Biodegradable FeMnSi Sputter-Coated Macroporous Polypropylene Membranes for the Sustained Release of Drugs
Abstract
:1. Introduction
2. Materials and Methods
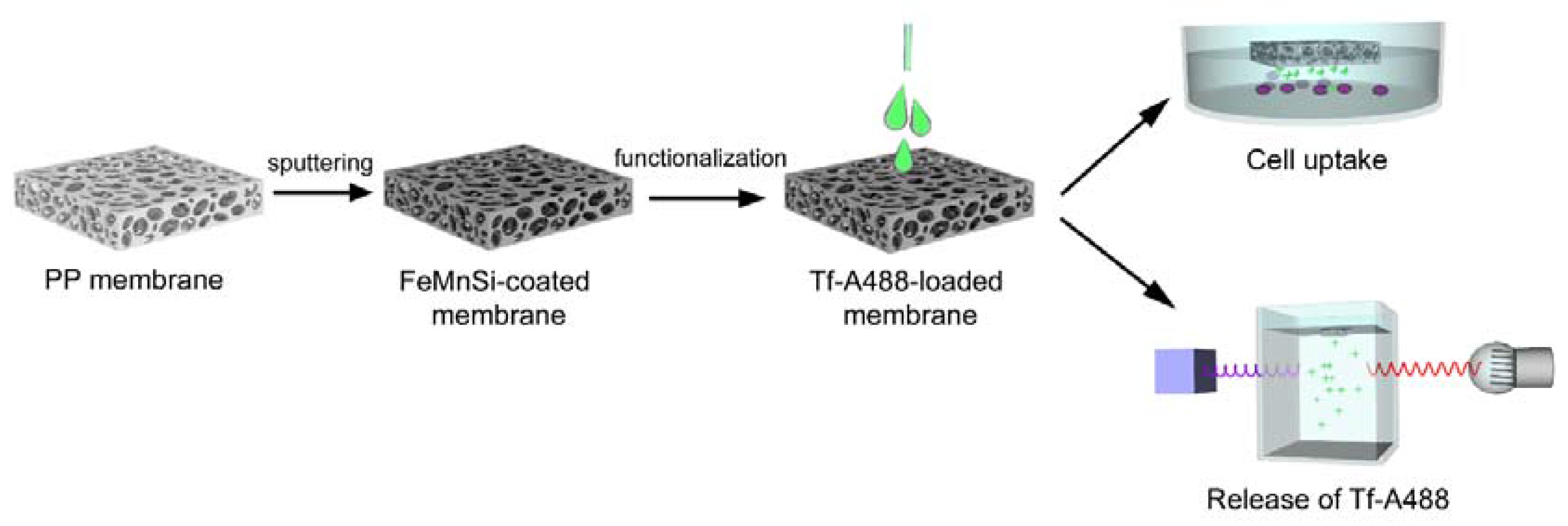
2.1. Preparation and Characterization of the Macroporous Fe- and FeMnSi-Coated PP Membranes
2.1.1. Sputtering of Fe and FeMnSi on Macroporous PP Membranes
2.1.2. Structural and Magnetic Characterization of the Fe- and FeMnSi-coated PP Membranes
2.1.3. Biodegradability Studies
2.1.4. Cell Culture
2.1.5. MTT Assay
2.2. Functionalization of the Macroporous FeMnSi-Coated PP Membranes
2.3. Characterization and Performance of the Tf-AF488-Loaded Membranes
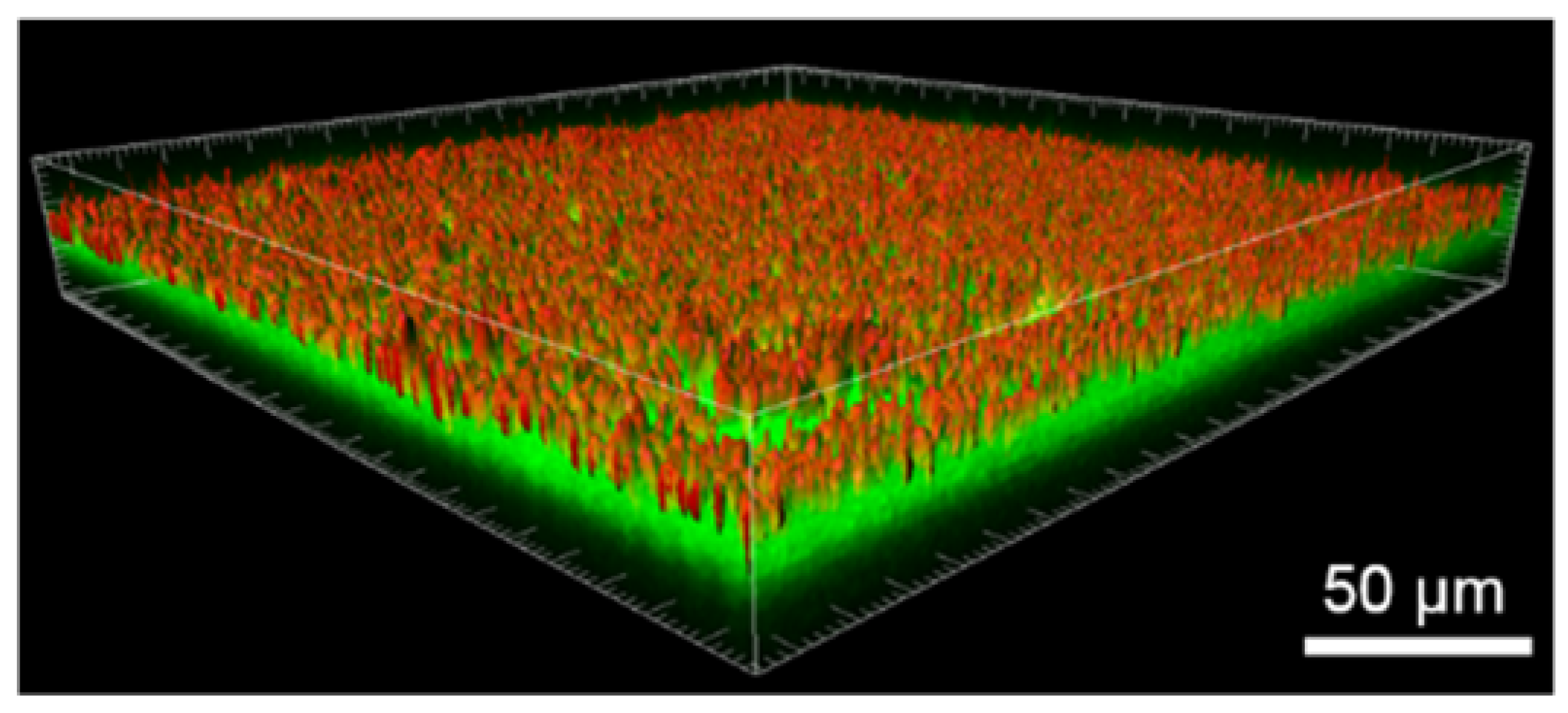
2.3.1. Distribution of Tf-AF488
2.3.2. Release of Tf-AF488
2.3.3. Tf-AF488 Uptake by Cells
3. Results and Discussion
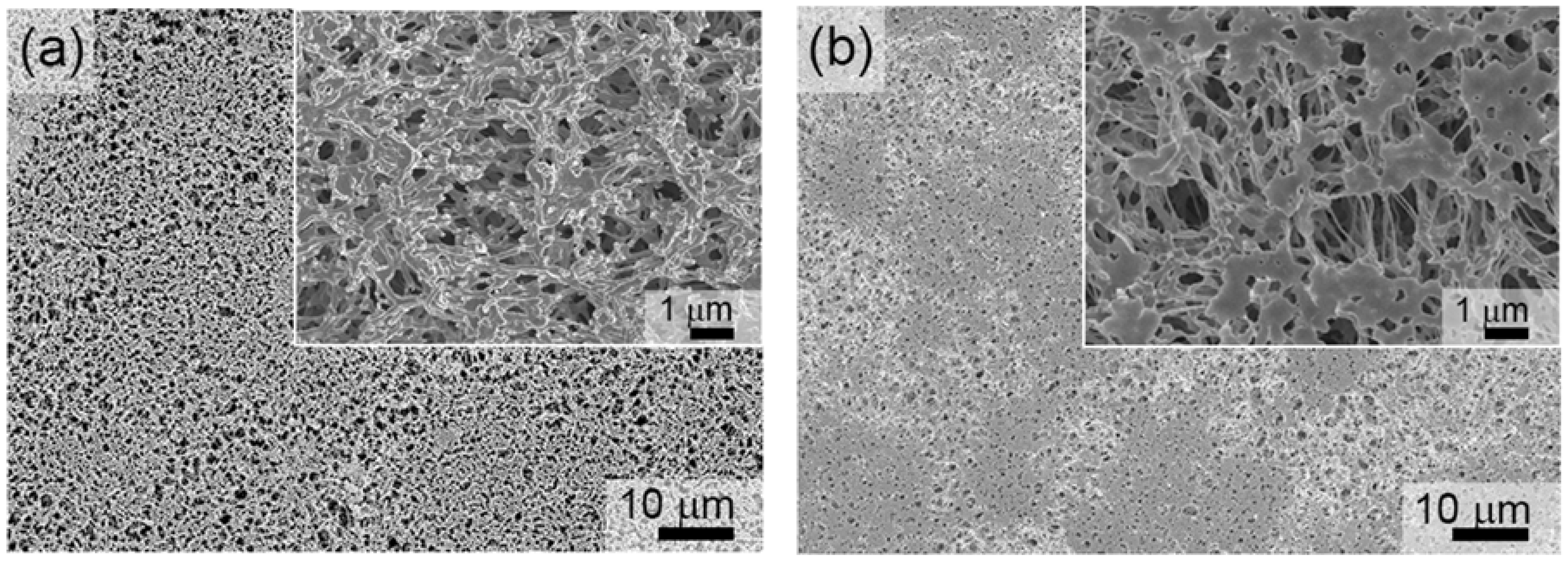
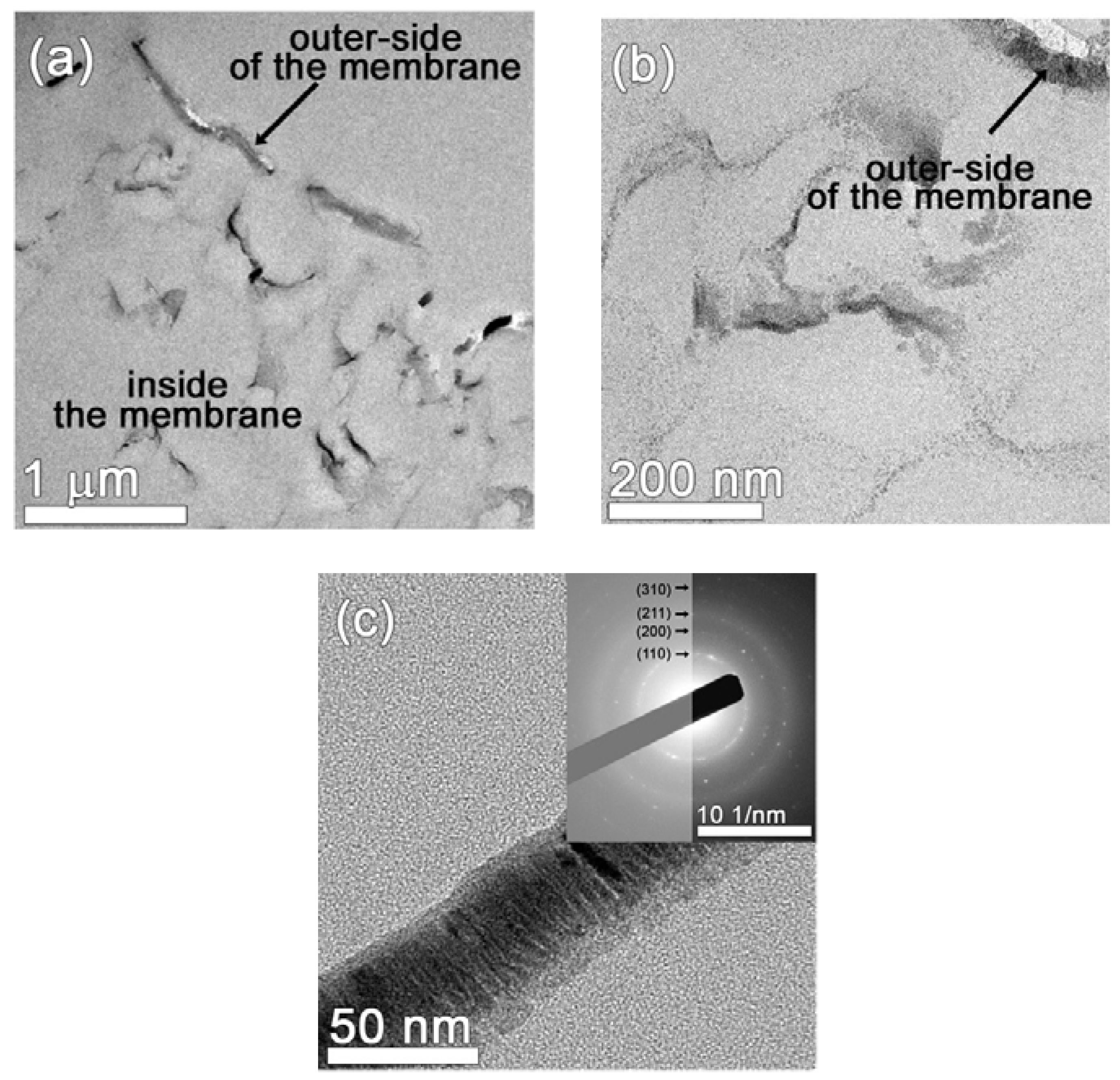
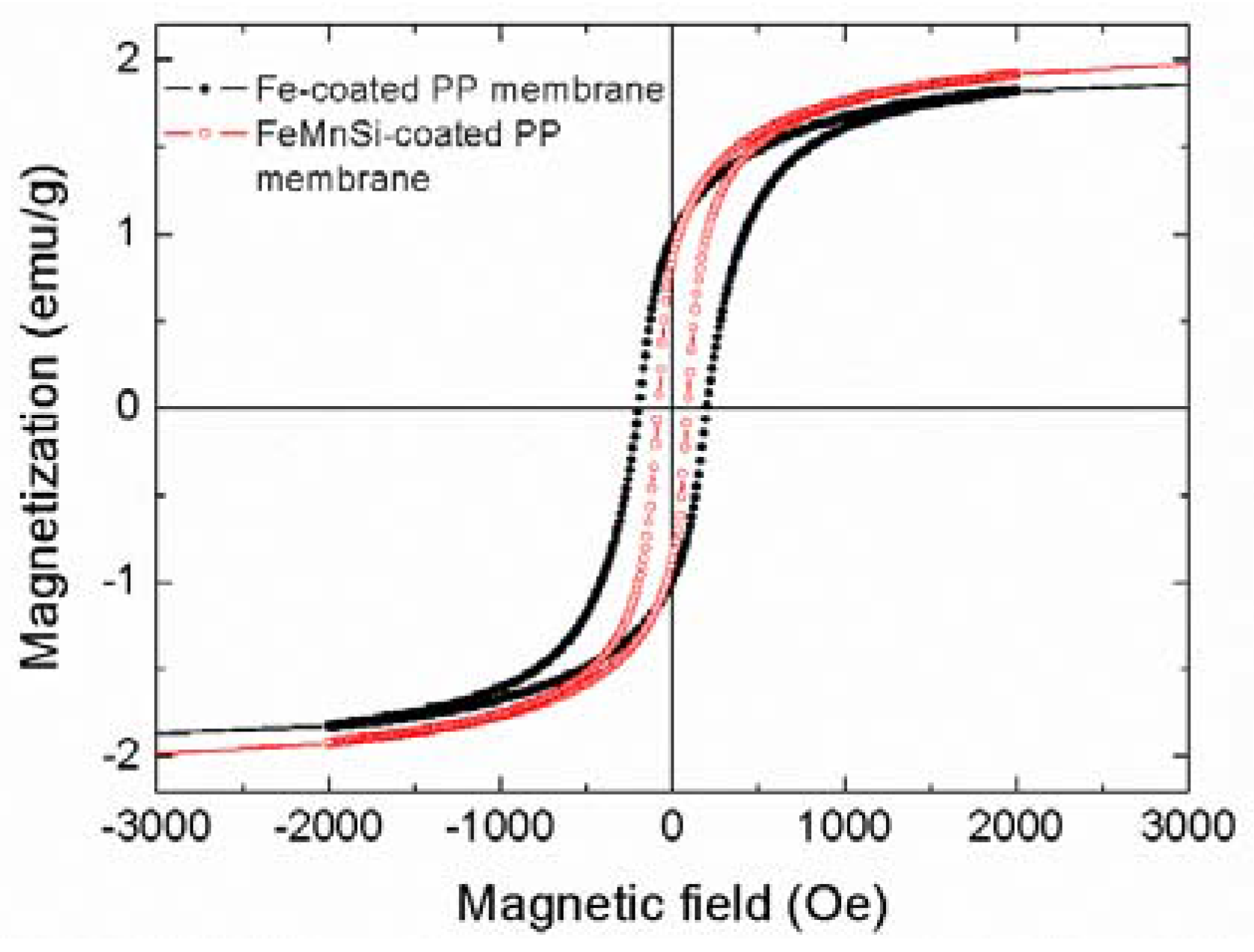
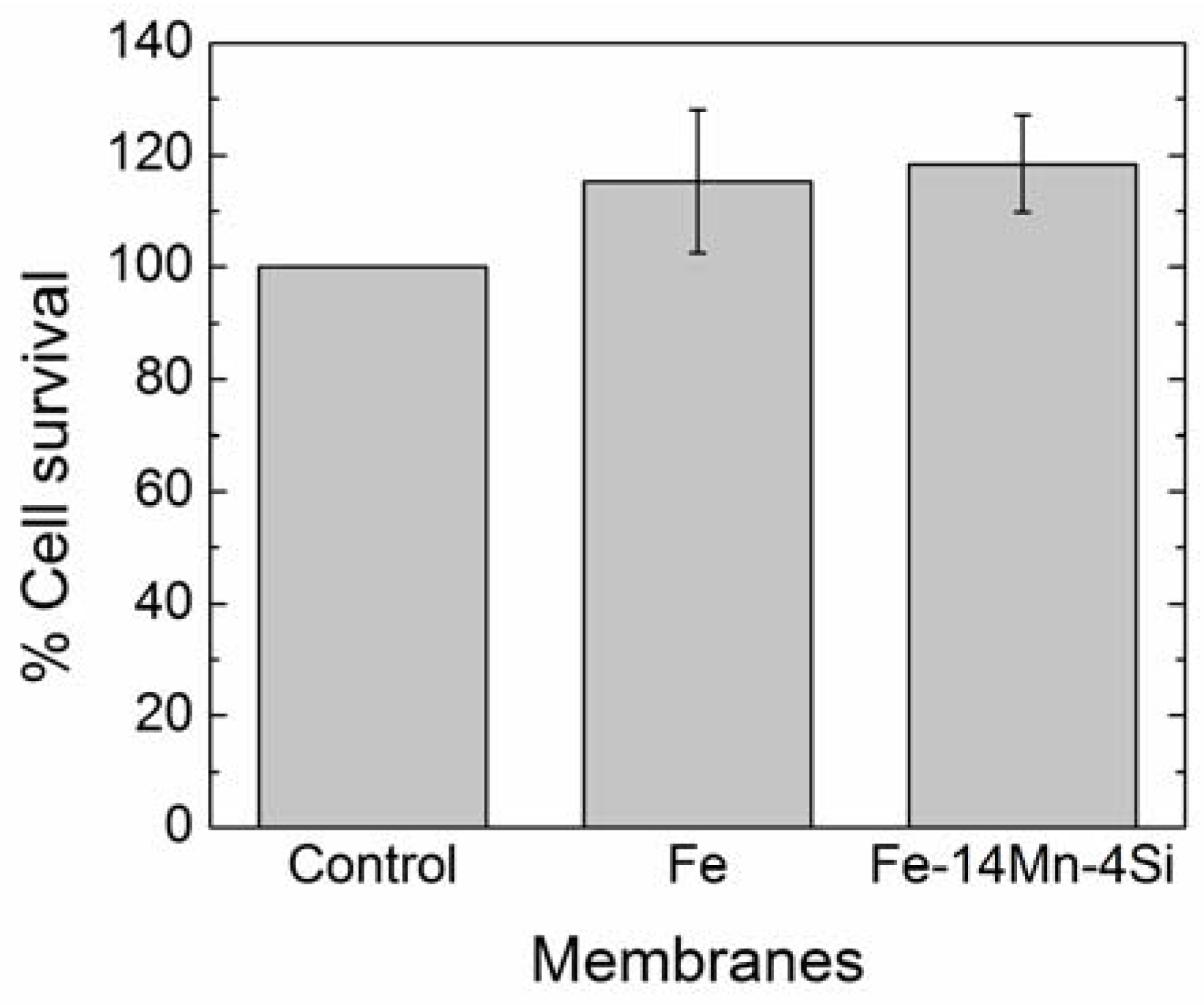
3.1. Characterization of the Fe- and FeMnSi-Coated Membranes
3.1.1. Structural and Morphological Characterization
3.1.2. Magnetic Characterization
3.1.3. Biodegradability
3.1.4. Cytotoxicity Results
3.2. Transferrin-Alexa Fluor 488 Functionalization of the FeMnSi-Coated Membranes
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Nelson, B.J.; Kaliakatsos, I.K.; Abbott, J.J. Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 2010, 12, 55–85. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Chen, Y.C.; Hackett, M.J.; Huang, L. Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol. Ther. 2007, 16, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E. BioMEMS and Biomedical Technology. In Therapeutic Micro/Nanotechnology; Desai, T., Bhatia, S., Eds.; Springer: New York, NY, USA, 2006; Volume III. [Google Scholar]
- Caka, M.; Türkcan, C.; Uygun, D.A.; Uygun, M.; Akgöl, S.; Denizli, A. Controlled release of curcumin from poly(HEMA-MAPA) membrane. Artif. Cells Nanomed. Biotechnol. 2017, 45, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Salazar, H.; Lima, A.C.; Lopes, A.C.; Botelho, G.; Lanceros-Mendez, S. Poly(vinylidene fluoride-trifluoroethylene)/NAY zeolite hybrid membranes as a drug release platform applied to ibuprofen release. Colloid. Sur. A 2015, 469, 93–99. [Google Scholar] [CrossRef]
- Temtem, M.; Pompeu, D.; Jaraquemada, G.; Cabrita, E.J.; Casimiro, T.; Aguiar-Ricardo, A. Development of PMMA membranes functionalized with hydroxypropyl-β-cyclodextrins for controlled drug delivery using a supercritical CO2-assisted technology. Int. J. Pharm. 2009, 376, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Minelli, E.B.; Bora, T.D.; Benini, A. Different microbial biofilm formation on polymethylmethacrylate [PMMA] bone cement loaded with gentamicin and vancomycin. Anaerobe 2011, 17, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Rochford, E.T.; Richards, R.G.; Moriarty, T.F. Influence of material on the development of device-associated infections. Clin. Microbiol. Infect. 2012, 18, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G.; Costerton, J.W. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J. Bone Jt. Surg. Am. 1985, 67, 264–273. [Google Scholar] [CrossRef]
- Kummer, M.P.; Abbott, J.J.; Kratochvil, B.E.; Borer, R.; Sengul, A.; Nelson, B.J. OctoMag: An electromagnetic system for 5-DOF wireless micromanipulation. IEEE Trans. Robot. 2010, 26, 1006–1017. [Google Scholar] [CrossRef]
- Zhang, L.; Peyer, K.E.; Nelson, B.J. Artificial bacterial fagella for micromanipulation. Lab Chip 2010, 10, 2203–2215. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.R.; Santos, G.; Barata, P.; Oliveira, R.; Lopes, C.M. Physical and chemical stimuli-responsive drug delivery systems: Targeted delivery and main routes of administration. Curr. Pharm. Des. 2013, 19, 7169–7184. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rezac, M.E.; Pfromm, P.H. Partial hydrogenation of soybean oil with minimal trans fat production using a Pt-decorated polymeric membrane reactor. J. Am. Oil Chem. Soc. 2009, 86, 93–101. [Google Scholar] [CrossRef]
- Himy, A.; Wagner, O.C. Stabilized Nickel-Zinc Battery. U.S. Patent 4327157A, 27 April 1982. [Google Scholar]
- Iakovlev, V.; Guelcher, S.; Bendavid, R. Degradation of polypropylene in vivo: A microscopic analysis of meshes explanted from patients. J. Biomed. Mater. Res. B 2017, 105, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, Y.F. Effects of alloying elements [Mn, Co, Al, W, Sn, B, C and S] on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011, 7, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J. Degradation performance of biodegradable Fe Mn C (Pd) alloys. Mat. Sci. Eng. C 2013, 33, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.P.; Blanquer, A.; Fornell, J.; Zhang, H.; Solsona, P.; Baró, M.D.; Suriñach, S.; Ibáñez, E.; García-Lecina, E.; Wei, X.; et al. Novel Fe–Mn–Si–Pd alloys: Insights into mechanical, magnetic, corrosion resistance and biocompatibility performances. J. Mat. Chem. B 2016, 4, 6402–6412. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mat. Sci. Eng. R 2014, 77, 1–34. [Google Scholar] [CrossRef]
- ASTM G31-72. Standard Practice for Laboratory Immersion Corrosion Testing of Metals; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Kawamoto, M.; Horibe, T.; Kohno, M.; Kawakami, K. A novel transferrin receptor-targeted hybrid peptide disintegrates cancer cell membrane to induce rapid killing of cancer cells. BMC Cancer 2011, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomater. 2010, 6, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H.; Purnama, A.; Dube, D.; Couet, J.; Mantovani, D. Fe–Mn alloys for metallic biodegradable stents: Degradation and cell viability studies. Acta Biomater. 2010, 6, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H.; Alamadari, H.; Mantovani, D.; Dubé, D. Iron-manganese: New class of metallic degradable biomaterials prepared by powder metallurgy. Powder Metall. 2008, 51, 38–45. [Google Scholar] [CrossRef]
- Moravej, M.; Purnama, A.; Fiset, M.; Couet, J.; Mantovani, D. Electroformed pure iron as a new biomaterial for degradable stents: In vitro degradation and preliminary cell viability studies. Acta Biomater. 2010, 6, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Baravalle, G.; Schober, D.; Huber, M.; Bayer, N.; Murphy, R.F.; Fuchs, R. Transferrin recycling and dextran transport to lysosomes is differentially affected by bafilomycin, nocodazole, and low temperature. Cell Tissue Res. 2005, 320, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.A. Monitoring of Respiration and Circulation; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
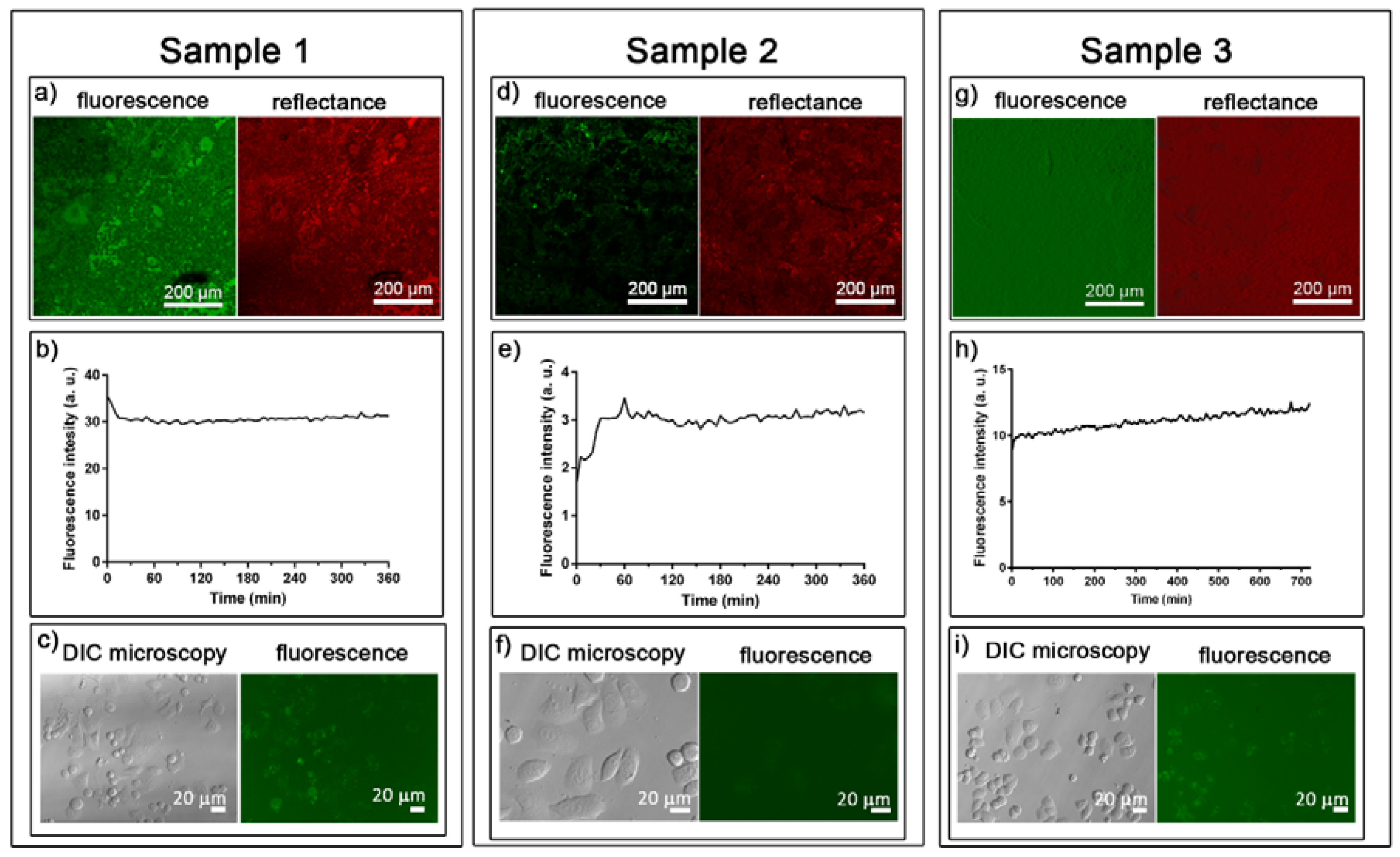
| Steps (# of Times) | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|
| Loading with Tf-AF488 | 1 | 1 | 3 |
| Cleaning with HBSS | 0 | 1 | 1 |
| Drying under vacuum | 1 | 1 | 1 |
| Stopper | 0 | 1 | 2 |
| Drying under vacuum | 0 | 1 | 1 |
| Membrane Type | [Fe] (μg L−1) | [Mn] (μg L−1) |
|---|---|---|
| PP membrane | <10 | <10 |
| Fe | <10 | <10 |
| Fe-14Mn-4Si | 19 ± 6 | 17 ± 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornell, J.; Soriano, J.; Guerrero, M.; Sirvent, J.D.D.; Ferran-Marqués, M.; Ibáñez, E.; Barrios, L.; Baró, M.D.; Suriñach, S.; Nogués, C.; et al. Biodegradable FeMnSi Sputter-Coated Macroporous Polypropylene Membranes for the Sustained Release of Drugs. Nanomaterials 2017, 7, 155. https://doi.org/10.3390/nano7070155
Fornell J, Soriano J, Guerrero M, Sirvent JDD, Ferran-Marqués M, Ibáñez E, Barrios L, Baró MD, Suriñach S, Nogués C, et al. Biodegradable FeMnSi Sputter-Coated Macroporous Polypropylene Membranes for the Sustained Release of Drugs. Nanomaterials. 2017; 7(7):155. https://doi.org/10.3390/nano7070155
Chicago/Turabian StyleFornell, Jordina, Jorge Soriano, Miguel Guerrero, Juan De Dios Sirvent, Marta Ferran-Marqués, Elena Ibáñez, Leonardo Barrios, Maria Dolors Baró, Santiago Suriñach, Carme Nogués, and et al. 2017. "Biodegradable FeMnSi Sputter-Coated Macroporous Polypropylene Membranes for the Sustained Release of Drugs" Nanomaterials 7, no. 7: 155. https://doi.org/10.3390/nano7070155





