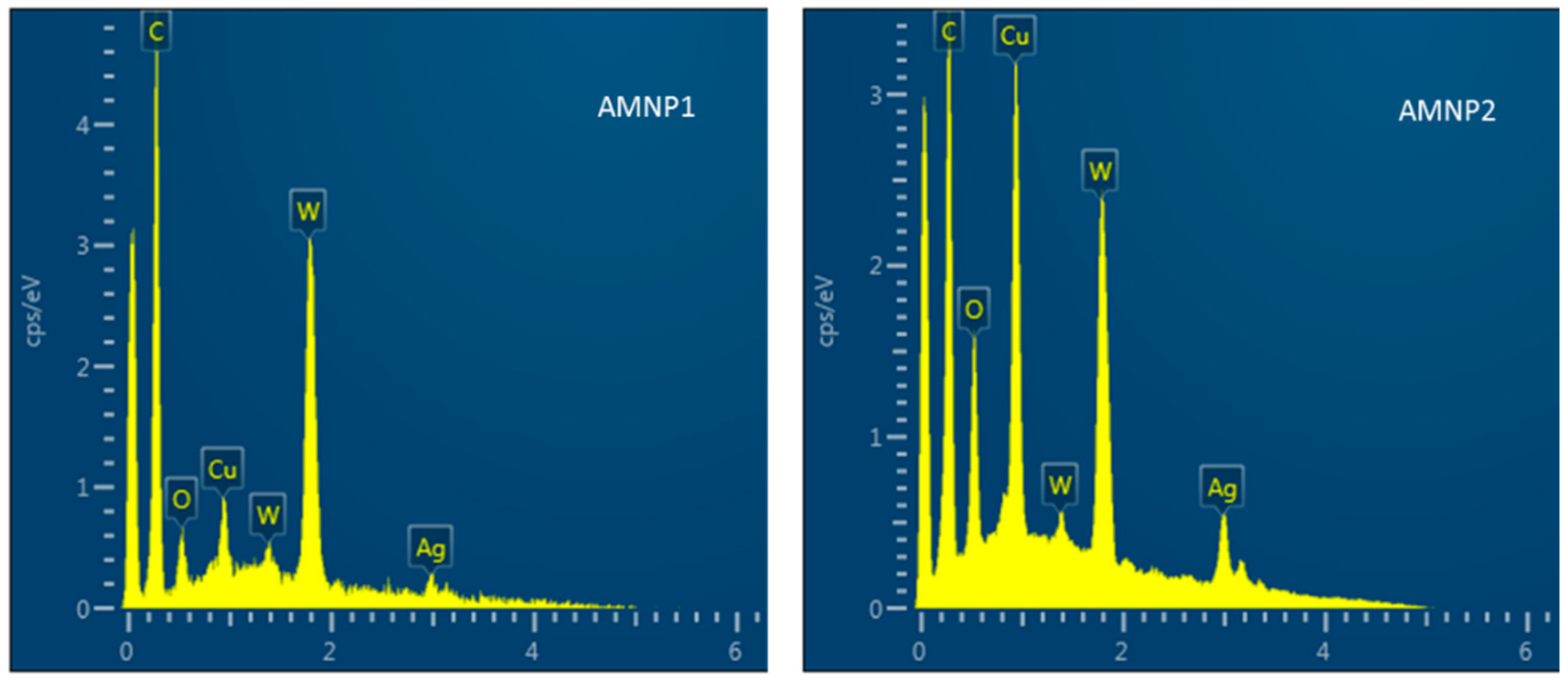
| AMNP1 | AMNP2 | ||
|---|---|---|---|
| Phases Detected | Details | Phases Detected | Details |
| Tungsten carbide (WC) | Hexagonal, Pm2 | Tungsten carbide (WC) | Hexagonal, Pm2 |
| Tungsten carbide (WC0.82) | Cubic, Fmm | ||
| Tungsten carbide (W2C) | orthorhombic, Pbcn | Copper oxide (CuO) | Cubic, Fmm |
| Tungsten (W) | β-W, cubic Pmn | Tungsten (W) | β-W, cubic Pmn |
| Silver (Ag) | cubic, Fmm | Silver (Ag) | cubic, Fmm |
| C | O | W | Ag | Cu | |
|---|---|---|---|---|---|
| AMNP0 | 42.7% | 8.7% | 47.3% | 0.5% | 0.8% |
| AMNP1 | 94.0% | 2.9% | 2.1% | 0.5% | 0.5% |
| AMNP2 | 77.7% | 5.5% | 6.7% | 4.7% | 5.4% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheong, Y.-K.; Calvo-Castro, J.; Ciric, L.; Edirisinghe, M.; Cloutman-Green, E.; Illangakoon, U.E.; Kang, Q.; Mahalingam, S.; Matharu, R.K.; Wilson, R.M.; et al. Characterisation of the Chemical Composition and Structural Features of Novel Antimicrobial Nanoparticles. Nanomaterials 2017, 7, 152. https://doi.org/10.3390/nano7070152
Cheong Y-K, Calvo-Castro J, Ciric L, Edirisinghe M, Cloutman-Green E, Illangakoon UE, Kang Q, Mahalingam S, Matharu RK, Wilson RM, et al. Characterisation of the Chemical Composition and Structural Features of Novel Antimicrobial Nanoparticles. Nanomaterials. 2017; 7(7):152. https://doi.org/10.3390/nano7070152
Chicago/Turabian StyleCheong, Yuen-Ki, Jesus Calvo-Castro, Lena Ciric, Mohan Edirisinghe, Elaine Cloutman-Green, Upulitha Eranka Illangakoon, Qiang Kang, Suntharavathanan Mahalingam, Rupy Kaur Matharu, Rory M. Wilson, and et al. 2017. "Characterisation of the Chemical Composition and Structural Features of Novel Antimicrobial Nanoparticles" Nanomaterials 7, no. 7: 152. https://doi.org/10.3390/nano7070152







