Controllable Charge Transfer in Ag-TiO2 Composite Structure for SERS Application
Abstract
:1. Introduction
2. Results and Discussion
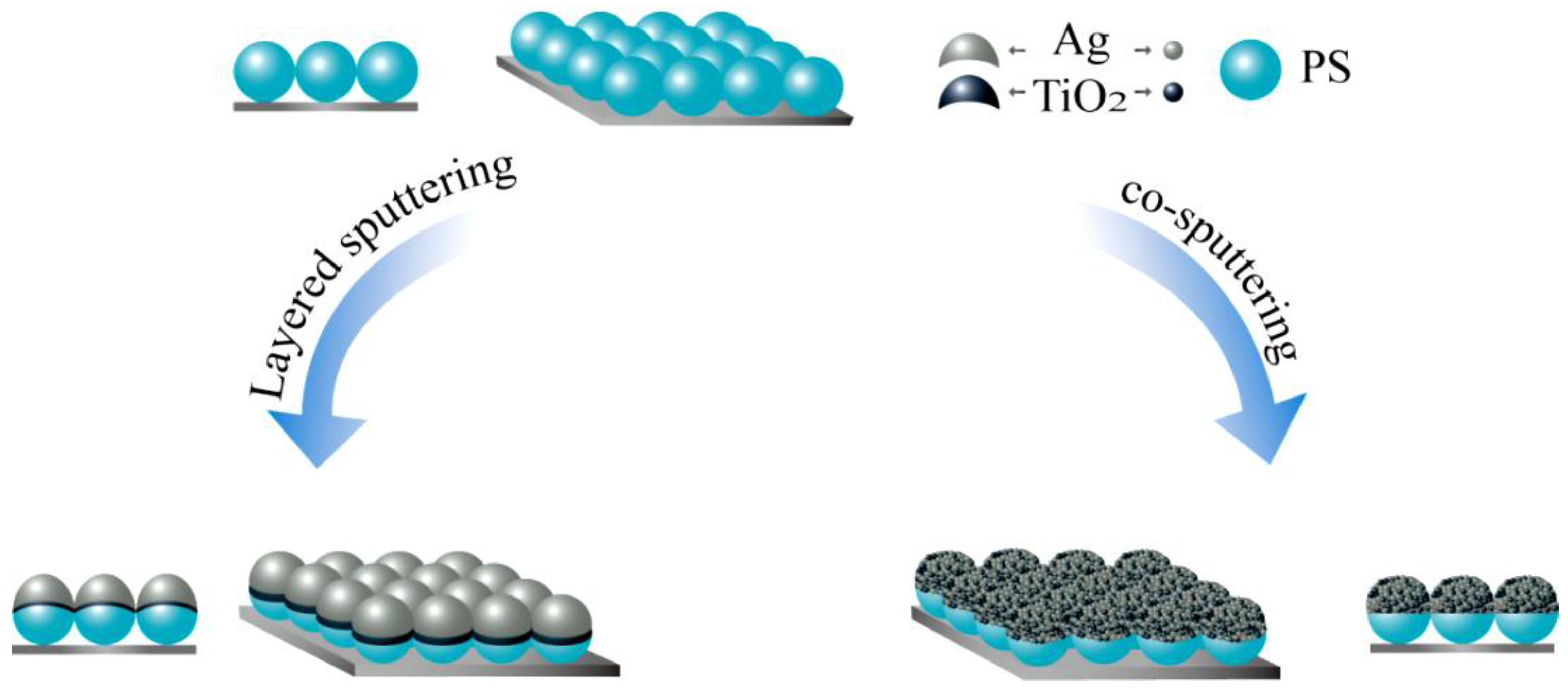
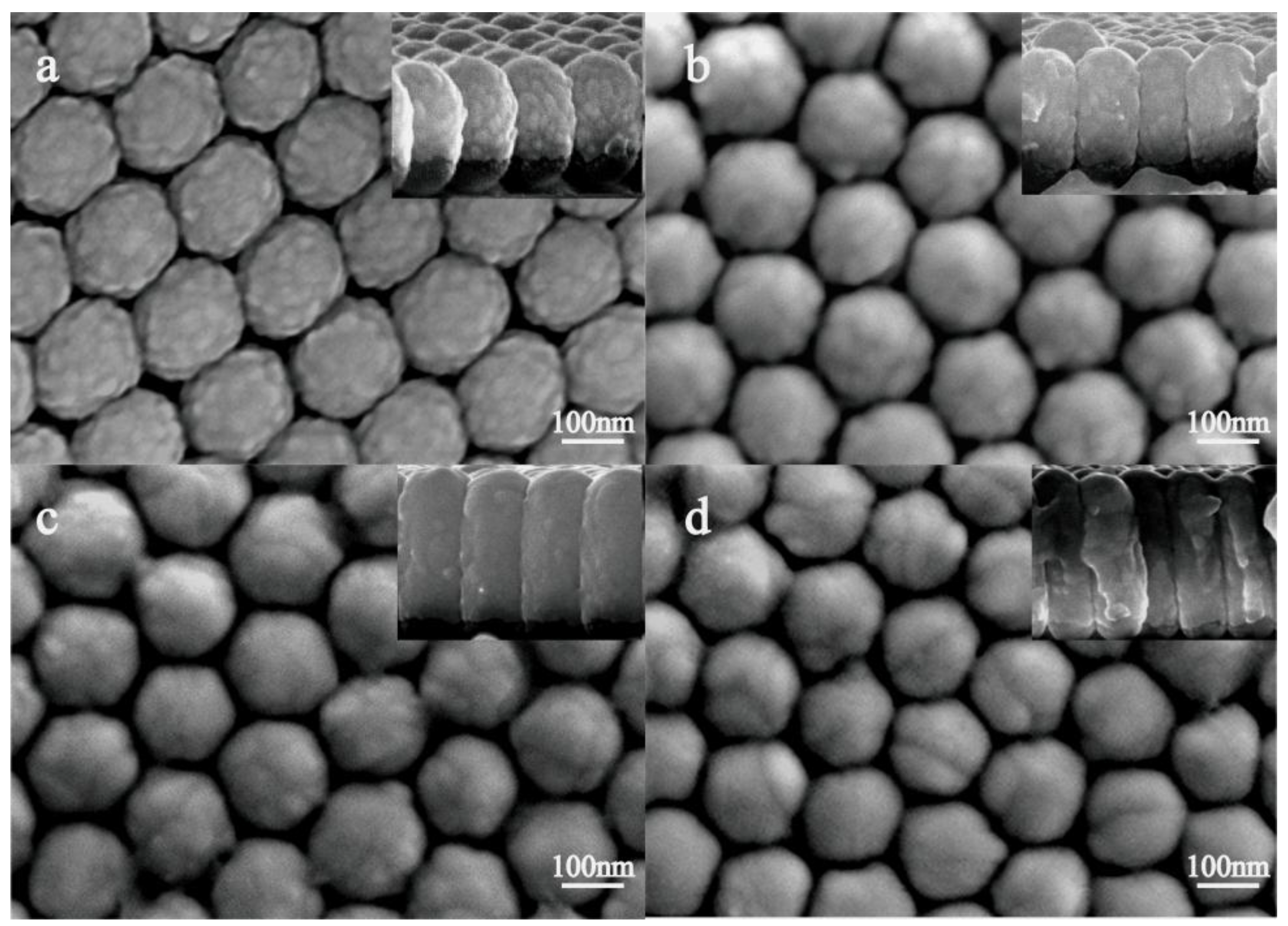
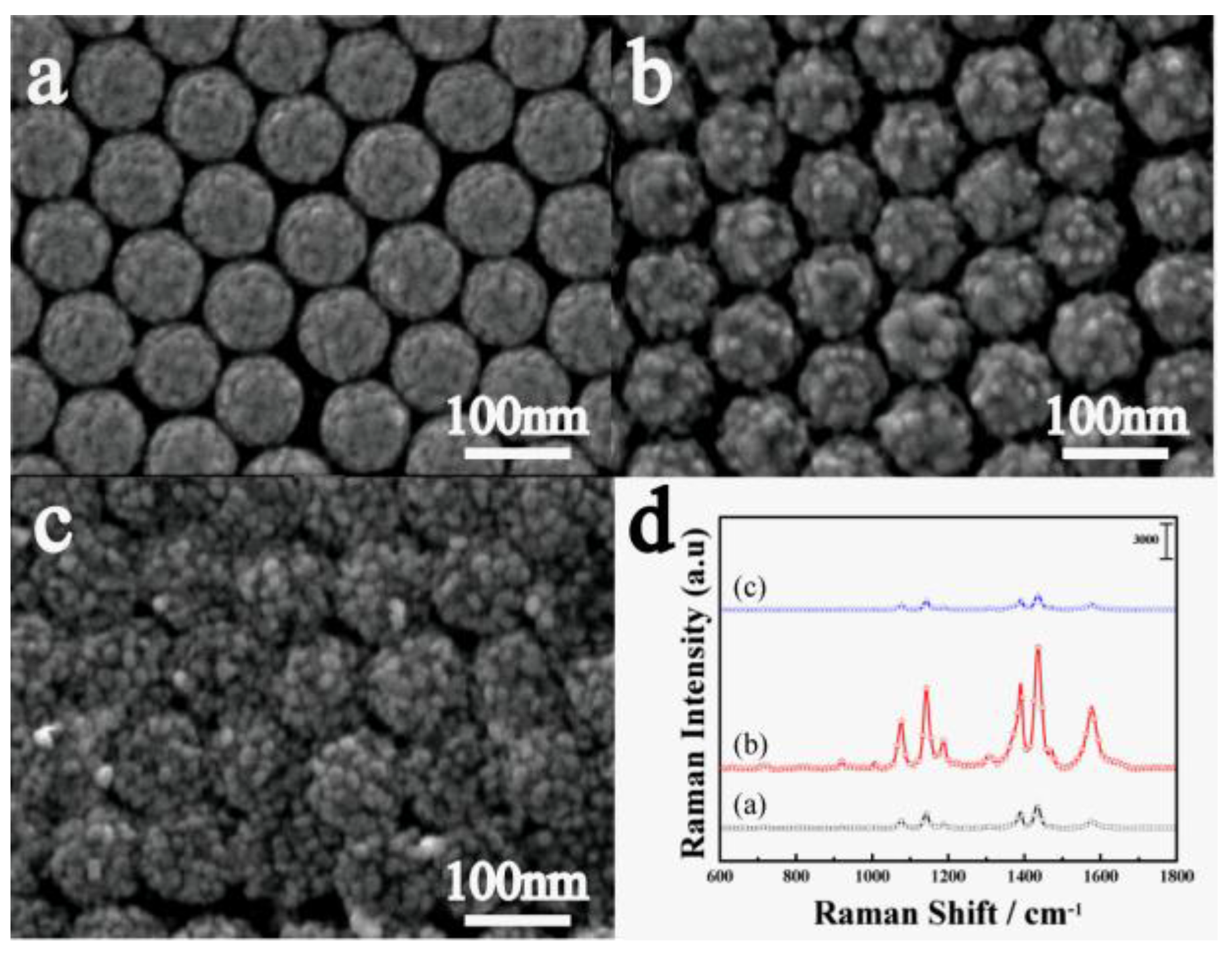
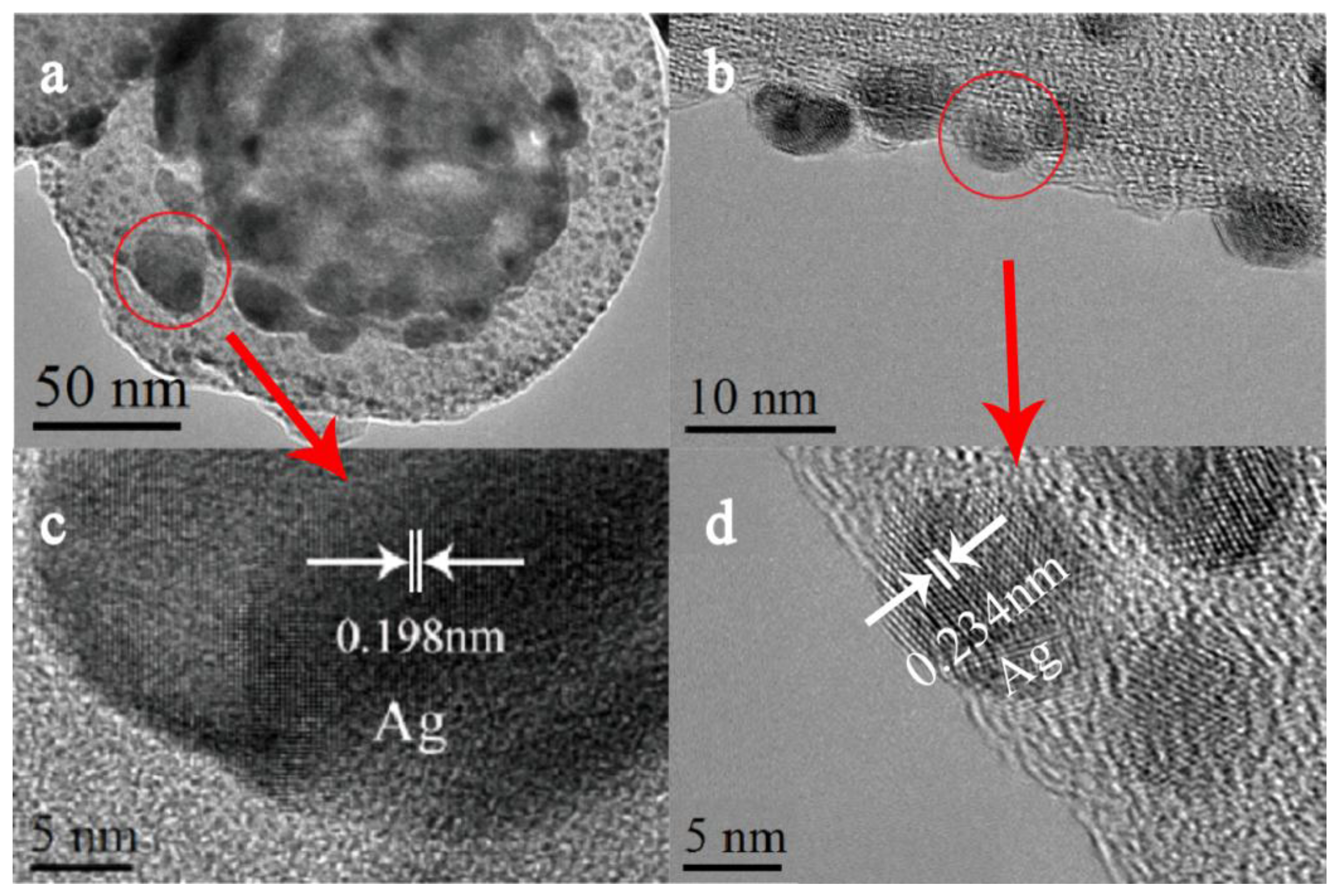
2.1. Preparation and Characterization of the Nano Composite Structure
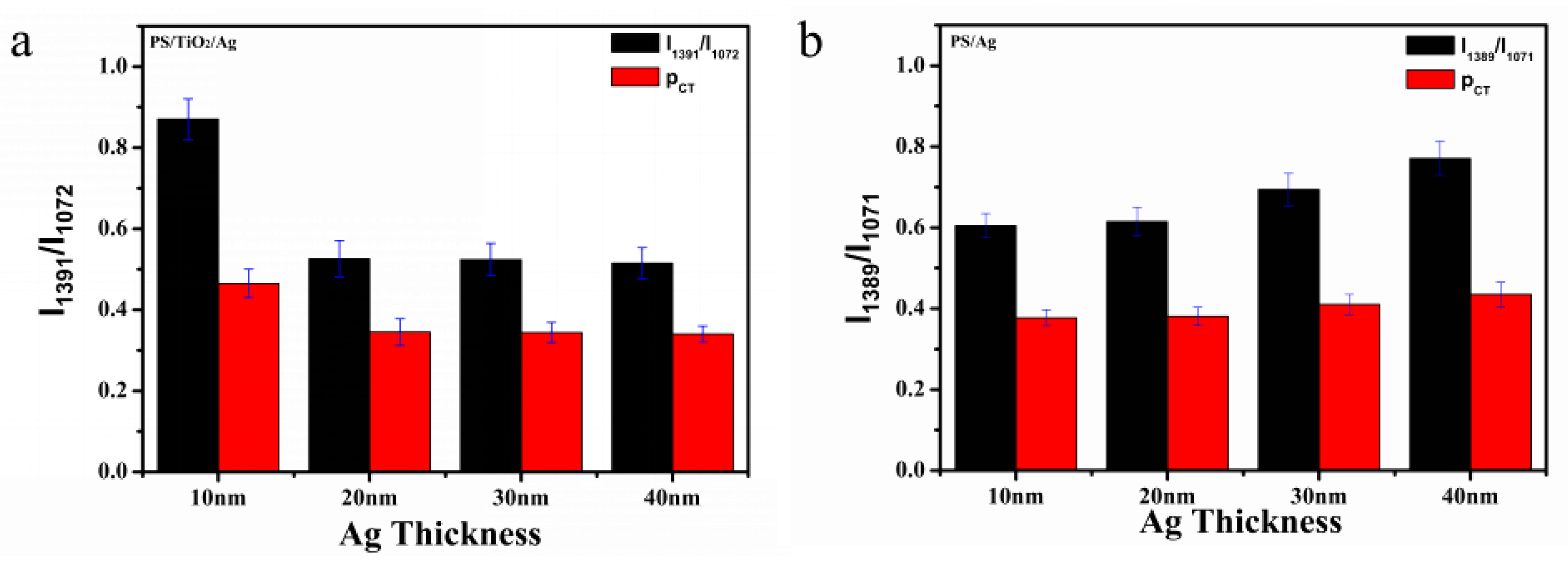
2.2. SERS Study of PS/TiO2/Ag and PS/Ag
2.3. Enhancement Factor (EF) of PS/TiO2/Ag and PS/TiO2:Ag
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fleischmann, M.; Hendra, P.J.; Mcquillan, A.J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Duyne, R.P.V. Surface Raman SpectroElectrochemistry Part I. Heterocyclic, Aromatic and Aliphatic Amines Adsorbed on the Anodized Silver Electrode. J. Electroanal. Chem. Interfac. Electrochem. 1997, 84, 1–20. [Google Scholar] [CrossRef]
- Ngo, H.T.; Wang, H.N.; Fales, A.M.; Vo-Dinh, T. Label-Free DNA Biosensor Based on SERS Molecular Sentinel on Nanowave Chip. Anal. Chem. 2013, 85, 6378–6383. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, Z.Y.; Zong, S.F.; Cui, Y.P. Rapid and Reproducible Analysis of Thiocyanate in Real Human Serum and Saliva Using a Droplet SERS-Microfluidic Chip. Biosens. Bioelectron. 2014, 62, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Sun, M.Z.; Xu, L.G.; Wang, L.B.; Kuang, H.; Xu, C.L. A SERS Active Gold Nanostar Dimer for Mercury Ion Detection. Chem. Commun. 2013, 49, 4989–4991. [Google Scholar] [CrossRef] [PubMed]
- Shanmukh, S.; Jones, L.; Driskell, J.; Zhao, Y.P.; Dluhy, R.; Tripp, R.A. Rapid and Sensitive Detection of Respiratory Virus Molecular Signatures Using a Silver Nanorod Array SERS Substrate. Nano Lett. 2006, 6, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.G.; Creighton, J.A. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. 1997, 99, 5215–5217. [Google Scholar] [CrossRef]
- He, S.J.; Liu, K.-K.; Su, S.; Yan, J.; Mao, X.H.; Wang, D.F.; He, Y.; Li, L.-J.; Song, S.P.; Fan, C.H. Graphene-Based High-Efficiency Surface-Enhanced Raman Scattering-Active Platform for Sensitive and Multiplex DNA Detection. Anal. Chem. 2012, 84, 4622–4627. [Google Scholar] [CrossRef] [PubMed]
- Bantz, K.C.; Meyer, A.F.; Wittenberg, N.J.; Im, H.; Kurtulus, Ö.; Lee, S.H.; Lindquist, N.C.; Oh, S.-H.; Haynes, C.L. Recent progress in SERS biosensing. Phys. Chem. Chem. Phys. 2011, 13, 11551–11567. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.X.; Li, J.F.; Wu, D.Y.; Yang, Z.L.; Ren, B.; Hu, J.W.; Chow, Y.L.; Tian, Z.Q. Characterization of surface water on Au core Pt-group metal shell nanoparticles coated electrodes by surface-enhanced Raman spectroscopy. Chem. Commun. 2007, 44, 4608–4610. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.B.; Jiang, X.; Ruan, W.D.; Zhao, B.; Xu, W.Q.; Lombard, J.R. Observation of Enhanced Raman Scattering for Molecules Adsorbed on TiO2 nanoparticles: Charge-Transfer Contribution. J. Phys. Chem. C 2008, 112, 20095–20098. [Google Scholar] [CrossRef]
- Wang, Y.X.; Song, W.; Ruan, W.D.; Yang, J.X.; Zhao, B.; Lombard, J.R. SERS Spectroscopy to Study an Adsorbate on Nanoscale Thin Film of CuO coated with Ag. J. Phys. Chem. C 2009, 113, 8065–8069. [Google Scholar] [CrossRef]
- Xu, W.G.; Mao, N.N.; Zhang, J. Graphene: A Plat form for Surface-Enhanced Raman Spectroscopy. Small 2013, 9, 1206–1224. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.B.; Jiang, X.; Ruan, W.D.; Yang, J.X.; Zhao, B.; Xu, W.Q.; Lombardi, J.R. Charge-Transfer-Induced Surface-Enhanced Raman Scattering on Ag–TiO2 Nanocomposites. J. Phys. Chem. C 2009, 113, 16226–16231. [Google Scholar] [CrossRef]
- Su, Y.; He, Q.; Yan, X.H.; Fei, J.B.; Cui, Y.; Li, J.B. Peptide Mesocrystals as Templates to Create an Au Surface with Stronger Surface-Enhanced Raman Spectroscopic Properties. Chem. A Eur. J. 2011, 17, 3370–3375. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhou, B.X. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Ghicov, A.; Schmuki, P. Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem. Commun. 2009, 20, 2791–2808. [Google Scholar] [CrossRef] [PubMed]
- Fattakhova-Rohlfing, D.; Zaleska, A.; Bein, T. Three-Dimensional Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9487–9558. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, Y.X.; Zhao, B. Surface-Enhanced Raman Scattering of 4-Mercaptopyridine on the Surface of TiO2 Nanofibers Coated with Ag Nanoparticles. J. Phys. Chem. C 2007, 111, 12786–12791. [Google Scholar] [CrossRef]
- Shaviv, E.; Schubert, O.; Alves-Santos, M.; Goldoni, G.; Felice, R.D.; Fatti, F.; Fatti, N.D.; Banin, U.; Sönnichsen, C. Absorption Properties of Metal–Semiconductor Hybrid, Nanoparticles. ACS Nano 2011, 5, 4712–4719. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, R.; Srivastava, R.S.; Misra, R.D.K. Comparative study of antimicrobial and photocatalytic activity in titaniaencapsulated composite nanoparticles with different dopants. Mater. Sci. Technol. 2008, 24, 589–595. [Google Scholar] [CrossRef]
- Rawat, J.; Rana, S.; Srivastava, R.S.; Misra, R.D.K. Anti-microbial activity of composite nanoparticles consisting of titania photocatalytic shell and nickel ferrite magnetic core. Mater. Sci. Eng. C 2007, 27, 540–545. [Google Scholar] [CrossRef]
- Rana, S.; Rawat, J.; Misra, R.D.K. Anti-microbial active composite nanoparticles with magnetic core and photocatalytic shell: TiO2–NiFe2O4 bio-material system. Acta Biomater. 2005, 1, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Lin, J.; Li, X.H.; Zhao, G.N.; Zhang, W.J. Silver nanoparticles deposited inverse opal film as a highly active and uniform SERS substrate. Appl. Surf. Sci. 2015, 347, 514–519. [Google Scholar] [CrossRef]
- Klaysri, R.; Praserthdam, P.; Kelly, P.J.; Ratova, M. Deposition of Visible Light-Active C-Doped Titania Films via Magnetron Sputtering Using CO2 as a Source of Carbon. Nanomaterials 2017, 7, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Fromm, D.P.; Sundaramurthy, A.; Kinkhabwala, A.; Schuck, P.J.; Kino, G.S.; Moerner, W.E. Exploring the Chemical Enhancement for Surface-Enhanced Raman Scattering with Au Bowtie Nanoantennas. J. Chem. Phys. 2006, 124, 061101. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.M.; Zhang, X.L.; Chen, S.T.; Fang, Y.; Yan, J.L.; Li, N.; Qi, P.X. Facile Synthesis of SERS Active Ag Nanoparticles in the Presence of Tri-N-Octylphosphine Sulfide. Appl. Surf. Sci. 2011, 257, 4935–4940. [Google Scholar] [CrossRef]
- Dong, B.; Wang, W.; Miller, D.L.; Li, C.Y. Polymer-Single-Crystal@Nanoparticle Nanosandwich for Surface Enhanced Raman Spectroscopy. J. Mater. Chem. 2012, 22, 15526–15529. [Google Scholar] [CrossRef]
- Xu, S.C.; Zhang, Y.X.; Luo, Y.Y.; Wang, S.; Ding, H.L.; Xu, J.M.; Li, G.H. Ag-Decorated TiO2 Nanograss for 3D SERS-Active Substrate with Visible Light Self-Cleaning and Reactivation. Analyst 2013, 138, 4519–4525. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Wang, C.X.; Yang, J.X.; Zhao, B.; Lombard, J.R. Nanoparticle Metal–Semiconductor Charge Transfer in ZnO/PATP/Ag Assemblies by Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 6093–6098. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, W.; Sui, H.M.; Kitahama, Y.; Ruan, W.D.; Ozaki, Y.; Zhao, B. Exploring the Effect of Intermolecular H-Bonding: A Study on Charge-Transfer Contribution to Surface-Enhanced Raman Scattering of p-Mercaptobenzoic Acid. J. Phys. Chem. C 2014, 118, 10191–10197. [Google Scholar] [CrossRef]
- Yang, L.; Wang, W.H.; Jiang, H.Y.; Zhang, Q.H.; Shan, H.H.; Zhang, M.; Zhu, K.; Lv, J.G.; He, G.; Sun, Z.Q. Improved SERS performance of single-crystalline TiO2 nanosheet arrays with coexposed {001} and {101} facets decorated with Ag nanoparticles. Sens. Actuators B Chem. 2017, 242, 932–939. [Google Scholar] [CrossRef]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C.; Tominaga, J.; Murakami, H.; Ohki, Y.; Yoshida, N.; Watanabe, T. A Plasmonic Photocatalyst Consisting of Silver Nanoparticles Embedded in Titanium Dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Ansari, S.A.; Amal, M.I.; Lee, J.; Cho, M.H. Highly visible light active Ag@TiO2 nanocomposites synthesized using an electrochemically active biofilm: A novel biogenic approach. Nanoscale 2013, 5, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering: Active nanomaterials and applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, J.R.; Birke, R.L. A Unified View of Surface-Enhanced Raman Scattering. Acc. Chem. Res. 2009, 42, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, J.R.; Birke, R.L. A Unified Approach to Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zou, X.Q.; Ren, W.; Wang, W.D.; Wang, E. Effect of Silver Nanoplates on Raman Spectra of p-Aminothiophenol Assembled on Smooth Macroscopic Gold and Silver Surface. J. Phys. Chem. C 2007, 111, 3259–3265. [Google Scholar] [CrossRef]
- Ren, B.; Lin, X.F.; Yang, Z.L.; Liu, G.K.; Aroca, R.F.; Mao, B.W.; Tian, Z.Q. Surface-Enhanced Raman Scattering in the Ultraviolet Spectral Region: UV-SERS on Rhodium and Ruthenium Electrodes. J. Am. Chem. Soc. 2003, 125, 9598–9599. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.M.; Fan, C.Z.; Wang, J.Q.; He, J.N.; Liang, E.; Chao, M. Surface enhanced Raman scattering of 4-aminothiophenol sandwiched between Ag nanocubes and smooth Pt substrate: The effect of the thickness of Pt film. J. Appl. Phys. 2014, 116, 044312. [Google Scholar] [CrossRef]
- Jiang, J.; Bosnick, K.; Maillard, M.; Brus, L. Single Molecule Raman Spectroscopy at the Junctions of Large Ag Nanocrystals. J. Phys. Chem. B 2003, 107, 9964–9972. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhao, X.Y.; Chen, L.; Chen, S.; Wei, M.B.; Gao, M.; Zhao, Y.; Wang, C.; Qu, X.; Zhang, Y.J.; et al. Ordered Nanocap Array Composed of SiO2-Isolated Ag Islands as SERS Platform. Langmuir 2014, 30, 15285–15291. [Google Scholar] [CrossRef] [PubMed]


| Wavenumber (cm−1) | Band Assignment | |
|---|---|---|
| PS/TiO2/Ag | PS/Ag | |
| 1577m | 1577m | υCC, 8b(b2) |
| 1472w | 1472w | υCC, 19a(a1) |
| 1440υs | 1439υs | υCC + δCH, 19b(b2) |
| 1390s | 1389s | δCH + υCC, 3(b2) |
| 1302w | 1301w | υCC + δCH, 14(b2) |
| 1188w | 1182w | δCH, 9a(a1) |
| 1141υs | 1140υs | δCH, 9b(b2) |
| 1072m | 1071m | υCS, 7a(a1) |
| 1008w | 1008w | γCC + γCCC, 18a(a1) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yan, C.; Chen, L.; Zhang, Y.; Yang, J. Controllable Charge Transfer in Ag-TiO2 Composite Structure for SERS Application. Nanomaterials 2017, 7, 159. https://doi.org/10.3390/nano7070159
Wang Y, Yan C, Chen L, Zhang Y, Yang J. Controllable Charge Transfer in Ag-TiO2 Composite Structure for SERS Application. Nanomaterials. 2017; 7(7):159. https://doi.org/10.3390/nano7070159
Chicago/Turabian StyleWang, Yaxin, Chao Yan, Lei Chen, Yongjun Zhang, and Jinghai Yang. 2017. "Controllable Charge Transfer in Ag-TiO2 Composite Structure for SERS Application" Nanomaterials 7, no. 7: 159. https://doi.org/10.3390/nano7070159






