Thermoelectric and Transport Properties of Delafossite CuCrO2:Mg Thin Films Prepared by RF Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Mg-Doped CuCrO2 (Target)
2.2. Preparation of Mg-Doped CuCrO2 Thin Films
2.3. Characterization
3. Results and Discussion
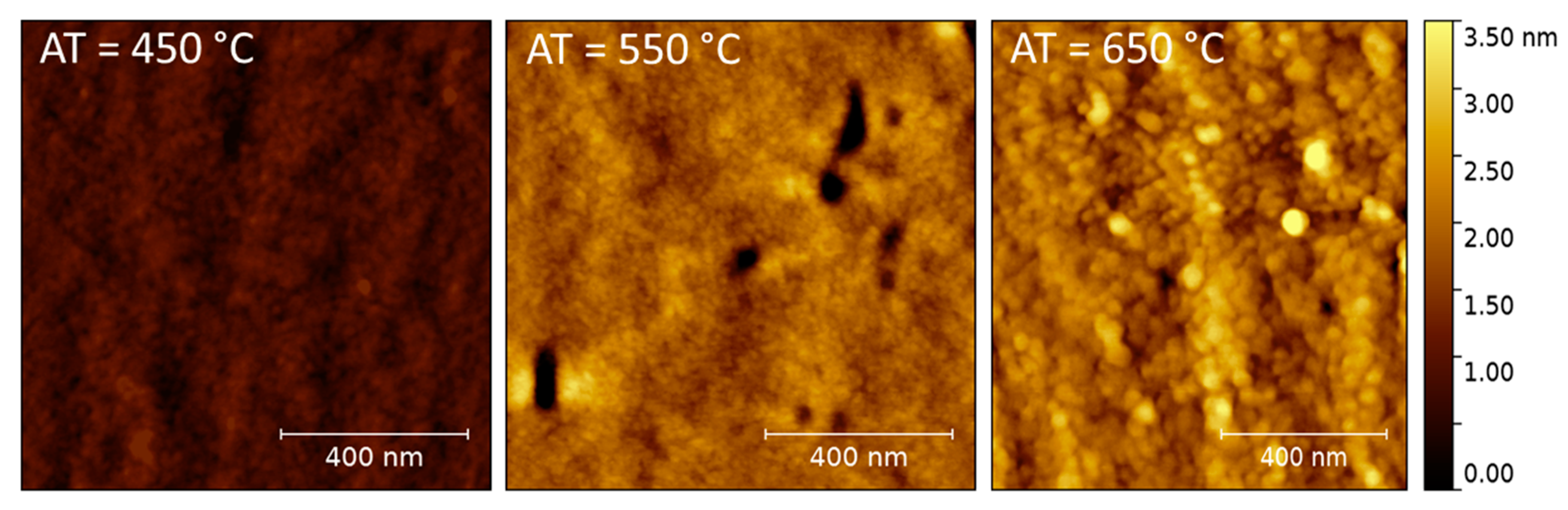
3.1. Structural and Microstructural Characterizations
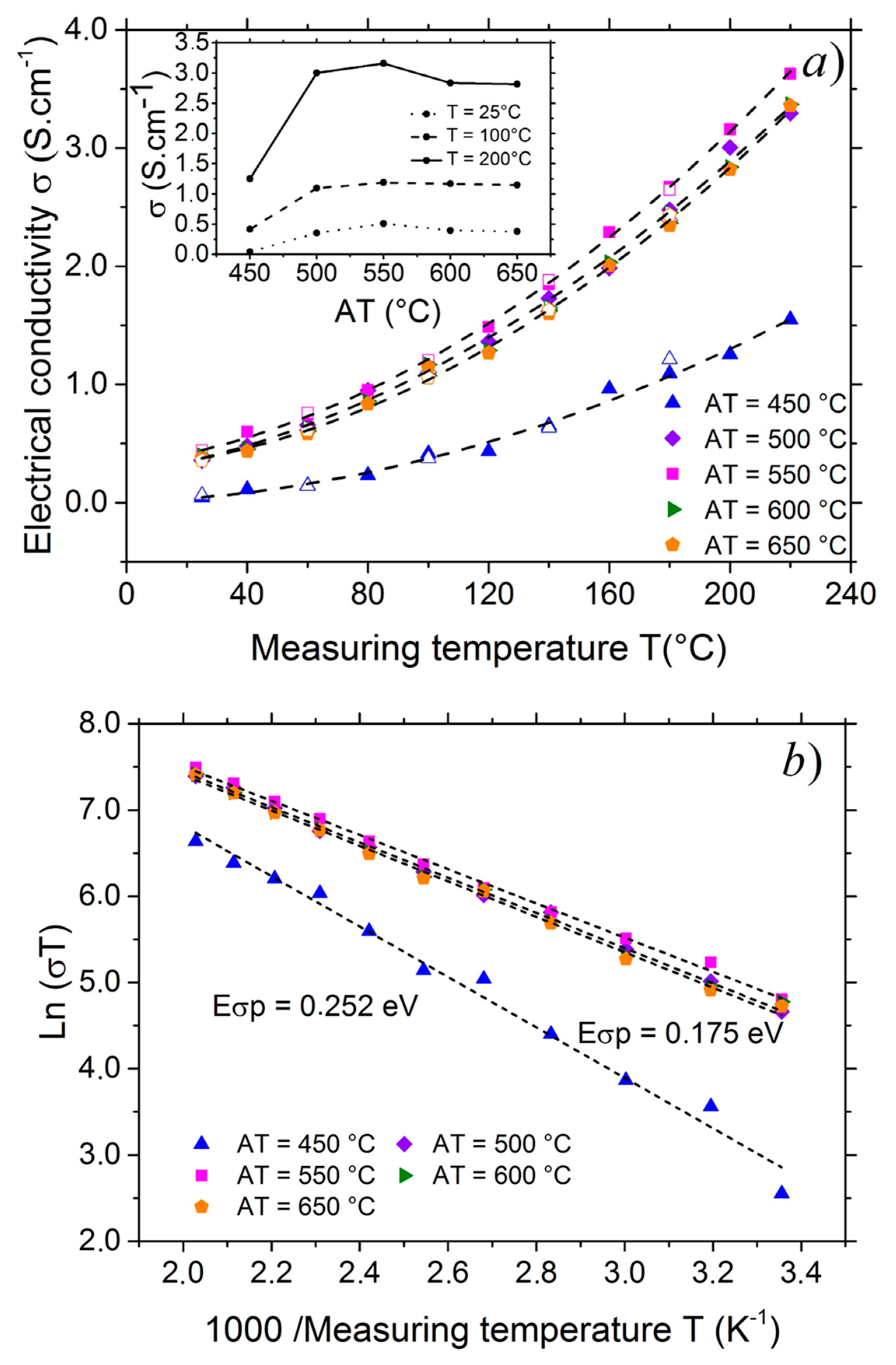
3.2. Transport Properties
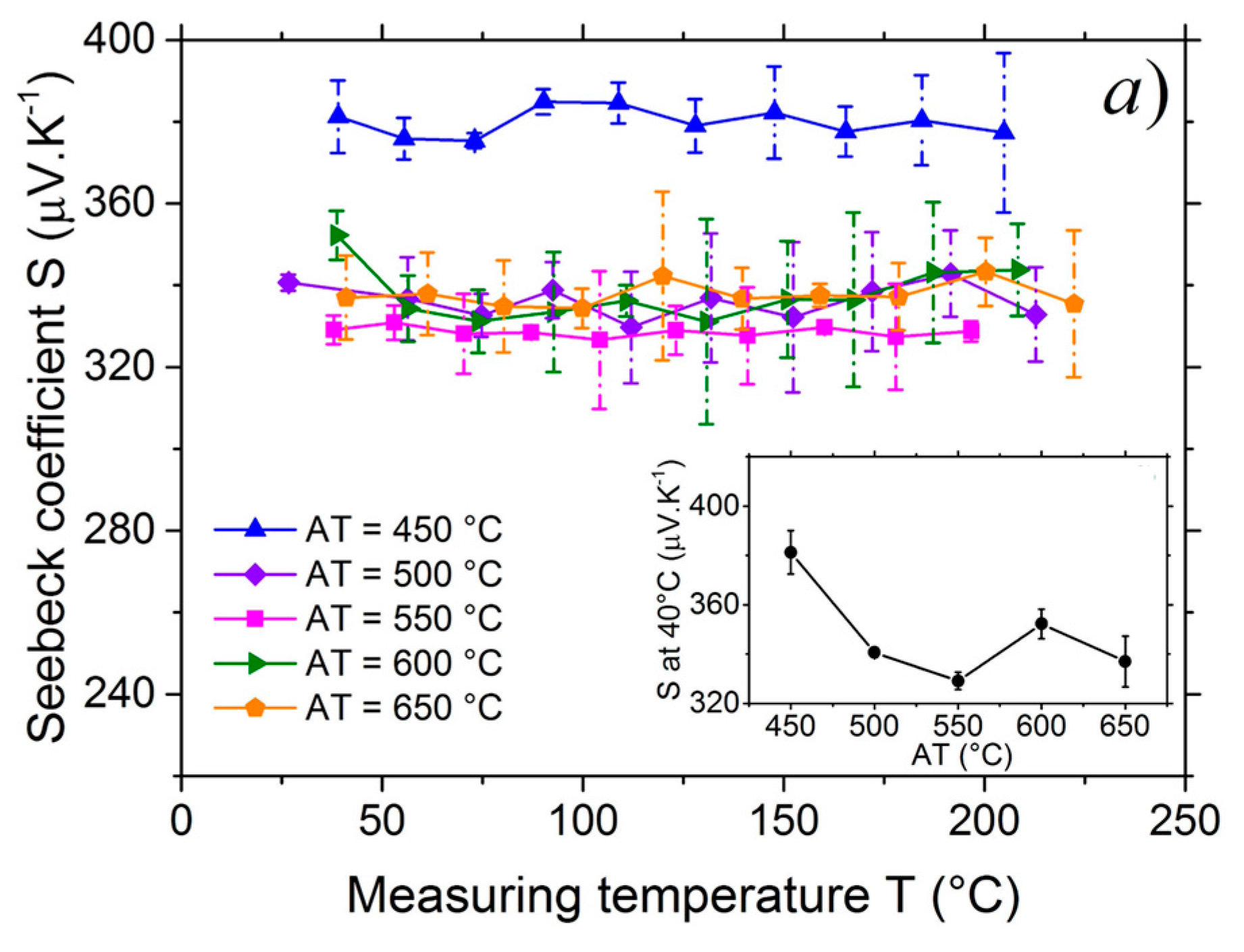
3.3. Seebeck Coefficient Measurement
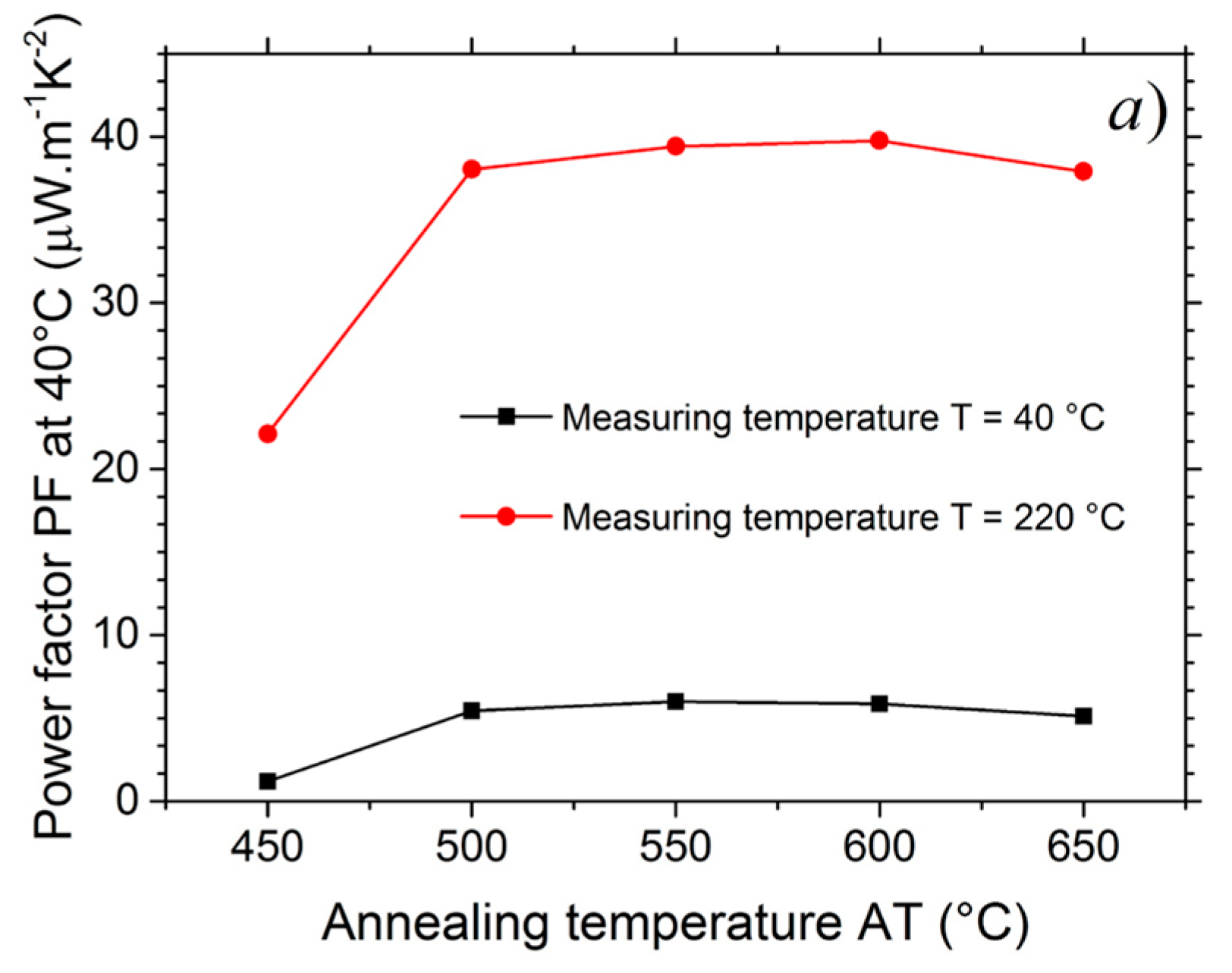
3.4. Power Factor (PF)
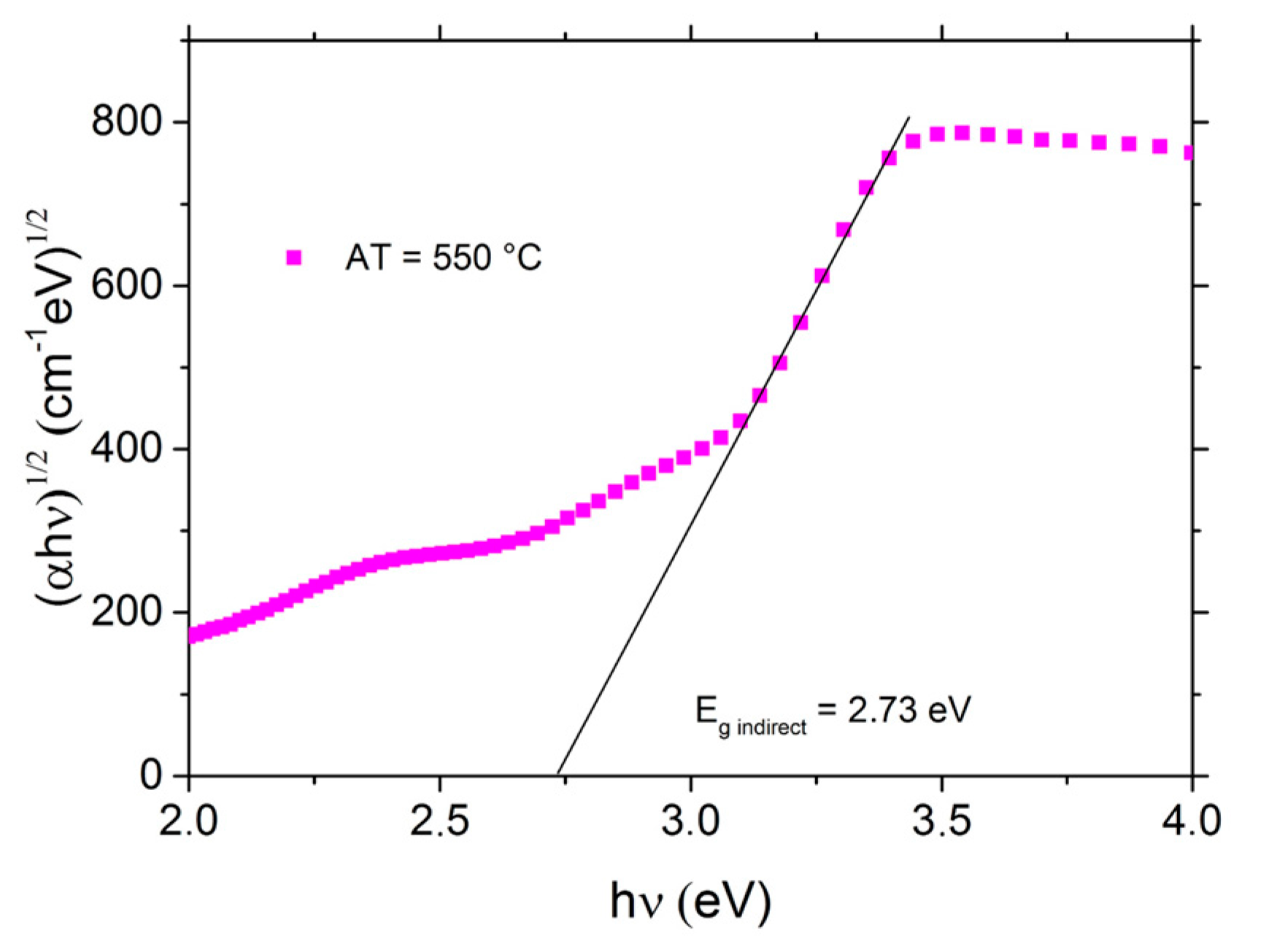
3.5. Optoelectrical Properties and Fermi Energy Level
3.6. Carrier Concentration and Hole Effective Mass
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rowe, D.M. CRC Handbook of Thermoelectrics; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Alam, H.; Ramakrishna, S. A review on the enhancement of figure of merit from bulk to nano-thermoelectric materials. Nano Energy 2013, 2, 190–212. [Google Scholar] [CrossRef]
- Martin, J.; Tritt, T.; Uher, C. High temperature Seebeck coefficient metrology. J. Appl. Phys. 2010, 108, 121101. [Google Scholar] [CrossRef]
- Aswal, D.K.; Basu, R.; Singh, A. Key issues in development of thermoelectric power generators: High figure-of-merit materials and their highly conducting interfaces with metallic interconnects. Energy Convers. Manag. 2016, 114, 50–67. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.G.; Lee, H.; Wang, D.Z.; Ren, Z.F.; Fleurial, J.-P.; Gogna, P. New Directions for Low-Dimensional Thermoelectric Materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Han, G.; Yang, L.; Cheng, L.; Zou, J. Nanostructured thermoelectric materials: Current research and future challenge. Prog. Nat. Sci. Mater. Int. 2012, 22, 535–549. [Google Scholar] [CrossRef]
- Cahill, D.G.; Fischer, H.E.; Klitsner, T.; Swartz, E.T.; Pohl, R.O. Thermal conductivity of thin films: Measurements and understanding. J. Vac. Sci. Technol. A Vac. Surf. Films 1989, 7, 1259–1266. [Google Scholar] [CrossRef]
- Loureiro, J.; Santos, J.R.; Nogueira, A.; Wyczisk, F.; Divay, L.; Reparaz, S.; Alzina, F.; Sotomayor Torres, C.M.; Cuffe, J.; Montemor, F.; et al. Nanostructured p-type Cr/V2O5 thin films with boosted thermoelectric properties. J. Mater. Chem. A 2014, 2, 6456–6462. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Siivola, E.; O’Quinn, B.; Coonley, K.; Colpitts, T.; Addepalli, P.; Napier, M.; Mantini, M. Nanostructured Superlattice Thin-Film Thermoelectric Devices. In Nanotechnology and the Environment; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2004; pp. 347–352. [Google Scholar]
- Benko, F.A.A.; Koffyberg, F.P.P. Preparation and opto-electronic properties of semi-conducting CuCrO2. Mater. Res. Bull. 1986, 21, 753–757. [Google Scholar] [CrossRef]
- Kawazoe, H.; Yasukawa, M.; Hyodo, H.; Kurita, M.; Yanagi, H.; Hosono, H. p-type electrical conduction in transparent thin films of CuAlO2. Nature 1997, 389, 939–942. [Google Scholar] [CrossRef]
- Nagarajan, R.; Duan, N.; Jayaraj, M.K.; Li, J.; Vanaja, K.A.; Yokochi, A.; Draeseke, A.; Tate, J.; Sleight, A.W. p-Type conductivity in the delafossite structure. Int. J. Inorg. Mater. 2001, 3, 265–270. [Google Scholar] [CrossRef]
- Yanagi, H.; Hase, T.; Ibuki, S.; Ueda, K.; Hosono, H. Bipolarity in electrical conduction of transparent oxide semiconductor CuInO2 with delafossite structure. Appl. Phys. Lett. 2001, 78, 1583–1585. [Google Scholar] [CrossRef]
- Banerjee, A.N.; Chattopadhyay, K.K. Recent developments in the emerging field of crystalline p-type transparent conducting oxide thin films. Prog. Cryst. Growth Charact. Mater. 2005, 50, 52–105. [Google Scholar] [CrossRef]
- Stadler, A. Transparent Conducting Oxides-An Up-To-Date Overview. Materials 2012, 5, 661–683. [Google Scholar] [CrossRef]
- Barnabé, A.; Thimont, Y.; Lalanne, M.; Presmanes, L.; Tailhades, P. P-type conducting transparent characteristics of delafossite Mg-doped CuCrO2 thin films preparation by RF-sputtering. J. Mater. Chem. C 2015, 3, 6012–6024. [Google Scholar] [CrossRef]
- Zhang, K.H.L.; Xi, K.; Blamire, M.G.; Egdell, R.G. P-type transparent conducting oxides. J. Phys. Condens. Matter 2016, 28, 383002. [Google Scholar] [CrossRef] [PubMed]
- Barquinha, P.; Martins, R.; Pereira, L.; Fortunato, E. Transparent Oxide Electronics: From Materials to Devices; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Wager, J.F.; Kesler, D.A.; Presley, D.A. Transparent Electronics; Springer: New York, NY, USA, 2008. [Google Scholar]
- Tonooka, K.; Kikuchi, N. Preparation of transparent CuCrO2: Mg/ZnO p-n junctions by pulsed laser deposition. Thin Solid Films 2006, 515, 2415–2418. [Google Scholar] [CrossRef]
- Hosono, H.; Ota, H.; Kawamura, K.; Sarukura, N.; Hirano, M. Light-Emitting Diode and Laser Diode Having N-Type ZnO Layer and P-Type Semiconductor Laser. U.S. Patent 6,806,503, 19 October 2004. [Google Scholar]
- Yanagi, H.; Ueda, K.; Ohta, H.; Orita, M.; Hirano, M.; Hosono, H. Fabrication of all oxide transparent p-n homojunction using bipolar CuInO2 semiconducting oxide with delafossite structure. Solid State Commun. 2002, 121, 15–18. [Google Scholar] [CrossRef]
- Ling, B.; Zhao, J.L.; Sun, X.W.; Tan, S.T.; Kyaw, A.K.K.; Divayana, Y.; Dong, Z.L. Color tunable light-emitting diodes based on p+-Si/p-CuAlO2 /n-ZnO nanorod array heterojunctions. Appl. Phys. Lett. 2010, 97, 13101. [Google Scholar] [CrossRef]
- Chiu, T.W.; Tonooka, K.; Kikuchi, N. Fabrication of ZnO and CuCrO2: Mg thin films by pulsed laser deposition with in situ laser annealing and its application to oxide diodes. Thin Solid Films 2008, 516, 5941–5947. [Google Scholar] [CrossRef]
- Tate, J.; Jayaraj, M.K.K.; Draeseke, A.D.D.; Ulbrich, T.; Sleight, A.W.W.; Vanaja, K.A.; Nagarajan, R.; Wager, J.F.F.; Hoffman, R.L.L. p-Type oxides for use in transparent diodes. Thin Solid Films 2002, 411, 119–124. [Google Scholar] [CrossRef]
- Renaud, A.; Cario, L.; Deniard, P.; Gautron, E.; Rocquefelte, X.; Pellegrin, Y.; Blart, E.; Odobel, F.; Jobic, S. Impact of Mg Doping on Performances of CuGaO2 Based p-Type Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 54–59. [Google Scholar] [CrossRef]
- Asemi, M.; Ghanaatshoar, M. Hydrothermal growth of one-dimensional Ce-doped TiO2 nanostructures for solid-state DSSCs comprising Mg-doped CuCrO2. J. Mater. Sci. 2017, 52, 489–503. [Google Scholar] [CrossRef]
- Zhu, T.; Deng, Z.; Fang, X.; Huo, Z.; Wang, S.; Dong, W.; Shao, J.; Tao, R.; Song, C.; Wang, L. High photovoltages of CuFeO2 based p-type dye-sensitized solar cells. J. Alloys Compd. 2016, 685, 836–840. [Google Scholar] [CrossRef]
- Jiang, T.; Bujoli-Doeuff, M.; Farré, Y.; Blart, E.; Pellegrin, Y.; Gautron, E.; Boujtita, M.; Cario, L.; Odobel, F.; Jobic, S. Copper borate as a photocathode in p-type dye-sensitized solar cells. RSC Adv. 2016, 6, 1549–1553. [Google Scholar] [CrossRef]
- Wuttig, A.; Krizan, J.W.; Gu, J.; Frick, J.J.; Cava, R.J.; Bocarsly, A.B. The effect of Mg-doping and Cu nonstoichiometry on the photoelectrochemical response of CuFeO2. J. Mater. Chem. A 2017, 5, 165–171. [Google Scholar] [CrossRef]
- Toyoda, K.; Hinogami, R.; Miyata, N.; Aizawa, M. Calculated Descriptors of Catalytic Activity for Water Electrolysis Anode: Application to Delafossite Oxides. J. Phys. Chem. C 2015, 119, 6495–6501. [Google Scholar] [CrossRef]
- Carcia, P.F.; Shannon, R.D.; Bierstedt, P.E.; Flippen, R.B. Oxygen electrocatalysis on Thin Film Metallic Oxide Electrodes with the Delafossite Structure. J. Electrochem. Soc. 1980, 127, 1974. [Google Scholar] [CrossRef]
- Dong, H.; Li, Z.; Xu, X.; Ding, Z.; Wu, L.; Wang, X.; Fu, X. Visible light-induced photocatalytic activity of delafossite AgMO2 (M=Al, Ga, In) prepared via a hydrothermal method. Appl. Catal. B Environ. 2009, 89, 551–556. [Google Scholar] [CrossRef]
- Ketir, W.; Rekhila, G.; Trari, M.; Amrane, A. Preparation, characterization and application of CuCrO2/ZnO photocatalysts for the reduction of Cr(VI). J. Environ. Sci. 2012, 24, 2173–2179. [Google Scholar] [CrossRef]
- Saadi, S.; Bouguelia, A.; Trari, M. Photocatalytic hydrogen evolution over CuCrO2. Sol. Energy 2006, 80, 272–280. [Google Scholar] [CrossRef]
- Rao, R.; Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Properties of Copper Chromite Catalysts in Hydrogenation Reactions. J. Catal. 1997, 171, 406–419. [Google Scholar] [CrossRef]
- Koriche, N.; Bouguelia, A.; Aider, A.; Trari, M. Photocatalytic hydrogen evolution over delafossite. Int. J. Hydrog. Energy 2005, 30, 693–699. [Google Scholar] [CrossRef]
- Ketir, W.; Bouguelia, A.; Trari, M. Visible Light Induced NO2 (-) Removal Over CuCrO2 Catalyst. Water Air Soil Pollut. 2009, 199, 115–122. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, Y.; Chi, M.; Park, J.N.; Stucky, G.D.; McFarland, E.W.; Gao, L. Mesoporous delafossite CuCrO2 and spinel CuCr2O4: Synthesis and catalysis. Nanotechnology 2013, 24, 345704. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Park, Y.B.; Kim, H.E.; Choi, Y.H.; Choi, S.H.; Lee, J.S. Oxygen-Intercalated CuFeO2 Photocathode Fabricated by Hybrid Microwave Annealing for Efficient Solar Hydrogen Production. Chem. Mater. 2016, 28, 6054–6061. [Google Scholar] [CrossRef]
- Lekse, J.W.; Underwood, M.K.; Lewis, J.P.; Matranga, C. Synthesis, Characterization, Electronic Structure, and Photocatalytic Behavior of CuGaO2 and CuGa1−xFexO2(x = 0.05, 0.10, 0.15, 0.20) Delafossites. J. Phys. Chem. C 2012, 116, 1865–1872. [Google Scholar] [CrossRef]
- Chiu, T.W.; Yang, Y.C.; Yeh, A.C.; Wang, Y.P.; Feng, Y.W. Antibacterial property of CuCrO2 thin films prepared by RF magnetron sputtering deposition. Vacuum 2013, 87, 174–177. [Google Scholar] [CrossRef]
- Doumerc, J.P.; Parent, C.; Chao, Z.J.; Le Flem, G.; Ammar, A. Luminescence of the Cu+ ion in CuLaO2. J. Less Common Met. 1989, 148, 333–337. [Google Scholar] [CrossRef]
- Jacob, A.; Parent, C.; Boutinaud, C.; Le Flem, G.; Doumerc, J.P.; Ammar, A.; Elazhari, M.; Elaatmani, M. Luminescent properties of Delafossite-type oxides LaCuO2 and YCuO2. Solid State Commun. 1997, 103, 529–532. [Google Scholar] [CrossRef]
- Byrne, D.; Cowley, A.; Bennett, N.; McGlynn, E. The luminescent properties of CuAlO2. J. Mater. Chem. C 2014, 2, 7859–7868. [Google Scholar] [CrossRef]
- Patzsch, J.; Balog, I.; Krauß, P.; Lehmann, C.W.; Schneider, J.J. Synthesis, characterization and p–n type gas sensing behaviour of CuFeO2 delafossite type inorganic wires using Fe and Cu complexes as single source molecular precursors. RSC Adv. 2014, 4, 15348. [Google Scholar] [CrossRef]
- Elgazzar, E.; Tataroğlu, A.; Al-Ghamdi, A.A.; Al-Turki, Y.; Farooq, W.A.; El-Tantawy, F.; Yakuphanoglu, F. Thermal sensors based on delafossite film/p-silicon diode for low-temperature measurements. Appl. Phys. A 2016, 122, 617. [Google Scholar] [CrossRef]
- Zheng, X.G.; Taniguchi, K.; Takahashi, A.; Liu, Y.; Xu, C.N. Room temperature sensing of ozone by transparent p-type semiconductor CuAlO2. Appl. Phys. Lett. 2004, 85, 1728–1729. [Google Scholar] [CrossRef]
- Zhou, S.; Fang, X.; Deng, Z.; Li, D.; Dong, W.; Tao, R.; Meng, G.; Wang, T. Room temperature ozone sensing properties of p-type CuCrO2 nanocrystals. Sens. Actuators B 2009, 143, 119–123. [Google Scholar] [CrossRef]
- Uhrmacher, M.; Attili, R.N.; Lieb, K.P.; Winzer, K.; Mekata, M. Perturbed Angular Correlation Measurements in 2D Spin-Frustrated CuFeO2. Phys. Rev. Lett. 1996, 76, 4829–4831. [Google Scholar] [CrossRef] [PubMed]
- Terada, N.; Mitsuda, S.; Oohara, Y.; Yoshizawa, H.; Takei, H. Anomalous magnetic excitation on triangular lattice antiferromagnet CuFeO2. J. Magn. Magn. Mater. 2004, 272–276, e997–e998. [Google Scholar] [CrossRef]
- Hoffman, R.; Wager, J. Transistor Device Having a Delafossite Material. U.S. Patent 7,026,713, 11 April 2006. [Google Scholar]
- Maignan, A.; Martin, C.; Frésard, R.; Eyert, V.; Guilmeau, E.; Hébert, S.; Poienar, M.; Pelloquin, D. On the strong impact of doping in the triangular antiferromagnet CuCrO2. Solid State Commun. 2009, 149, 962–967. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Stamenov, P.; Alaria, J.; Venkatesan, M.; Coey, J.M.D. Magnetoresistance of CuCrO2-based delafossite films. J. Phys. Conf. Ser. 2010, 200, 052021. [Google Scholar] [CrossRef]
- Shu, J.; Zhu, X.; Yi, T. CuCrO2 as anode material for lithium ion batteries. Electrochim. Acta 2009, 54, 2795–2799. [Google Scholar] [CrossRef]
- Huang, X.; Ni, C.; Zhao, G.; Irvine, J.T.S. Oxygen storage capacity and thermal stability of the CuMnO2–CeO2 composite system. J. Mater. Chem. A 2015, 3, 12958–12964. [Google Scholar] [CrossRef]
- Díaz-García, A.K.; Lana-Villarreal, T.; Gómez, R. Sol–gel copper chromium delafossite thin films as stable oxide photocathodes for water splitting. J. Mater. Chem. A 2015, 3, 19683–19687. [Google Scholar] [CrossRef]
- Taddee, C.; Kamwanna, T.; Amornkitbamrung, V. Characterization of transparent superconductivity Fe-doped CuCrO2 delafossite oxide. Appl. Surf. Sci. 2016, 380, 237–242. [Google Scholar] [CrossRef]
- Suriwong, T.; Thongtem, T.; Thongtem, S. Thermoelectric and optical properties of CuAlO2 synthesized by direct microwave heating. Curr. Appl. Phys. 2014, 14, 1257–1262. [Google Scholar] [CrossRef]
- Hayashi, K.; Nozaki, T.; Kajitani, T. Structure and High Temperature Thermoelectric Properties of Delafossite-Type Oxide CuFe1−xNixO2 (0 ≤ x ≤ 0.05). Jpn. J. Appl. Phys. 2007, 46, 5226–5229. [Google Scholar] [CrossRef]
- Park, K.; Ko, K.Y.; Kwon, H.C.; Nahm, S. Improvement in thermoelectric properties of CuAlO2 by adding Fe2O3. J. Alloys Compd. 2007, 437, 1–6. [Google Scholar] [CrossRef]
- Isawa, K.; Yaegashi, Y.; Ogota, S.; Nagano, M.; Sudo, S.; Yamada, K.; Yamauchi, H. Thermoelectric power of delafossite-derived compounds, RCuO2 + δ (R = Y, La, Pr, Nd, Sm, and Eu). Phys. Rev. B 1998, 57, 7950–7954. [Google Scholar] [CrossRef]
- Banerjee, A.N.; Maity, R.; Ghosh, P.K.; Chattopadhyay, K.K. Thermoelectric properties and electrical characteristics of sputter-deposited P-CuAlO2 thin films. Thin Solid Films 2005, 474, 261–266. [Google Scholar] [CrossRef]
- Park, K.; Ko, K.Y.; Seo, W.-S. Thermoelectric properties of CuAlO2. J. Eur. Ceram. Soc. 2005, 25, 2219–2222. [Google Scholar] [CrossRef]
- Guilmeau, E.; Maignan, A.; Martin, C. Thermoelectric Oxides: Effect of Doping in Delafossites and Zinc Oxide. J. Electron. Mater. 2009, 38, 1104–1107. [Google Scholar] [CrossRef]
- Nozaki, T.; Hayashi, K.; Kajitani, T.; Nozaki, K.; Hayashi, K.; Kajitani, T. Electronic Structure and Thermoelectric Properties of the Delafossite-Type Oxides CuFe1−xNixO2. J. Electron. Mater. 2009, 38, 1282–1286. [Google Scholar] [CrossRef]
- Ruttanapun, C. Effects of Pd substitution on the thermoelectric and electronic properties of delafossite Cu1−xPdxFeO2 (x = 0.01, 0.03 and 0.05). J. Solid State Chem. 2014, 215, 43–49. [Google Scholar] [CrossRef]
- Powar, S.; Xiong, D.; Daeneke, T.; Ma, M.T.; Gupta, A.; Lee, G.; Makuta, S.; Tachibana, Y.; Chen, W.; Spiccia, L.; et al. Improved Photovoltages for p-Type Dye-Sensitized Solar Cells Using CuCrO2 Nanoparticles. J. Phys. Chem. C 2014, 118, 16375–16379. [Google Scholar] [CrossRef]
- Hayashi, K.; Sato, K.I.; Nozaki, K.; Kajitani, T. Effect of Doping on Thermoelectric Properties of Delafossite-Type Oxide CuCrO2. Jpn. J. Appl. Phys. 2008, 57, 59–63. [Google Scholar] [CrossRef]
- Ono, Y.; Satoh, K.; Nozaki, T.; Kajitani, T. Structural, Magnetic and Thermoelectric Properties of Delafossite-type Oxide, CuCr1−xMgxO2 (0 ≤ x ≤ 0.05). Jpn. J. Appl. Phys 2007, 46, 1071–1075. [Google Scholar] [CrossRef]
- Okuda, T.; Jufuku, N.; Hidaka, S.; Terada, N. Magnetic, transport, and thermoelectric properties of the delafossite oxides CuCr1−xMgxO2 (0 ≤ x ≤ 0.04 ). Phys. Rev. B 2005, 72, 144403. [Google Scholar] [CrossRef]
- Farrell, L.; Norton, E.; Smith, C.M.; Caffrey, D.; Shvets, I.V.; Fleischer, K. Synthesis of nanocrystalline Cu deficient CuCrO2—A high figure of merit p-type transparent semiconductor. J. Mater. Chem. C 2016, 126, 126–134. [Google Scholar] [CrossRef]
- Lunca Popa, P.; Crêpellière, J.; Leturcq, R.; Lenoble, D. Electrical and optical properties of Cu–Cr–O thin films fabricated by chemical vapour deposition. Thin Solid Films 2016, 612, 194–201. [Google Scholar] [CrossRef]
- Ngo, T.N.M.; Palstra, T.T.M.; Blake, G.R. Crystallite size dependence of thermoelectric performance of CuCrO2. RSC Adv. 2016, 6, 91171–91178. [Google Scholar] [CrossRef]
- Gotzendorfer, S.; Bywalez, R.; Lobmann, P. Preparation of p-type conducting transparent CuCrO2 and CuAl0.5Cr0.5O2 thin films by sol-gel processing. J. Sol-Gel Sci. Technol. 2009, 52, 113–119. [Google Scholar] [CrossRef]
- Lin, F.; Gao, C.; Zhou, X.; Shi, W.; Liu, A. Magnetic, electrical and optical properties of p-type Fe-doped CuCrO2 semiconductor thin films. J. Alloys Compd. 2013, 581, 502–507. [Google Scholar] [CrossRef]
- Yu, R.-S.; Hu, D.-H. Formation and characterization of p-type semiconductor CuCrO2 thin films prepared by a sol–gel method. Ceram. Int. 2015, 41, 9383–9391. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Huang, C. Development of a fast annealing process to prepare transparent conductive Mg-doped CuCrO2 thin films. Thin Solid Films 2016, 605, 180–185. [Google Scholar] [CrossRef]
- Meng, Q.; Lu, S.S.; Lu, S.S.; Xiang, Y. Preparation of p-type CuCr1−xMgxO2 bulk with improved thermoelectric properties by sol-gel method. J. Sol-Gel Sci. Technol. 2012, 63, 1–7. [Google Scholar] [CrossRef]
- Ruttanapun, C.; Maensiri, S. Effects of spin entropy and lattice strain from mixed-trivalent Fe3+/Cr3+ on the electronic, thermoelectric and optical properties of delafossite CuFe1−xCrxO2. J. Phys. D Appl. Phys. 2015, 48, 495103. [Google Scholar] [CrossRef]
- Nagarajan, R.; Draeseke, A.D.; Sleight, A.W.; Tate, J. P-type conductivity in CuCr1−xMgxO2 films and powders. J. Appl. Phys. 2001, 89, 8022–8025. [Google Scholar] [CrossRef]
- Tripathi, T.S.; Niemelä, J.-P.; Karppinen, M. Atomic layer deposition of transparent semiconducting oxide CuCrO2 thin films. J. Mater. Chem. C 2015, 3, 8364–8371. [Google Scholar] [CrossRef]
- Chikoidze, E.; Boshta, M.; Gomaa, M.; Tchelidze, T.; Daraselia, D.; Japaridze, D.; Shengelaya, A.; Dumont, Y.; Neumann-Spallart, M. Control of p-type conduction in Mg doped monophase CuCrO2 thin layers. J. Phys. D Appl. Phys. 2016, 49, 205107. [Google Scholar] [CrossRef]
- Rastogi, A.C.; Lim, S.H.; Desu, S.B. Structure and optoelectronic properties of spray deposited Mg doped p-CuCrO2 semiconductor oxide thin films. J. Appl. Phys. 2008, 104, 023712. [Google Scholar] [CrossRef]
- Lim, S.H.; Desu, S.; Rastogi, A.C. Chemical spray pyrolysis deposition and characterization of P-type CuCr1−xMgxO2 transparent oxide semiconductor thin films. J. Phys. Chem. Solids 2008, 69, 2047–2056. [Google Scholar] [CrossRef]
- Le Trong, H.; Bui, T.M.A.; Presmanes, L.; Barnabé, A.; Pasquet, I.; Bonningue, C.; Tailhades, P. Preparation of iron cobaltite thin films by RF magnetron sputtering. Thin Solid Films 2015, 589, 292–297. [Google Scholar] [CrossRef]
- Lalanne, M.; Barnabé, A.; Mathieu, F.; Tailhades, P. Synthesis and Thermostructural Studies of a CuFe1−xCrxO2 Delafossite Solid Solution with 0 ≤ x ≤ 1. Inorg. Chem. 2009, 48, 6065–6071. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.W.; Tsai, S.W.; Wang, Y.P.; Hsu, K.H. Preparation of p-type conductive transparent CuCrO2:Mg thin films by chemical solution deposition with two-step annealing. Ceram. Int. 2012, 38, S673–S676. [Google Scholar] [CrossRef]
- Lin, C.; Wang, J. Sol–gel preparation of delafossite CuCr1-x Mgx O2 thin films by nitrate salts. Mater. Lett. 2016, 165, 111–114. [Google Scholar] [CrossRef]
- Götzendörfer, S.; Löbmann, P. Influence of single layer thickness on the performance of undoped and Mg-doped CuCrO2 thin films by sol–gel processing. J. Sol-Gel Sci. Technol. 2011, 57, 157–163. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Watson, G.W. Understanding the p-type defect chemistry of CuCrO2. J. Mater. Chem. 2011, 21, 3655. [Google Scholar] [CrossRef]
- Ingram, B.J.; Gonzá Lez, G.B.; Mason, T.O.; Shahriari, D.Y.; Barnabé, A.; Ko, D.; Poeppelmeier, K.R. Transport and Defect Mechanisms in Cuprous Delafossites. 1. Comparison of Hydrothermal and Standard Solid-State Synthesis in CuAlO2. Chem. Mater. 2004, 14, 5616–5622. [Google Scholar] [CrossRef]
- Ingram, B.J.; Bertoni, M.I.; Poeppelmeier, K.R.; Mason, T.O. Point defects and transport mechanisms in transparent conducting oxides of intermediate conductivity. Thin Solid Films 2005, 486, 86–93. [Google Scholar] [CrossRef]
- Norton, E.; Farrell, L.; Callaghan, S.D.; McGuinness, C.; Shvets, I.V.; Fleischer, K. X ray spectroscopic studies of the electronic structure of chromium based p type transparent conducting oxides. Phys. Rev. B 2016, 93, 115302. [Google Scholar] [CrossRef]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Jonker, G.H. The application of combined conductivity and Seebeck effet plots for the analysis of semiconductor properties. Philips Res. Rep. 1968, 23, 131–138. [Google Scholar]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Ebraheem, S.; El-Saied, A. Band Gap Determination from Diffuse Reflectance Measurements of Irradiated Lead Borate Glass System Doped with TiO2 by Using Diffuse Reflectance Technique. Mater. Sci. Appl. 2013, 4, 324–329. [Google Scholar]
- Kaya, I.C.; Sevindik, M.A.; Akyıldız, H. Characteristics of Fe- and Mg-doped CuCrO2 nanocrystals prepared by hydrothermal synthesis. J. Mater. Sci. Mater. Electron. 2016, 27, 2404–2411. [Google Scholar] [CrossRef]
- Mott, N.F. Conduction in Non-Crystalline Materials; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Chaikin, P.M.; Beni, G. Thermopower in the correlated hopping regime. Phys. Rev. B 1976, 13, 647–651. [Google Scholar] [CrossRef]
- Heikes, R.R.; Ure, R.W. Thermoelectricity: Science and Engineering; Interscience Publishers: Olney, UK, 1961. [Google Scholar]
- Scanlon, D.O.; Godinho, K.G.; Morgan, B.J.; Watson, G.W. Understanding conductivity anomalies in Cu-I-based delafossite transparent conducting oxides: Theoretical insights. J. Chem. Phys. 2010, 132, 24707. [Google Scholar] [CrossRef] [PubMed]





| Target material | 3 at % Mg-doped CuCrO2 |
| Substrate | Fused quartz |
| Power (W·cm−2) | 0.9 |
| Magnetron | Yes |
| Argon pressure P (Pa) | 0.5 |
| Target to substrate distance d (cm) | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinnarasa, I.; Thimont, Y.; Presmanes, L.; Barnabé, A.; Tailhades, P. Thermoelectric and Transport Properties of Delafossite CuCrO2:Mg Thin Films Prepared by RF Magnetron Sputtering. Nanomaterials 2017, 7, 157. https://doi.org/10.3390/nano7070157
Sinnarasa I, Thimont Y, Presmanes L, Barnabé A, Tailhades P. Thermoelectric and Transport Properties of Delafossite CuCrO2:Mg Thin Films Prepared by RF Magnetron Sputtering. Nanomaterials. 2017; 7(7):157. https://doi.org/10.3390/nano7070157
Chicago/Turabian StyleSinnarasa, Inthuga, Yohann Thimont, Lionel Presmanes, Antoine Barnabé, and Philippe Tailhades. 2017. "Thermoelectric and Transport Properties of Delafossite CuCrO2:Mg Thin Films Prepared by RF Magnetron Sputtering" Nanomaterials 7, no. 7: 157. https://doi.org/10.3390/nano7070157





