First-Principles Study of Structural, Electronic and Magnetic Properties of Metal-Centered Tetrahexahedral V15+ Cluster
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
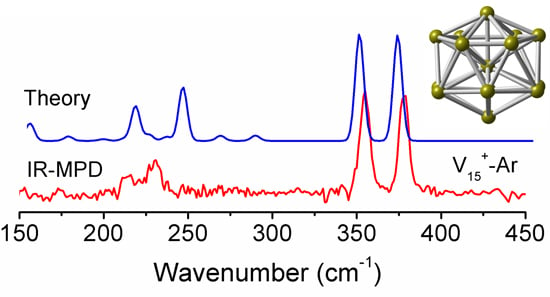
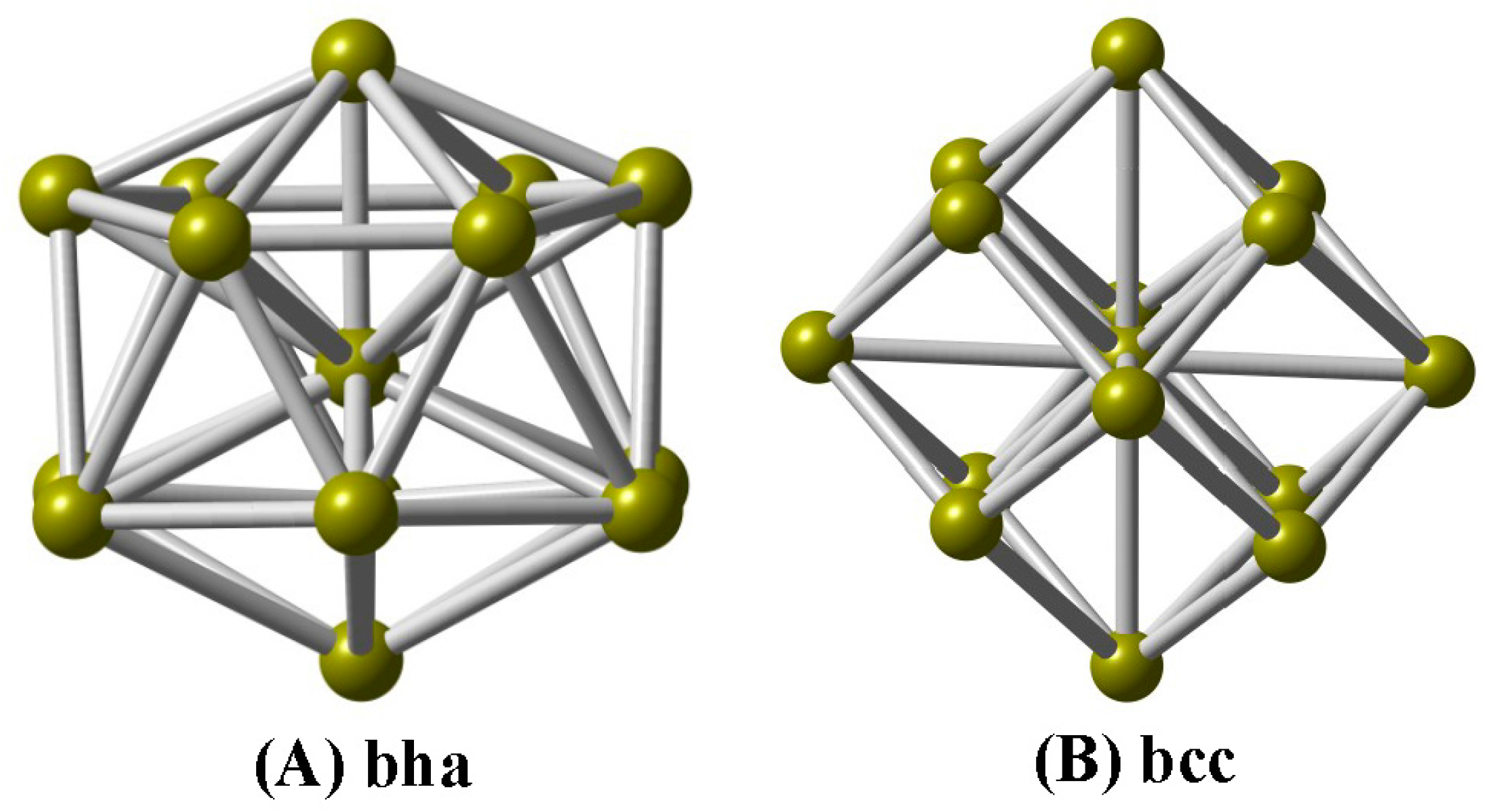
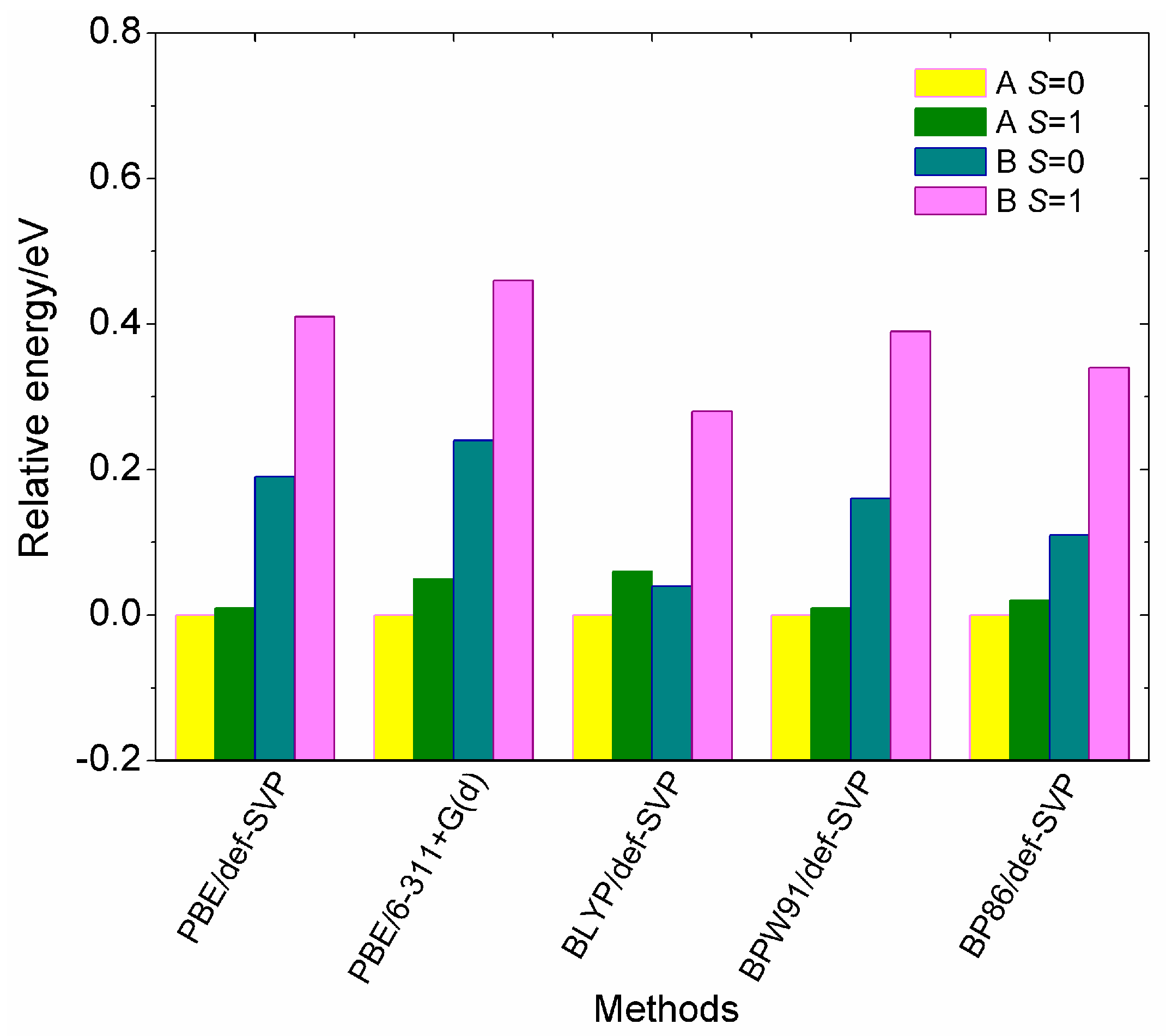
3.1. Structural Determination
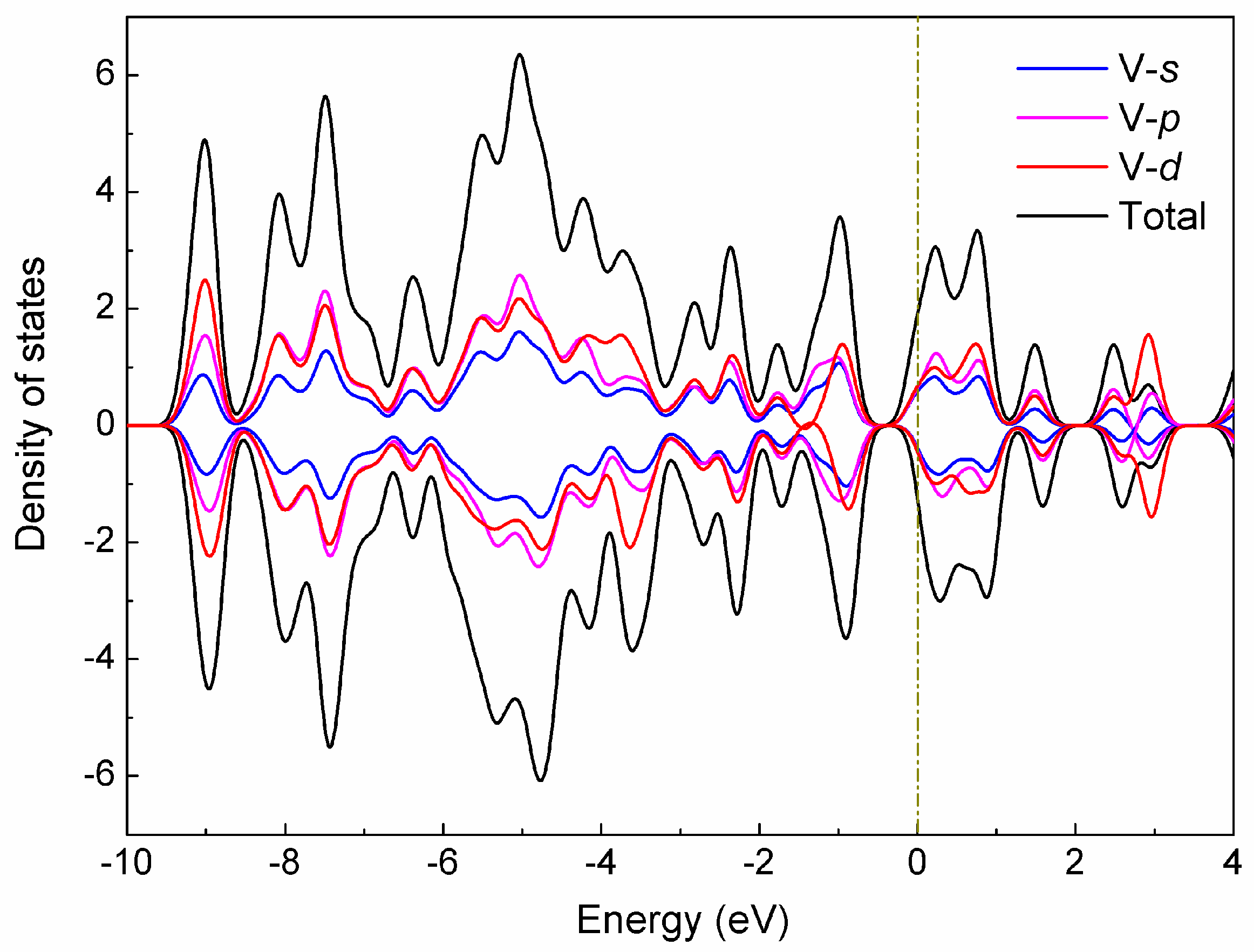
3.2. Electronic Structures
3.3. Chemical Bonding Analyses
3.4. Magnetic Moments and Charge Transfers
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Michaelian, K.; Rendón, N.; Garzón, I.L. Structure and energetics of Ni, Ag, and Au nanoclusters. Phys. Rev. B 1999, 60, 2000. [Google Scholar] [CrossRef]
- Schaefer, B.; Pal, R.; Khetrapal, N.S.; Amsler, M.; Sadeghi, A.; Zeng, V.B.X.C.; Goedecker, S.; Wang, L.-S. Isomerism and structural fluxionality in the Au26 and Au26− nanoclusters. ACS Nano 2014, 8, 7413–7422. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Zhai, H.J.; Wang, L.S. Au20: A tetrahedral cluster. Science 2003, 299, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Bowlan, J.; Harding, D.J.; Jalink, J.; Kirilyuk, A.; Meijer, G.; Fielicke, A. Communication: Structure of magnetic lanthanide clusters from far-ir spectroscopy:Tbn+ (n = 5–9). J. Chem. Phys. 2013, 138, 031102. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Huang, W.; Mei, W.-N.; Wang, L.-S.; Wu, Q.; Zeng, X.-C. Structural evolution of medium-sized gold clusters Aun− (n = 36, 37, 38): Appearance of bulk-like face centered cubic fragment. J. Phys. Chem. C 2014, 118, 6887–6892. [Google Scholar] [CrossRef]
- Du, J.; Sun, X.; Jiang, G. A theoretical study on Tan+ cluster cations: Structural assignments, stability, and electronic properties. J. Chem. Phys. 2012, 136, 094311. [Google Scholar] [CrossRef] [PubMed]
- Gruene, P.; Rayner, D.M.; Redlich, B.; van der Meer, A.F.G.; Lyon, J.T.; Meijer, G.; Fielicke, A. Structures of neutral Au7, Au19, and Au20 clusters in the gas phase. Science 2008, 321, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tian, H.; Hao, C.; Zhao, J.; Wang, Q.; Liu, E.; Dong, C. First-principles calculations of elastic moduli of Ti-Mo-Nb alloys using a cluster-plus-glue-atom model for stable solid solutions. J. Mater. Sci. 2013, 48, 3138–3146. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Mishra, B.K.; Deka, R.C. Effect of double aluminium doping on the structure, stability and electronic properties of small gold clusters. J. Mater. Sci. 2015, 50, 4586–4599. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.A.; Shibuta, Y.; Wales, D.J. Global minima of transition metal clusters described by finnis–sinclair potentials: A comparison with semiempirical molecular orbital theory. Philos. Mag. 2009, 89, 3311–3332. [Google Scholar] [CrossRef]
- Lozano, X.L.; Mottet, C.; Weissker, H.-C. Effect of alloying on the optical properties of Ag–Au nanoparticles. J. Phys. Chem. C 2013, 117, 3062–3068. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer: Berlin, Germany, 1995. [Google Scholar]
- Koppen, J.V.; Hapka, M.; Szczęśniak, M.M.; Chałasiński, G. Optical absorption spectra of gold clusters Aun (n = 4, 6, 8,12, 20) from long-range corrected functionals with optimal tuning. J. Chem. Phys. 2012, 137, 114302. [Google Scholar] [CrossRef] [PubMed]
- Billas, I.M.L.; Becker, J.A.; Châtelain, A.; de Heer, W.A. Magnetic moments of iron clusters with 25 to 700 atoms and their dependence on temperature. Phys. Rev. Lett. 1993, 71, 4067. [Google Scholar] [CrossRef] [PubMed]
- Iseda, M.; Nishio, T.; Han, S.Y.; Yoshida, H.; Terasaki, A.; Kondow, T. Electronic structure of vanadium cluster anions as studied by photoelectron spectroscopy. J. Chem. Phys. 1997, 106, 2182. [Google Scholar] [CrossRef]
- Bell, A.T. The impact of nanoscience on heterogeneous catalysis. Science 2003, 299, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Valden, M.; Lai, X.; Goodman, D.W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 1998, 281, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, X.; Zhang, H. Probing the activity of Ni13, Cu13, and Ni12Cu clusters towards the ammonia decomposition reaction by density functional theory. J. Mater. Sci. 2017, 52, 3162–3168. [Google Scholar] [CrossRef]
- Mariscal, M.M.; Oviedo, O.A.; Leiva, E.P.M. Metal Clusters and Nanoalloys: From Modeling to Applications; Springer: New York, NY, USA, 2013. [Google Scholar]
- Lee, K.; Callaway, J. Electronic structure and magnetism of small V and Cr clusters. Phys. Rev. B 1993, 48, 15358. [Google Scholar] [CrossRef]
- Douglass, D.C.; Bucher, J.P.; Bloomfield, L.A. Magnetic studies of free nonferromagnetic clusters. Phys. Rev. B 1992, 45, 6341. [Google Scholar] [CrossRef]
- Sun, H.; Luo, Y.-H.; Zhao, J.; Wang, G. Structural, electronic, and magnetic properties of small vanadium clusters. Phys. Status Solid B 1999, 215, 1127–1135. [Google Scholar] [CrossRef]
- Xu, J.; Rodgers, M.T.; Griffin, J.B.; Armentrout1, P.B. Guided ion beam studies of the reactions of Vn+ (n = 2–17) with O2: Bond energies and dissociation pathways. J. Chem. Phys. 1998, 108, 9339. [Google Scholar] [CrossRef]
- Jaeger, T.D.; Fielicke, A.; von Helden, G.; Meijer, G.; Duncan, M.A. Infrared spectroscopy of water adsorption on vanadium cluster cations (Vx+; x = 3–18). Chem. Phys. Lett. 2004, 392, 409–414. [Google Scholar] [CrossRef]
- Zakin, M.R.; Cox, D.M.; Whetten, R.L.; Trevor, D.J.; Kaldor, A. Effect of hydrogen chemisorption on the photoionization threshold of isolated transition metal clusters. Chem. Phys. Lett. 1987, 135, 223–228. [Google Scholar] [CrossRef]
- Fielicke, A.; von Helden, G.; Meijer, G. Far-infrared spectroscopy of isolated transition metal clusters. Eur. Phys. J. D 2005, 34, 83–88. [Google Scholar] [CrossRef]
- Ratsch, C.; Fielicke, A.; Kirilyuk, A.; Behler, J.; von Helden, G.; Meijer, G.; Scheffler, M. Structure determination of small vanadium clusters by density-functional theory in comparison with experimental far-infrared spectra. J. Chem. Phys. 2005, 122, 124302. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Sun, Q.; Liu, F.; Wang, G.; Lain, K.D. Tight-binding study of the structural and magnetic properties of vanadium clusters. Phys. B Condens. Matter 1995, 215, 377–382. [Google Scholar]
- Taneda, A.; Shimizu, T.; Kawazoe, Y. Stable disordered structures of vanadium clusters. J. Phys. Condens. Matter 2001, 13, L305–L312. [Google Scholar] [CrossRef]
- Fielicke, A.; Kirilyuk, A.; Ratsch, C.; Behler, J.; Scheffler, M.; von Helden, G.; Meijer, G. Structure determination of isolated metal clusters via far-infrared spectroscopy. Phys. Rev. Lett. 2004, 93, 023401. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Desai, S.R.; Wang, L.-S. Evolution of the electronic structure of small vanadium clusters from molecular to bulklike. Phys. Rev. Lett. 1996, 77, 2436. [Google Scholar] [CrossRef] [PubMed]
- Su, C.X.; Hales, D.A.; Armentrout, P.B. Collision-induced dissociation of Vn+ (n = 2–20) with Xe: Bond energies, dissociation pathways, and structures. J. Chem. Phys. 1993, 99, 6613. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D. 01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Erratum: Generalized gradient approximation made simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822. [Google Scholar] [CrossRef]
- Perdew, J.P. Erratum: Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 34, 7406. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. Cclib: A library for packageindependent computational chemistry algorithms. J. Comp. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Zubarev, D.Y.; Boldyrev, A.I. Developing paradigms of chemical bonding: Adaptive natural density partitioning. Phys. Chem. Chem. Phys. 2008, 10, 5207–5217. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhao, J.; Wang, J.; Wang, B.; Lu, Q.; Wang, G. Growth behavior and magnetic properties of SinFe (n = 2–14) clusters. Phys. Rev. B 2006, 73, 125439. [Google Scholar] [CrossRef]
- Zhao, W.-J.; Wang, Y.-X. Geometries, stabilities, and electronic properties of FeGen (n = 9–16) clusters: Density-functional theory investigations. Chem. Phys. 2008, 352, 291–296. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic population analysis on LCAO–MO molecular wave functions. I. J. Chem. Phys. 1955, 23, 1833–1838. [Google Scholar] [CrossRef]
- Zubarev, D.Y.; Boldyrev, A.I. Revealing intuitively assessable chemical bonding patterns in organic aromatic molecules via adaptive natural density partitioning. J. Org. Chem. 2008, 73, 9251–9258. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, H.; Song, X.Y.J. Exploring the chemical bonding, infrared and UV–vis absorption spectra of OH radicals adsorption on the smallest fullerene. Spectrochim. Acta Part A 2015, 144, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.-J.; Zhao, Y.-F.; Li, W.-L.; Chen, Q.; Bai, H.; Hu, H.-S.; Piazza, Z.A.; Tian, W.-J.; Lu, H.-G.; Wu, Y.-B.; et al. Observation of an all-boron fullerene. Nat. Chem. 2014, 6, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Popov, I.A.; Li, W.-L.; Piazza, Z.A.; Boldyrev, A.I.; Wang, L.-S. Complexes between planar boron clusters and transition metals: A photoelectron spectroscopy and ab initio study of CoB12− and RhB12−. J. Phys. Chem. A 2014, 118, 8098–8105. [Google Scholar] [CrossRef] [PubMed]
- Galeev, T.R.; Boldyrev, A.I. Recent advances in aromaticity and antiaromaticity in transition-metal systems. Annu. Rep. Prog. Chem. Sect. C 2011, 107, 124–147. [Google Scholar] [CrossRef]
- Zubarev, D.Y.; Boldyrev, A.I. Deciphering chemical bonding in golden cages. J. Phys. Chem. A 2009, 113, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, Z.; Li, S. The nature of structure and bonding between transition metal and mixed Si-Ge tetramers: A 20-electron superatom system. J. Comput. Chem. 2016, 37, 2316–2323. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ren, H.; Huang, X.; Li, S. First-Principles Study of Structural, Electronic and Magnetic Properties of Metal-Centered Tetrahexahedral V15+ Cluster. Nanomaterials 2017, 7, 164. https://doi.org/10.3390/nano7070164
Li X, Ren H, Huang X, Li S. First-Principles Study of Structural, Electronic and Magnetic Properties of Metal-Centered Tetrahexahedral V15+ Cluster. Nanomaterials. 2017; 7(7):164. https://doi.org/10.3390/nano7070164
Chicago/Turabian StyleLi, Xiaojun, Hongjiang Ren, Xinwei Huang, and Shuna Li. 2017. "First-Principles Study of Structural, Electronic and Magnetic Properties of Metal-Centered Tetrahexahedral V15+ Cluster" Nanomaterials 7, no. 7: 164. https://doi.org/10.3390/nano7070164