Citrus Pectin-Derived Carbon Microspheres with Superior Adsorption Ability for Methylene Blue
Abstract
:1. Introduction
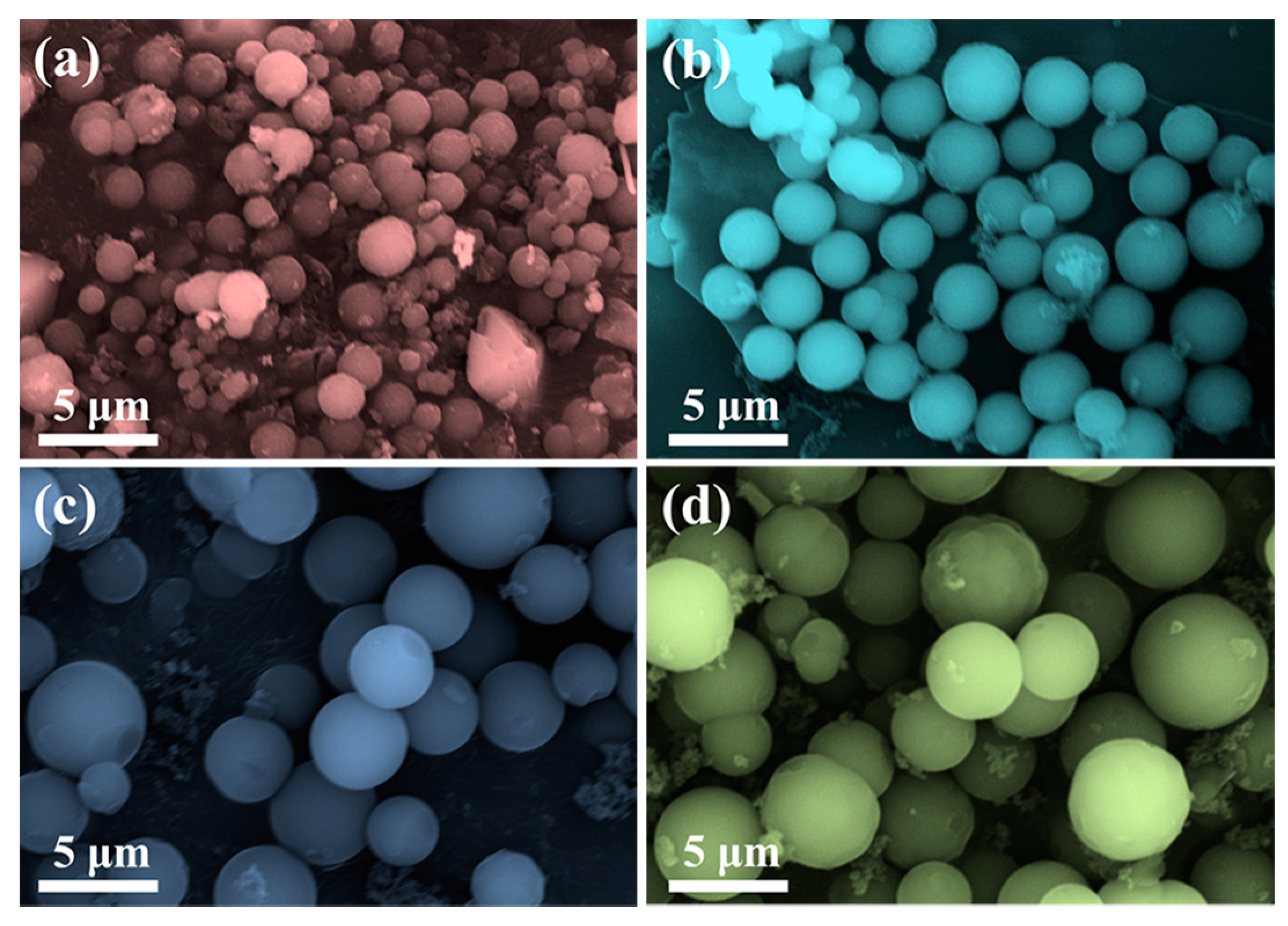
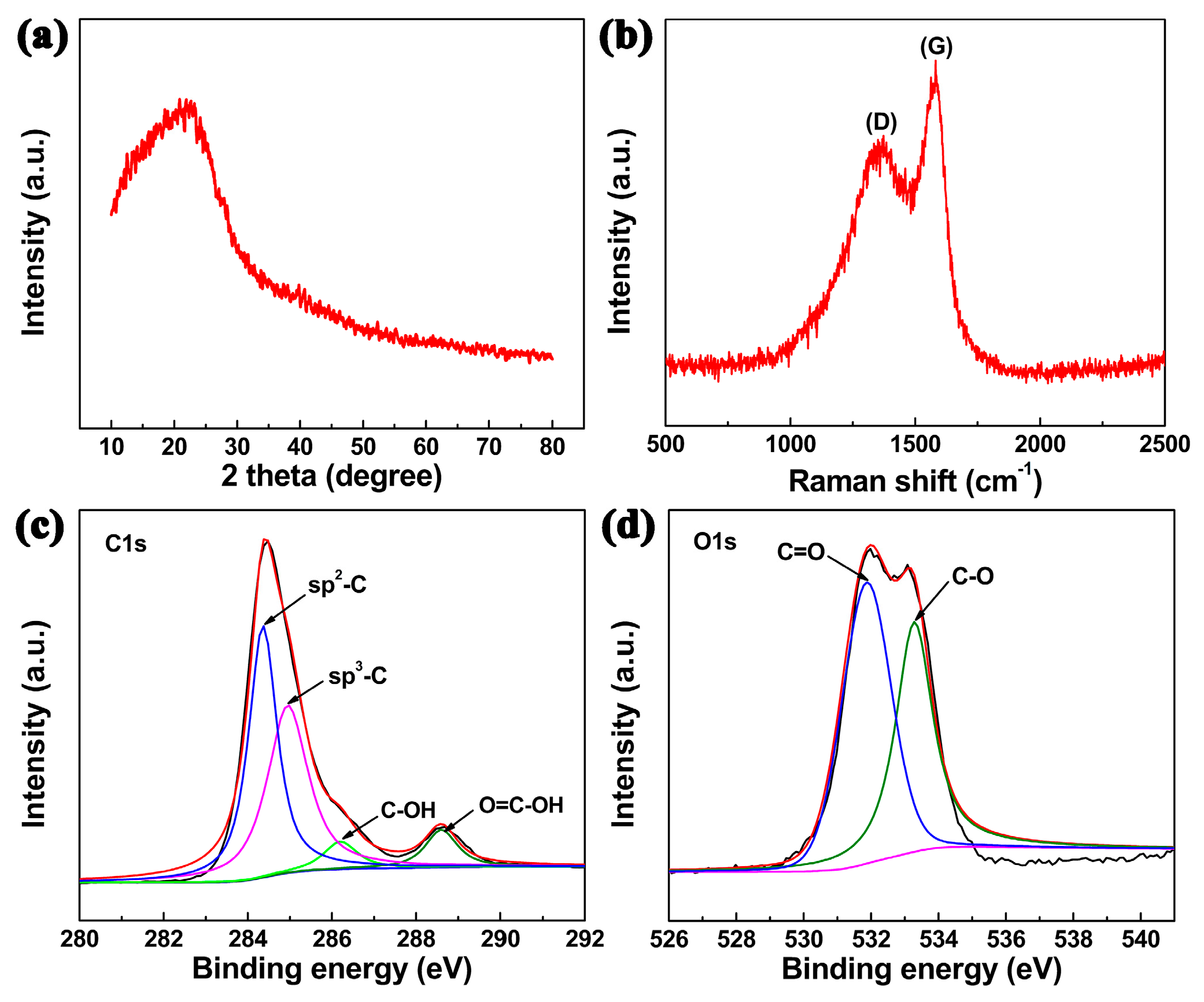
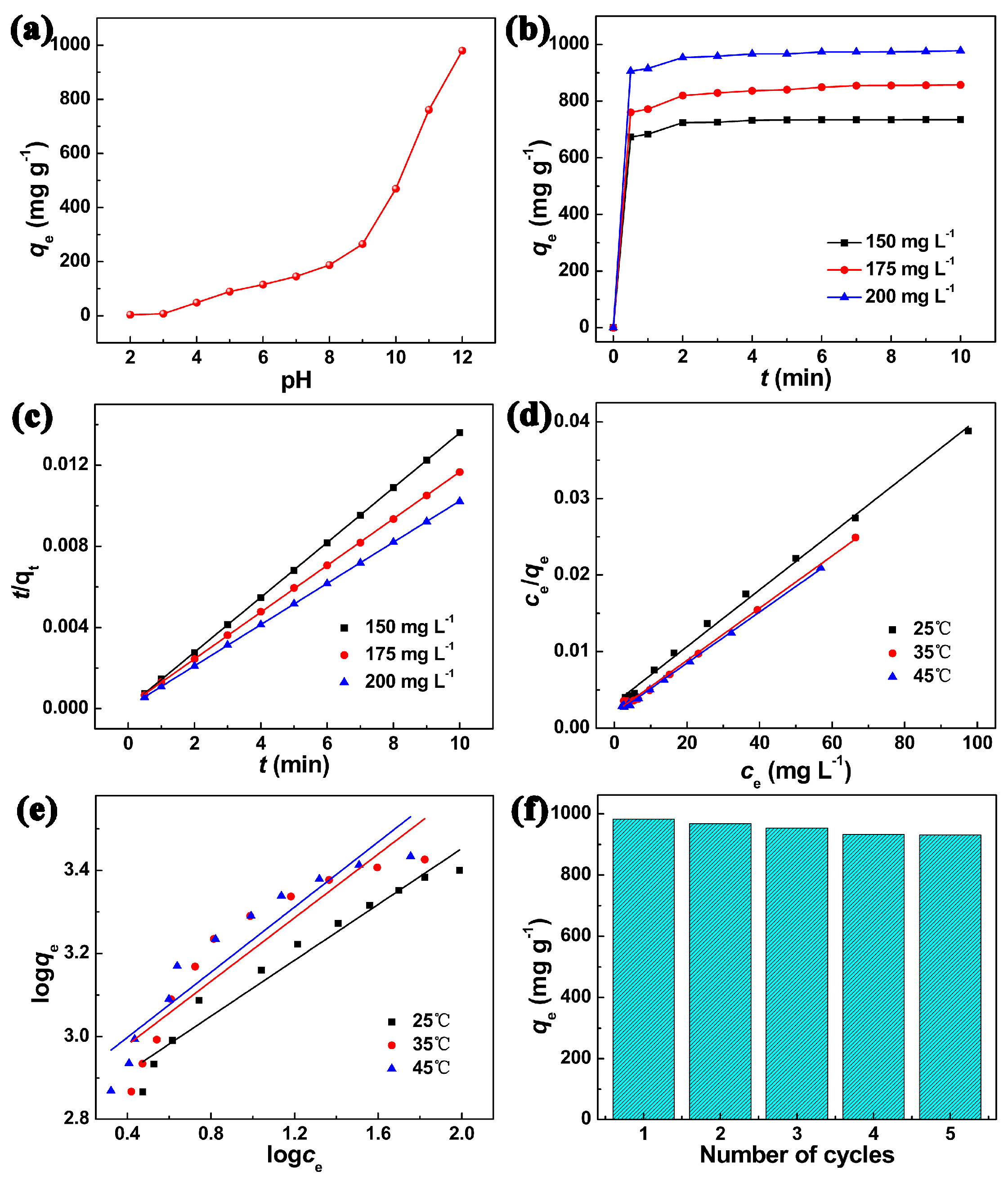
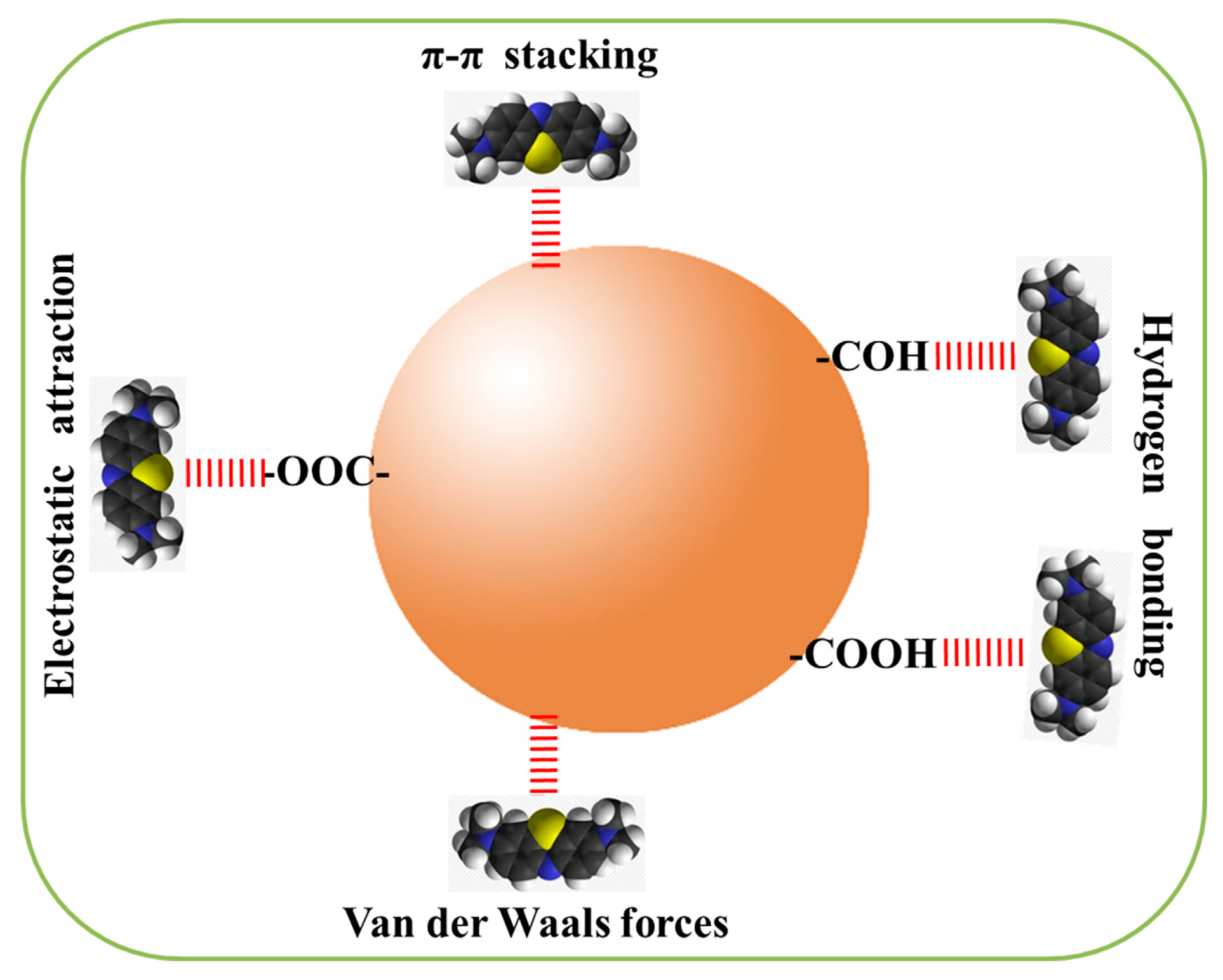
2. Results and Discussion
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, W.; Zhang, L.Y.; Zhao, X.J.; Zhou, Z. Citrus pectin derived ultrasmall Fe3O4@C nanoparticles as a high-performance adsorbent toward removal of methylene blue. J. Mol. Liq. 2016, 222, 995–1002. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, X.; Chen, X. Synthesis and utilization of a novel carbon nanotubes supported nanocables for the adsorption of dyes from aqueous solutions. J. Solid State Chem. 2015, 229, 342–349. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.Y.; Zhao, X.J.; Zhou, Z. Citrus pectin derived porous carbons as a superior adsorbent toward removal of methylene blue. J. Solid State Chem. 2016, 243, 101–105. [Google Scholar] [CrossRef]
- Dai, J.; Sun, J.; Xie, A.; He, J.; Li, C.; Yan, Y. Designed preparation of 3D hierarchically porous carbon material via solvothermal route and in situ activation for ultrahigh-efficiency dye removal: Adsorption isotherm, kinetics and thermodynamics characteristics. RSC Adv. 2016, 6, 3446–3457. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, Y.; Wang, K.; Xu, R. Low-Cost Carbon Nanospheres for Efficient Removal of Organic Dyes from Aqueous Solutions. Ind. Eng. Chem. Res. 2012, 51, 13438–13444. [Google Scholar] [CrossRef]
- Robati, D.; Mirza, B.; Ghazisaeidi, R.; Rajabi, M.; Moradi, O.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Adsorption behavior of methylene blue dye on nanocomposite multi-walled carbon nanotube functionalized thiol (MWCNT-SH) as new adsorbent. J. Mol. Liq. 2016, 216, 830–835. [Google Scholar] [CrossRef]
- Bedin, K.C.; Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. KOH-activated carbon prepared from sucrose spherical carbon: Adsorption equilibrium, kinetic and thermodynamic studies for Methylene Blue removal. Chem. Eng. J. 2016, 286, 476–484. [Google Scholar] [CrossRef]
- Chang, B.; Guan, D.; Tian, Y.; Yang, Z.; Dong, X. Convenient synthesis of porous carbon nanospheres with tunable pore structure and excellent adsorption capacity. J. Hazard. Mater. 2013, 262, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Zhang, W.L.; Zhou, Z.Q. Sodium hydroxide-mediated hydrogel of citrus pectin for preparation of fluorescent carbon dots for bioimaging. Colloids Surf. B Biointerfaces 2014, 123, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, X.J.; Jiang, Y.; Zhou, Z. Citrus pectin derived silver nanoparticles and their antibacterial activity. Inorg. Nano-Met. Chem. 2017, 47, 15–20. [Google Scholar] [CrossRef]
- Grohman, K.; Cameron, R.; Kim, Y.; Widmer, W.; Luzio, G. Extraction and recovery of pectic fragments from citrus processing waste for co-production with ethanol. J. Chem. Technol. Biotechnol. 2013, 88, 395–407. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Liu, S. Carboxyl-rich carbon microspheres prepared from pentosan with high adsorption capacity for heavy metal ions. Mater. Res. Bull. 2014, 60, 516–523. [Google Scholar] [CrossRef]
- Jia, Z.; Li, Z.; Li, S.; Li, Y.; Zhu, R. Adsorption performance and mechanism of methylene blue on chemically activated carbon spheres derived from hydrothermally-prepared poly (vinyl alcohol) microspheres. J. Mol. Liq. 2016, 220, 56–62. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, Q. Preparation of carbon microspheres decorated with silver nanoparticles and their ability to remove dyes from aqueous solution. J. Hazard. Mater. 2015, 283, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, P.F.; Yang, Z.J.; Jiang, T.W.; Yao, K.L.; Han, R.; Huo, X.X.; Xiong, Y.Y. Bi-functional porous carbon spheres derived from pectin as electrode material for supercapacitors and support material for Pt nanowires towards electrocatalytic methanol and ethanol oxidation. Electrochim. Acta 2015, 163, 140–148. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; He, T.; You, L.; Shen, X. Assessment of Potential Capability of Water Bamboo Leaves on the Adsorption Removal Efficiency of Cationic Dye from Aqueous Solutions. J. Polym. Environ. 2016, 24, 148–158. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, W.; Zhou, Z.; Li, C.M. γ-Fe2O3 nanocrystals-anchored macro/meso-porous graphene as a highly efficient adsorbent toward removal of methylene blue. J. Colloid Interface Sci. 2016, 476, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, Z.; Zhang, L.; Tian, Y.; Cui, G.; Yan, S. A cost-effective porous carbon derived from pomelo peel for the removal of methyl orange from aqueous solution. Colloid Surf. A-Physicochem. Eng. Asp. 2016, 489, 191–199. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.; Ma, X. Adsorption characteristics of methylene blue onto low cost biomass material lotus leaf. Chem. Eng. J. 2011, 171, 1–8. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Tan, J.; Nan, X.; Liu, S. Hydrothermal synthesis of magnetic mesoporous carbon microspheres from carboxymethylcellulose and nickel acetate. Appl. Surf. Sci. 2015, 332, 354–361. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhou, Z. Citrus Pectin-Derived Carbon Microspheres with Superior Adsorption Ability for Methylene Blue. Nanomaterials 2017, 7, 161. https://doi.org/10.3390/nano7070161
Zhang W, Zhou Z. Citrus Pectin-Derived Carbon Microspheres with Superior Adsorption Ability for Methylene Blue. Nanomaterials. 2017; 7(7):161. https://doi.org/10.3390/nano7070161
Chicago/Turabian StyleZhang, Wenlin, and Zhiqin Zhou. 2017. "Citrus Pectin-Derived Carbon Microspheres with Superior Adsorption Ability for Methylene Blue" Nanomaterials 7, no. 7: 161. https://doi.org/10.3390/nano7070161



