The Electrosome: A Surface-Displayed Enzymatic Cascade in a Biofuel Cell’s Anode and a High-Density Surface-Displayed Biocathodic Enzyme
Abstract
:1. Introduction
2. Results and Discussion
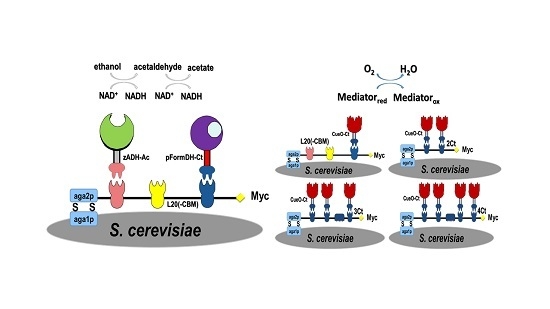
2.1. Choice of Enzymatic Cascade
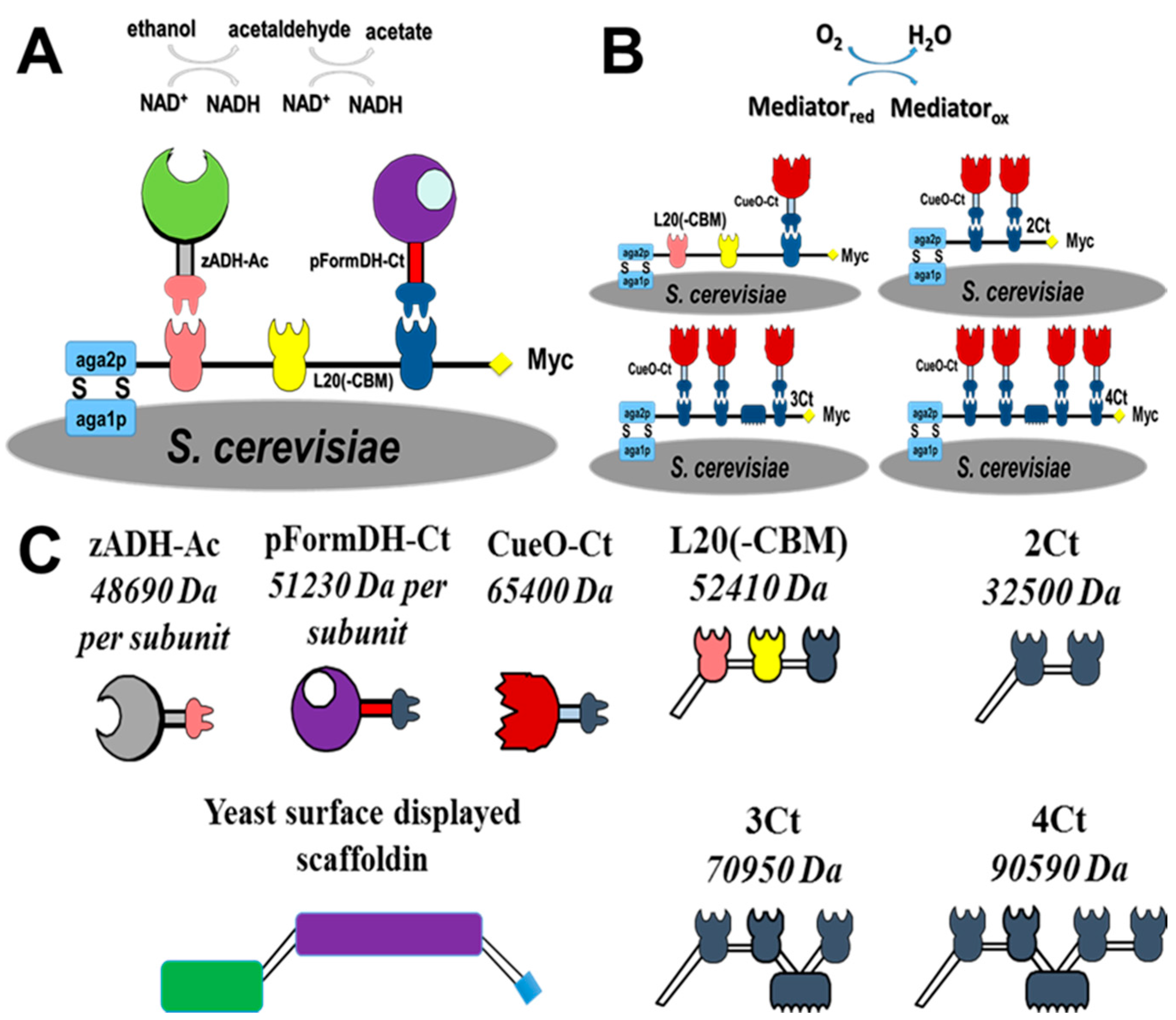
2.2. Expression of Enzymes and Scaffoldin
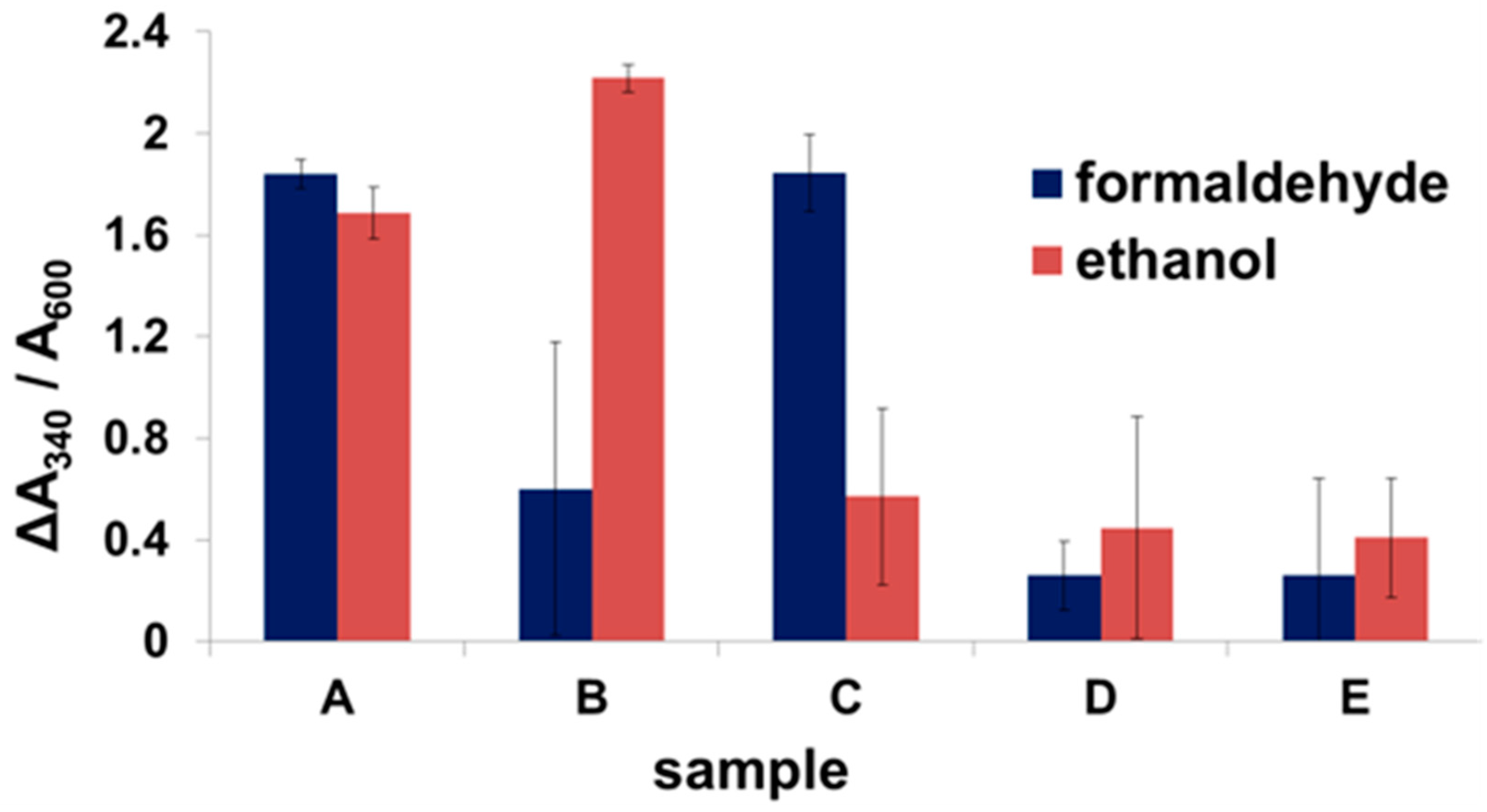
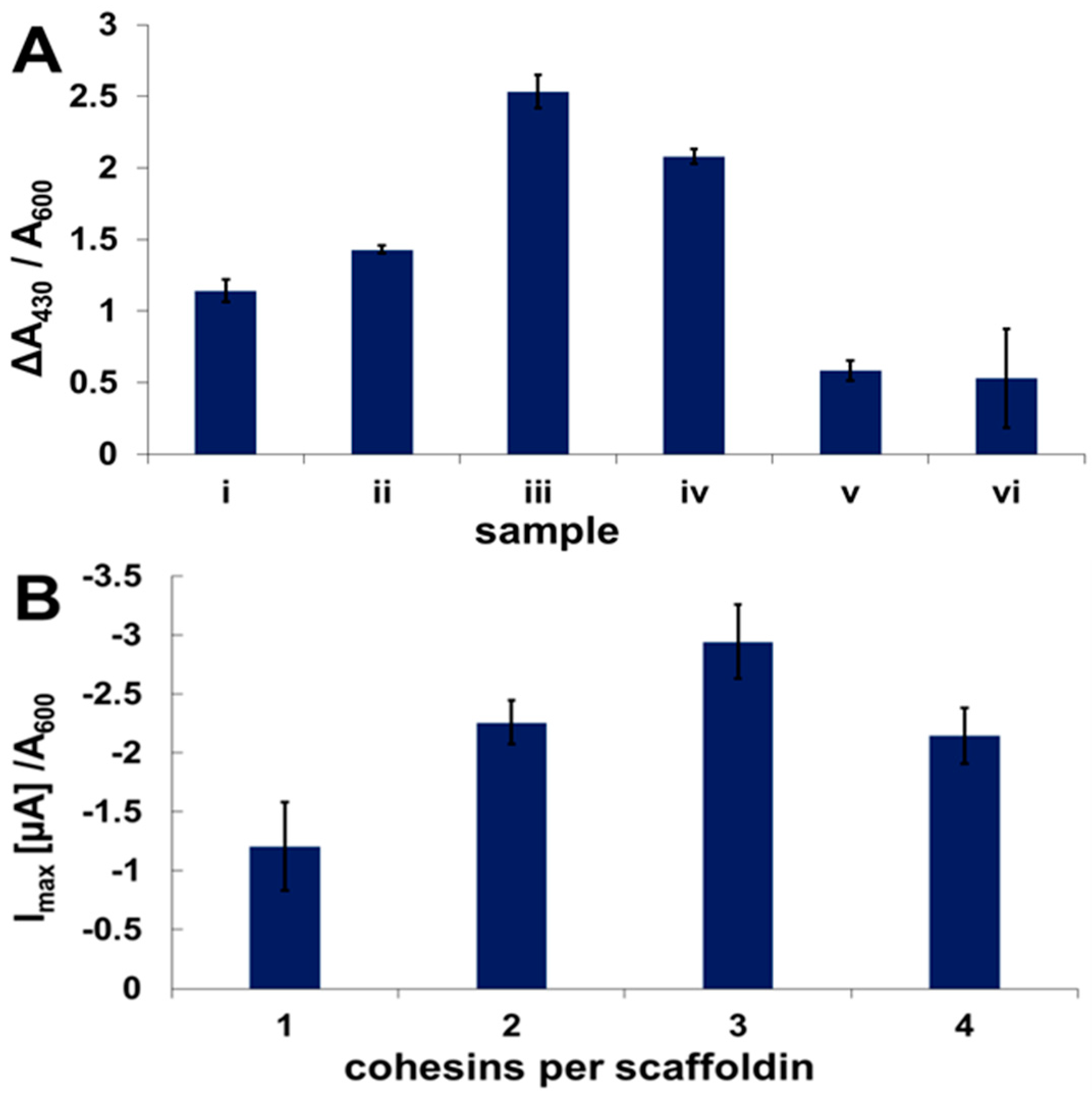
2.3. Enzyme Binding to Surface-Displayed Scaffoldins
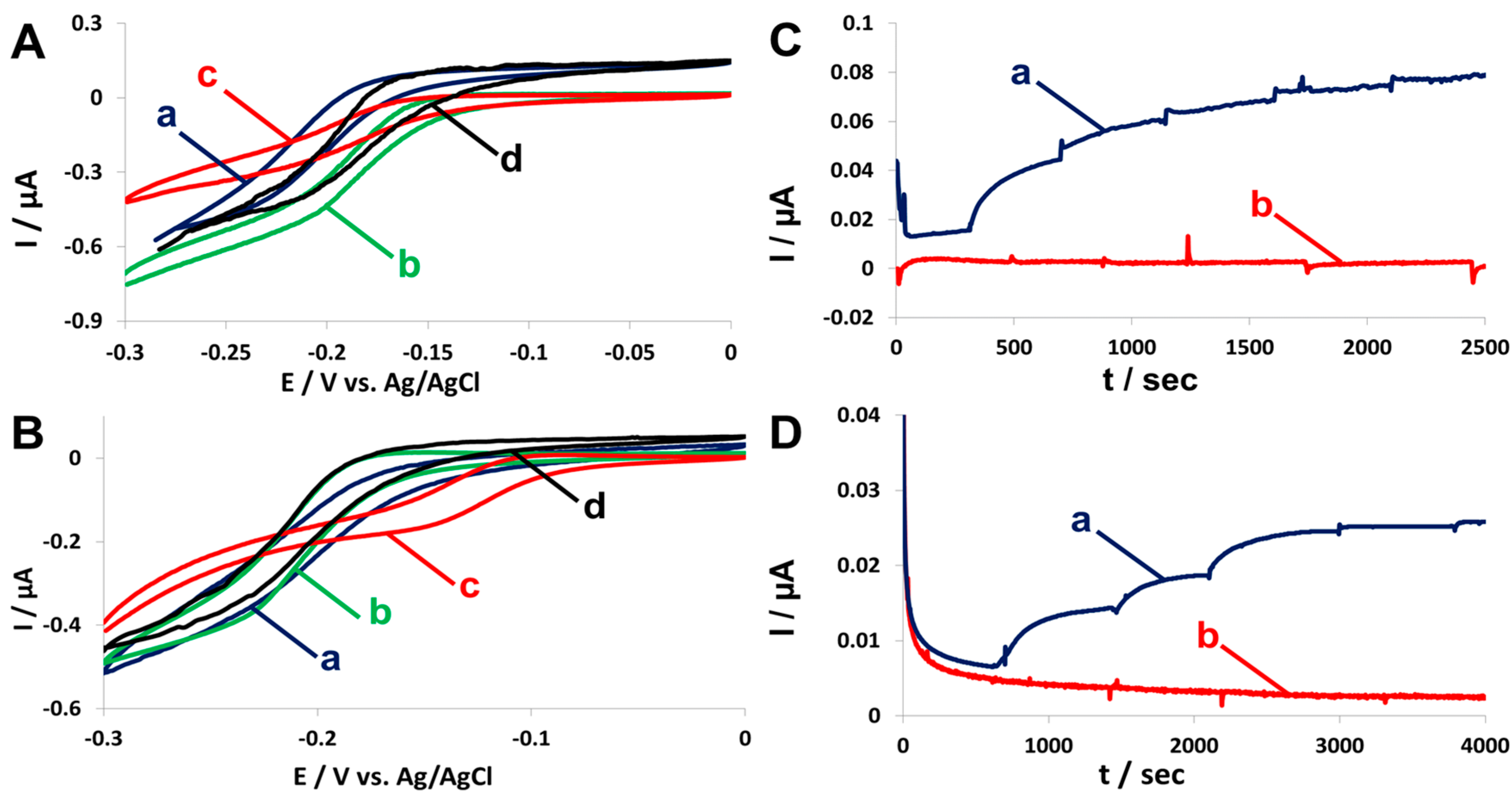
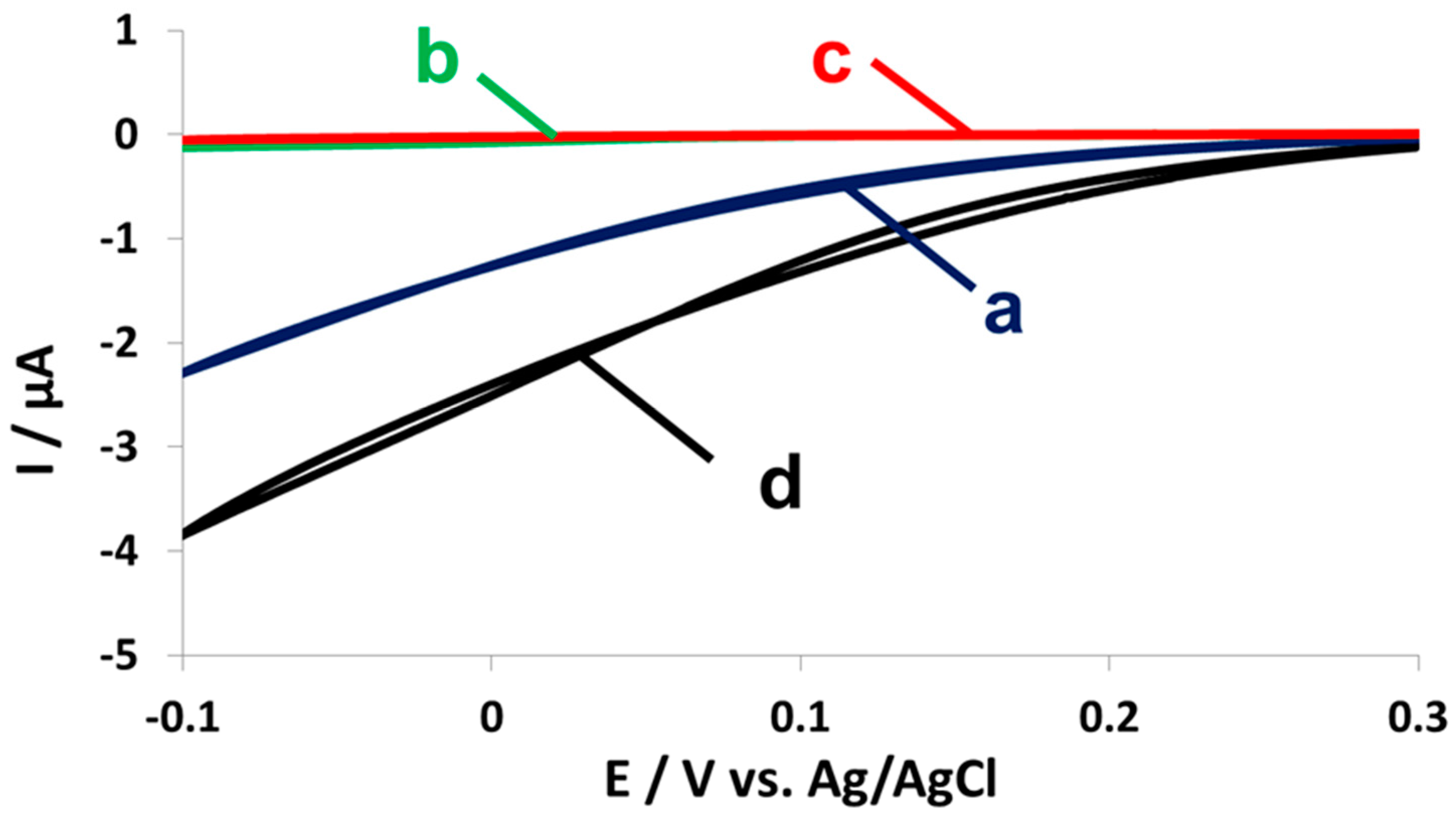
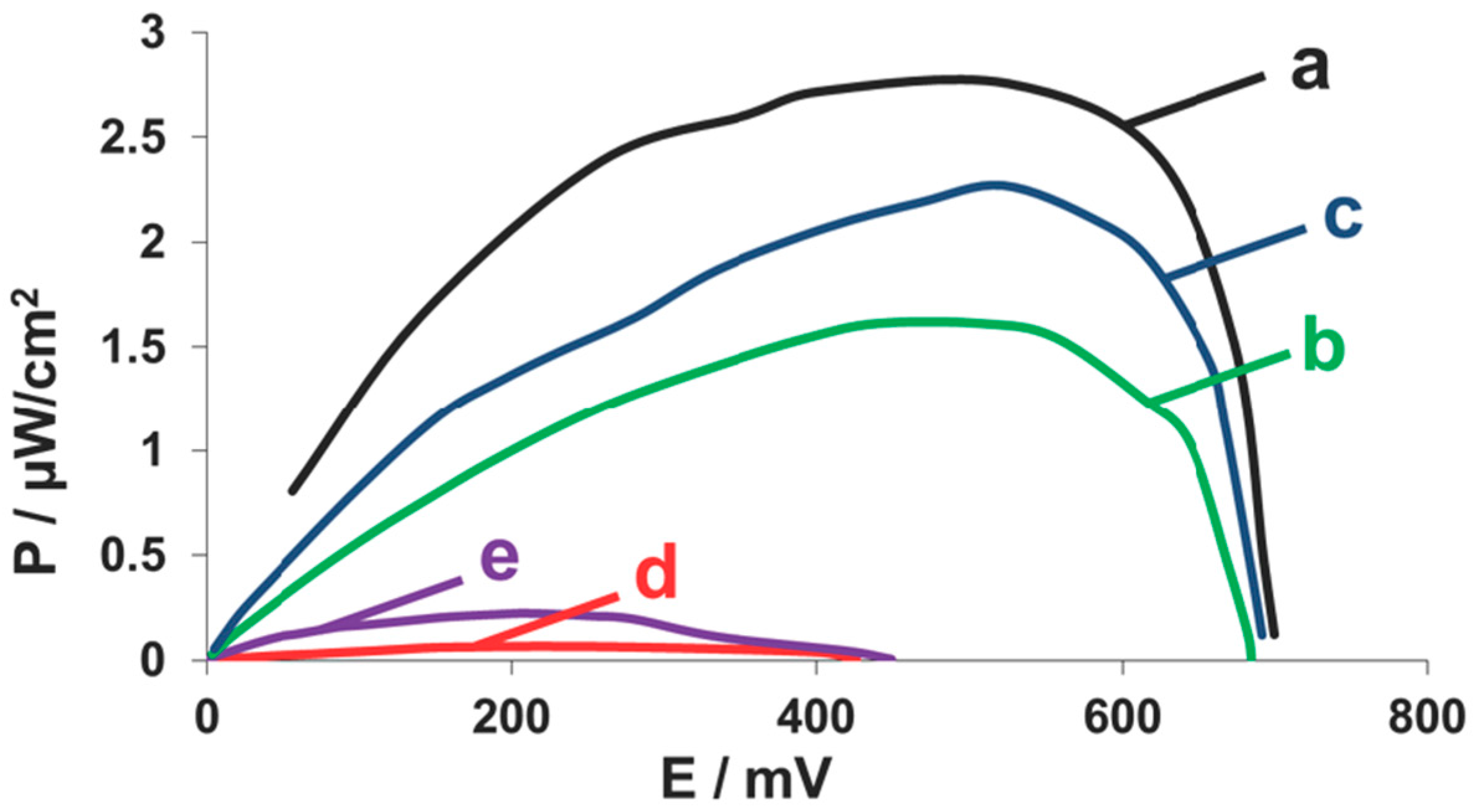
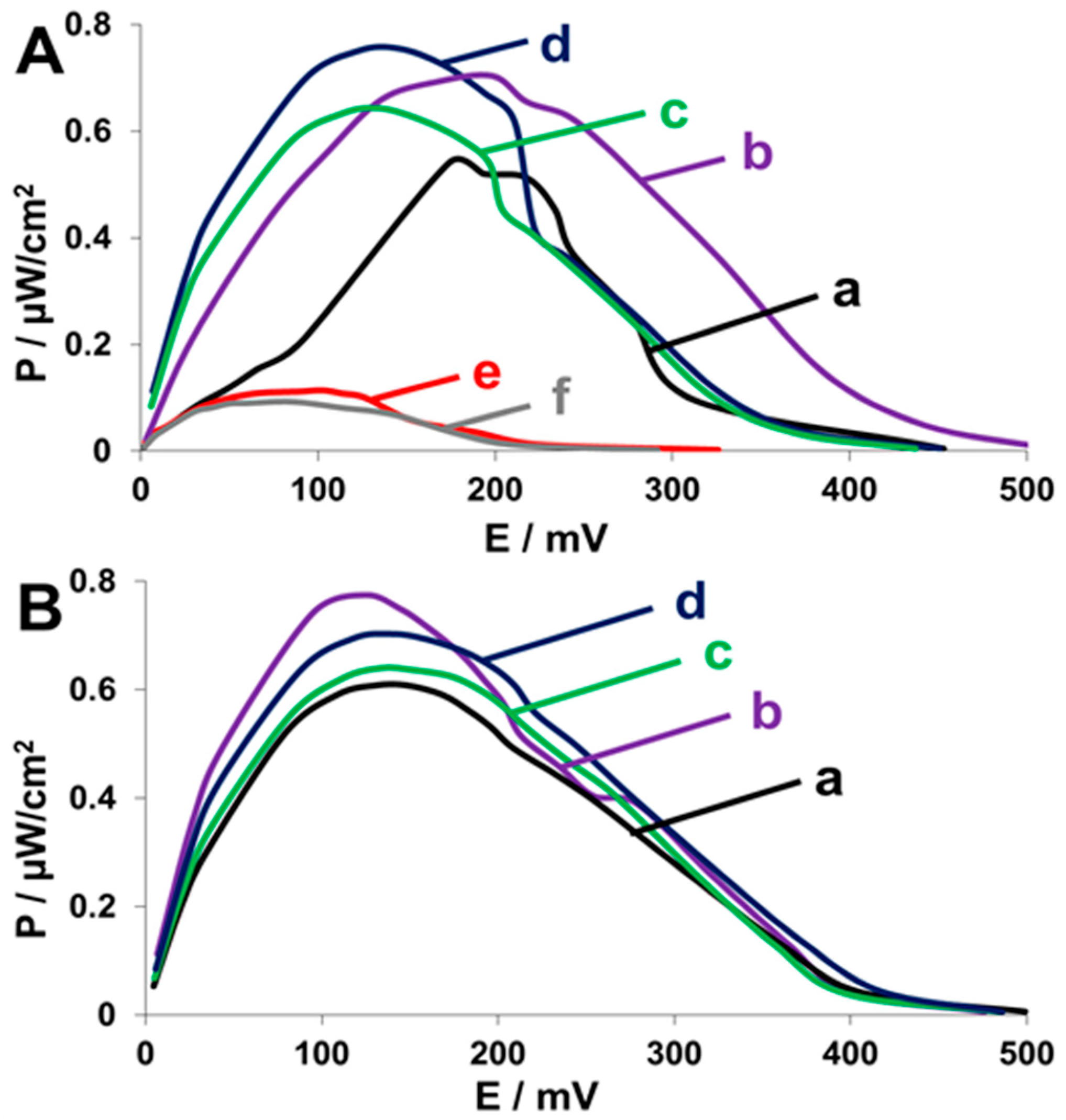
2.4. Fuel Cell Assembly and Characterization
3. Conclusions
4. Materials and Methods
4.1. Strains and Constructs
4.2. Protein Expression
4.3. Enzyme Activity Assays
4.4. Construction of YSD of Chimeric Scaffoldins
4.5. Enzyme Binding to Scaffoldin
4.6. Cyclic Voltammetry (CV) and Chronoamperometry (CA)
4.7. Biofuel-Cell Assembly and Characterization
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MFC | microbial fuel cell |
| YSD | yeast surface-display |
| ADH | alcohol dehydrogenase |
| AldDH | aldehyde dehydrogenase |
| FormDH | formaldehyde dehydrogenase |
| GOx | glucose oxidase |
| zADH | Zymomonas mobilis alcohol dehydrogenase |
| pFormDH | Pseudomonas putida formaldehyde dehydrogenase |
| CueO | Escherichia coli copper oxidase |
| BOD | bilirubin oxidase |
| Ac | Acetivibrio cellulolyticus |
| Ct | Clostridium thermocellum |
| CBM | Cellulose-binding module |
| CV | cyclic voltammetry |
| CA | chronoamperometry |
| MB | methylene blue |
| NAD+ | β-Nicotinamide adenine dinucleotide |
| OPD | o-phenylenediamine dihydrochloride |
| DMSO | dimethyl sulfoxide |
| IPTG | Isopropyl β-d-1-thiogalactopyranoside |
| PEG | polyethylene glycol |
| WT | wild type |
| EtOH | ethanol |
| MeOH | methanol |
| FACS | Fluorescence Activated Cell Sorting |
References
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Cracknell, J.A.; Vincent, K.A.; Armstrong, F.A. Enzymes as working or inspirational electrocatalysts for fuel cells and electrolysis. Chem. Rev. 2008, 108, 2439–2461. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.J.; Svoboda, V.; Lau, C.; Martin, G.; Minteer, S.D. Enzyme catalysed biofuel cells. Energy Environ. Sci. 2008, 1, 320–337. [Google Scholar] [CrossRef]
- Fishilevich, S.; Amir, L.; Fridman, Y.; Aharoni, A.; Alfonta, L. Surface-display of Redox Enzymes in Microbial Fuel Cells. J. Am. Chem. Soc. 2009, 131, 12052–12053. [Google Scholar] [CrossRef] [PubMed]
- Szczupak, A.; Alfonta, L. The Use of Yeast Surface-display in Biofuel Cells. In Yeast Surface-Display: Methods, Protocols, and Applications; Liu, B., Ed.; Springer: New York, NY, USA, 2015; pp. 261–268. [Google Scholar]
- Boder, E.T.; Wittrup, K.D. Yeast surface-display for screening combinatorial polypeptide libraries. Nat. Biotechnol. 1997, 15, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Szczupak, A.; Kol-Kalman, D.; Alfonta, L. A hybrid biocathode: Surface-display of O2-reducing enzymes for microbial fuel cell applications. Chem. Commun. 2012, 48, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Amir, L.; Carnally, S.A.; Rayo, J.; Rosenne, S.; Melamed Yerushalmi, S.; Schlesinger, O.; Meijler, M.M.; Alfonta, L. Surface-display of a redox enzyme and its site-specific wiring to gold electrodes. J. Am. Chem. Soc. 2013, 135, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Lattemann, C.T.; Maurer, J.; Gerland, E.; Meyer, T.F. Autodisplay: Functional Display of Active beta-Lactamase on the Surface of Escherichia coli by the AIDA-I Autotransporter. J. Bacteriol. 2000, 182, 3726–3733. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Ravenna, Y.; Alfonta, L. Layer-by-layer assembly of a redox enzyme displayed on the surface of elongated bacteria. Chem. Commun. 2015, 51, 2633–2636. [Google Scholar] [CrossRef] [PubMed]
- Bahartan, K.; Amir, L.; Israel, A.; Lichtenstein, R.G.; Alfonta, L. In situ fuel processing in a microbial fuel cell. ChemSusChem 2012, 5, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Gal, I.; Schlesinger, O.; Amir, L.; Alfonta, L. Bioelectrochemistry Yeast surface-display of dehydrogenases in microbial fuel-cells. Bioelectrochemistry 2016, 112, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Tam, T.K.; Sun, F.; You, C.; Zhang, Y.P. A high-energy-density sugar biobattery based on a synthetic enzymatic pathway. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Campbell, E.; Yu, J.; Minteer, S.D.; Banta, S. Complete oxidation of methanol in biobattery devices using a hydrogel created from three modified dehydrogenases. Angew. Chem. Int. Ed. 2013, 52, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Sokic-Lazic, D.; de Andrade, A.R.; Minteer, S.D. Utilization of enzyme cascades for complete oxidation of lactate in an enzymatic biofuel cell. Electrochim. Acta 2011, 56, 10772–10775. [Google Scholar] [CrossRef]
- Tasca, F.; Gorton, L.; Kujawa, M.; Patel, I.; Harreither, W.; Peterbauer, C.K.; Ludwig, R.; Nöll, G. Increasing the coulombic efficiency of glucose biofuel cell anodes by combination of redox enzymes. Biosens. Bioelectron. 2010, 25, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Wheeldon, I.; Minteer, S.D.; Banta, S.; Barton, S.C.; Atanassov, P.; Sigman, M. Substrate channelling as an approach to cascade reactions. Nat. Chem. 2016, 8, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, K.; Giroud, F.; Minteer, S.D. Improved Bioelectrocatalytic Oxidation of Sucrose in a Biofuel Cell with an Enzyme Cascade Assembled on a DNA Scaffold. J. Electrochem. Soc. 2014, 161, H930–H933. [Google Scholar] [CrossRef]
- Bayer, E.A.; Chanzy, H.; Lamed, R.; Shoham, Y. Cellulose, cellulases and cellulosomes. Curr. Opin. Struct. Biol. 1998, 8, 548–557. [Google Scholar] [CrossRef]
- Bayer, E.A.; Shimon, L.J.; Shoham, Y.; Lamed, R. Cellulosomes-structure and ultrastructure. J. Struct. Biol. 1998, 124, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Dias, F.M.V.; Prates, J.A.M.; Nagy, T.; Gilbert, H.J.; Davies, G.J.; Ferreira, L.M.A.; Romão, M.J.; Fontes, C.M.G.A. Cellulosome assembly revealed by the crystal structure of the cohesin-dockerin complex. Proc. Natl. Acad. Sci. USA 2003, 100, 13809–13814. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Dias, F.M.V.; Nagy, T.; Prates, J.A.M.; Proctor, M.R.; Smith, N.; Bayer, E.A.; Davies, G.J.; Ferreira, L.M.A.; Romão, M.J.; et al. Evidence for a dual binding mode of dockerin modules to cohesins. Proc. Natl. Acad. Sci. USA 2007, 104, 3089–3094. [Google Scholar] [CrossRef] [PubMed]
- Mechaly, A.; Fierobe, H.P.; Belaich, A.; Belaich, J.P.; Lamed, R.; Shoham, Y.; Bayer, E.A. Cohesin-dockerin interaction in cellulosome assembly: A single hydroxyl group of a dockerin domain distinguishes between nonrecognition and high affinity recognition. J. Biol. Chem. 2001, 276, 9883–9888. [Google Scholar] [CrossRef] [PubMed]
- Pagés, S.; Bélaïch, A.; Bélaïch, J.P.; Morag, E.; Lamed, R.; Shoham, Y.; Bayer, E.A. Species-specificity of the cohesin-dockerin interaction between Clostridium thermocellum and Clostridium cellulolyticum: Prediction of specificity determinants of the dockerin domain. Proteins Struct. Funct. Genet. 1997, 29, 517–527. [Google Scholar] [CrossRef]
- Bayer, E.A.; Morag, E.; Lamed, R. The cellulosome—A treasure-trove for biotechnology. Trends Biotechnol. 1994, 12, 379–386. [Google Scholar] [CrossRef]
- Fierobe, H.P.; Mingardon, F.; Mechaly, A.; Belaich, A.; Rincon, M.T.; Pagès, S.; Lamed, R.; Tardif, C.; Belaich, J.P.; Bayer, E.A. Action of designer cellulosomes on homogeneous versus complex substrates: Controlled incorporation of three distinct enzymes into a defined trifunctional scaffoldin. J. Biol. Chem. 2005, 280, 16325–16334. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Moraïs, S.; Lamed, R.; Bayer, E.A. Adaptor Scaffoldins: An Original Strategy for Extended Designer Cellulosomes, Inspired from Nature. mBio 2016, 7, e00083-16. [Google Scholar] [CrossRef] [PubMed]
- Moraïs, S.; Morag, E.; Barak, Y.; Goldman, D.; Hadar, Y.; Lamed, R.; Shoham, Y.; Wilson, D.B.; Bayer, E.A. Deconstruction of lignocellulose into soluble sugars by native and designer cellulosomes. MBio 2012, 3, e00508-12. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Myung, S.; Zhang, Y.P. Facilitated Substrate Channeling in a Self-Assembled Trifunctional Enzyme Complex. Angew. Chem. Int. Ed. 2012, 51, 8787–8790. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Zhang, Y.-H.P. Self-assembly of synthetic metabolons through synthetic protein scaffolds: One-step purification, co-immobilization, and substrate channeling. ACS Synth. Biol. 2013, 2, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-L.; DaSilva, N.A.; Chen, W. Functional display of complex cellulosomes on the yeast surface via adaptive assembly. ACS Synth. Biol. 2013, 2, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Sun, J.; Zhao, H. Yeast surface-display of trifunctional minicellulosomes for simultaneous saccharification and fermentation of cellulose to ethanol. Appl. Environ. Microbiol. 2010, 76, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Moraïs, S.; Shterzer, N.; Lamed, R.; Bayer, E.A.; Mizrahi, I. A combined cell-consortium approach for lignocellulose degradation by specialized Lactobacillus plantarum cells. Biotechnol. Biofuels 2014, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Banta, S.; Chen, W. Functional assembly of a multi-enzyme methanol oxidation cascade on a surface-displayed trifunctional scaffold for enhanced NADH production. Chem. Commun. 2013, 49, 3766–3768. [Google Scholar] [CrossRef] [PubMed]
- Boumans, H.; Grivell, L.A.; Berden, J.A. The Respiratory Chain in Yeast Behaves as a Single Functional Unit. J. Biol. Chem. 1998, 273, 4872–4877. [Google Scholar] [CrossRef] [PubMed]
- Outten, F.W.; Huffman, D.L.; Hale, J.A.; Halloran, T.V.O. The Independent cue and cus Systems Confer Copper Tolerance during Aerobic and Anaerobic Growth in Escherichia coli. J. Biol. Chem. 2001, 276, 30670–30677. [Google Scholar] [CrossRef] [PubMed]
- Vazana, Y.; Barak, Y.; Unger, T.; Peleg, Y.; Shamshoum, M.; Ben-Yehezkel, T.; Mazor, Y.; Shapiro, E.; Lamed, R.; Bayer, E.A. A synthetic biology approach for evaluating the functional contribution of designer cellulosome components to deconstruction of cellulosic substrates. Biotechnol. Biofuels 2013, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Vazana, Y.; Morais, S.; Barak, Y.; Lamed, R.; Bayer, E.A. Designer cellulosomes for enhanced hydrolysis of cellulosic substrates. Methods Enzymol. 2012, 510, 429–452. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczupak, A.; Aizik, D.; Moraïs, S.; Vazana, Y.; Barak, Y.; Bayer, E.A.; Alfonta, L. The Electrosome: A Surface-Displayed Enzymatic Cascade in a Biofuel Cell’s Anode and a High-Density Surface-Displayed Biocathodic Enzyme. Nanomaterials 2017, 7, 153. https://doi.org/10.3390/nano7070153
Szczupak A, Aizik D, Moraïs S, Vazana Y, Barak Y, Bayer EA, Alfonta L. The Electrosome: A Surface-Displayed Enzymatic Cascade in a Biofuel Cell’s Anode and a High-Density Surface-Displayed Biocathodic Enzyme. Nanomaterials. 2017; 7(7):153. https://doi.org/10.3390/nano7070153
Chicago/Turabian StyleSzczupak, Alon, Dror Aizik, Sarah Moraïs, Yael Vazana, Yoav Barak, Edward A. Bayer, and Lital Alfonta. 2017. "The Electrosome: A Surface-Displayed Enzymatic Cascade in a Biofuel Cell’s Anode and a High-Density Surface-Displayed Biocathodic Enzyme" Nanomaterials 7, no. 7: 153. https://doi.org/10.3390/nano7070153