Overcoming the Intrinsic Difference between Hydrophilic CH3NH3PbI3 and Hydrophobic C60 Thin Films to Improve the Photovoltaic Performance
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.H.; Tseng, Z.L.; Wu, C.G. Planar heterojunction perovskite/PC71BM solar cells with enhanced open-circuit voltage via a (2/1)-step spin-coating process. J. Mater. Chem. A 2014, 2, 15897–15903. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Huang, F.; Huang, W.; Dkhissi, Y.; Zhu, Y.; Etherigde, J.; Gray-Weale, A.; Bach, U.; Cheng, Y.B.; Spiccia, L. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. 2014, 126, 10056–10061. [Google Scholar] [CrossRef]
- Wolf, S.D.; Holovsky, J.; Moon, S.J.; Loper, P.; Niesen, B.; Ledinsky, M.; Haug, F.J.; Yum, J.H.; Ballif, C. Organometallic halide perovskites: Sharp optical absorption edge and its relation to photovoltaic performance. J. Phys. Chem. Lett. 2014, 5, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kondo, T. Bandgap and exciton binding energies in lead-iodide-based natural quantum-well crystals. Sci. Technol. Adv. Mater. 2003, 4, 599–604. [Google Scholar] [CrossRef]
- Lin, Q.; Armin, A.; Nagiri, R.C.R.; Burn, P.L.; Meredith, P. Electro-optics of perovskite solar cells. Nat. Photonics 2015, 9, 106–112. [Google Scholar] [CrossRef]
- Yang, Y.; Ostrowski, D.P.; France, R.M.; Zhu, K.; Lagemaat, J.; van de Lagemaat, J.; Luther, J.M.; Beard, M.C. Observation of a hot-phonon bottleneck in lead-iodide perovskites. Nat. Photonics 2016, 10, 53–59. [Google Scholar] [CrossRef]
- Chen, C.C.; Chang, S.H.; Chen, L.C.; Tsai, C.L.; Cheng, H.M.; Huang, W.C.; Chen, W.N.; Lu, Y.C.; Tseng, Z.L.; Chiu, K.Y.; et al. Interplay between nucleation and crystal growth during the formation of CH3NH3PbI3 thin films and their application in solar cells. Sol. Energy Mater. Sol. Cells 2017, 159, 583–589. [Google Scholar] [CrossRef]
- Stranks, S.D.; Burlakov, V.M.; Leijtens, T.; Ball, J.M.; Goriely, A.; Snaith, H.J. Recombination kinetics in organic-inorganic perovskites excitations, free charge, and subgap states. Phys. Rev. Appl. 2014, 2, 034007. [Google Scholar] [CrossRef]
- Jiang, Y.; Wen, X.; Benda, A.; Sheng, R.; Ho-Baillie, A.W.Y.; Huang, S.; Huang, F.; Cheng, Y.B.; Green, M.A. Time-resolved fluorescence anisotropy study of organic lead halide perovskite. Sol. Energy Mater. Sol. Cells 2016, 151, 102–112. [Google Scholar] [CrossRef]
- Chen, C.C.; Chang, S.H.; Chen, L.C.; Kao, F.S.; Cheng, H.M.; Yeh, S.C.; Chen, C.T.; Wu, W.T.; Tseng, Z.L.; Chuang, C.L.; et al. Improving the efficiency of inverted mixed-organic-cation perovskite absorber based photovoltaics by tailing the surface roughness of PEDOT:PSS thin film. Sol. Energy 2016, 134, 445–451. [Google Scholar] [CrossRef]
- Zhao, T.; Shi, W.; Xi, J.; Wang, D.; Shuai, Z. Intrinsic and extrinsic charge transport in CH3NH3PbI3 perovskites predicted from first-principles. Sci. Rep. 2016, 6, 19968. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Lin, K.-F.; Cheng, H.-M.; Chen, C.-C.; Wu, W.-T.; Chen, W.-N.; Wu, P.-J.; Chen, S.-H.; Wu, C.-G. Influence of organic cations on high-performance CH3NH3PbI3 based photovoltaics. Sol. Energy Mater. Sol. Cells 2016, 145, 375–381. [Google Scholar] [CrossRef]
- Guo, Z.; Manser, J.S.; Wan, Y.; Kamat, P.V.; Huang, L. Spatial and temporal imaging of long-range charge transport in perovskite thin films by ultrafast microscopy. Nat. Commun. 2015, 6, 7471. [Google Scholar] [CrossRef] [PubMed]
- Milot, R.L.; Eperon, G.E.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Temperature-dependent charge-carrier dynamics in CH3NH3PbI3 perovskite thin films. Adv. Funct. Mater. 2015, 25, 6218–6227. [Google Scholar] [CrossRef]
- Wang, J.L.H.; Li, H.; Zuo, C.; Wang, H. Thermal stability and optimal photoinduced hydrophilicity of mesoporous TiO2 thin films. J. Phys. Chem. C 2012, 116, 9517–9525. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskite as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.-Y.; Chiang, Y.-F.; Lee, M.-H.; Peng, S.-R.; Guo, T.-F.; Chen, P.; Wen, T.-C. CH3NH3PbI3 perovskite/fullerene planar-heterojunction hybrid solar cells. Adv. Mater. 2013, 25, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- National Renewable Energy Laboratory (NREL) Best Research-Cell Efficiency Char. Available online: http://www.nrel.gov/ncpv/images/efficiency_chart.jpg (accessed on 29 June 2016).
- Rao, H.; Ye, S.; Sun, W.; Yan, W.; Li, Y.; Peng, H.; Liu, Z.; Bian, Z.; Li, Y.; Huang, C. A 19% efficiency achieved in CuOx-based inverted CH3NH3PbIx−3Clx solar cells by an effective Cl doping method. Nano Energy 2016, 27, 51–57. [Google Scholar] [CrossRef]
- Bi, C.; Shao, Y.; Yuan, Y.; Xiao, Z.; Wang, C.; Gao, Y.; Huang, J. Understanding the formation and evolution of interdiffusion grown organolead halide perovskite thin films by thermal annealing. J. Mater. Chem. A 2014, 2, 18508–18514. [Google Scholar] [CrossRef]
- You, J.; Yang, Y.; Hong, Z.; Song, T.-B.; Meng, L.; Liu, Y.; Jiang, C.; Zhou, H.; Chang, W.-H.; Li, G.; et al. Moisture assisted perovskite film growth for high performance solar cells. Appl. Phys. Lett. 2014, 105, 183902. [Google Scholar] [CrossRef]
- Lin, K.-F.; Chang, S.H.; Wang, K.-H.; Cheng, H.-M.; Chiu, K.Y.; Lee, K.-M.; Chen, S.-H.; Wu, C.-G. Unraveling the high performance of tri-iodide perovskite absorber based photovoltaics with a non-polar solvent washing treatment. Sol. Energy Mater. Sol. Cells 2015, 141, 309–314. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.-Y.; Jang, I.-H.; Kang, S.M.; Choi, M.; Park, N.-G. Highly reproducible perovskite solar cells with average efficiency of 18.3% and best efficiency of 19.7% fabricated via lewis based adduct of lead(II) iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Zhang, W.-H.; Qu, J. Solvent engineering of spin-coating solutions for planar-structured high-efficiency perovskite solar cells. Chin. J. Catal. 2015, 36, 1183–1190. [Google Scholar] [CrossRef]
- Cohen, B.-E.; Etgar, L. Parameters that control and influence the organo-metal halide perovskite crystallization and morphology. Front. Optoelectron. 2016, 9, 44–52. [Google Scholar] [CrossRef]
- Yoon, H.; Kang, M.S.; Lee, J.-K.; Choi, M. Hysteresis-free low-temperature-processed planar perovskite solar cells with 19.1% efficiency. Energy Environ. Sci. 2016, 9, 2262–2266. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Qin, C.; Yang, X.; Yasuda, T.; Islam, A.; Zhang, K.; Peng, W.; Chen, W.; Han, L. A dopant-free hole-transporting material for efficient and stable perovskite solar cells. Energy Environ. Sci. 2014, 7, 2963–2967. [Google Scholar] [CrossRef]
- Hau, S.K.; Yip, H.-L.; Acton, O.; Baek, N.S.; Ma, H.; Jen, A.K.-Y. Interfacial modification to improve inverted polymer solar cells. J. Mater. Chem. 2008, 18, 5113–5119. [Google Scholar] [CrossRef]
- Bi, C.; Wang, Q.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef] [PubMed]
- Tikhonravov, A.V.; Trubetskov, M.K.; Tikhonravov, A.A.; Duparre, A. Effects of interface roughness on the spectral properties of thin films and multilayers. Appl. Opt. 2003, 42, 5140–5148. [Google Scholar] [CrossRef] [PubMed]
- Leijtens, T.; Eperon, G.E.; Barker, A.J.; Grancini, G.; Zhang, W.; Ball, J.M.; Kandada, A.R.S.; Snaith, H.J.; Petrozza, A. Carrier trapping and recombination: The role of defect physics in enhancing the open circuit voltage of metal halide perovskite solar cells. Energy Environ. Sci. 2016, 9, 3472–3481. [Google Scholar] [CrossRef]
- Shao, Y.; Xiao, Z.; Bi, C.; Yuan, Y.; Huang, J. Origin and elimination of photocurrent hysteresis by fullerene passivation in CH3NH3PbI3 planar heterojunction solar cells. Nat. Commun. 2014, 5, 5784. [Google Scholar] [CrossRef] [PubMed]
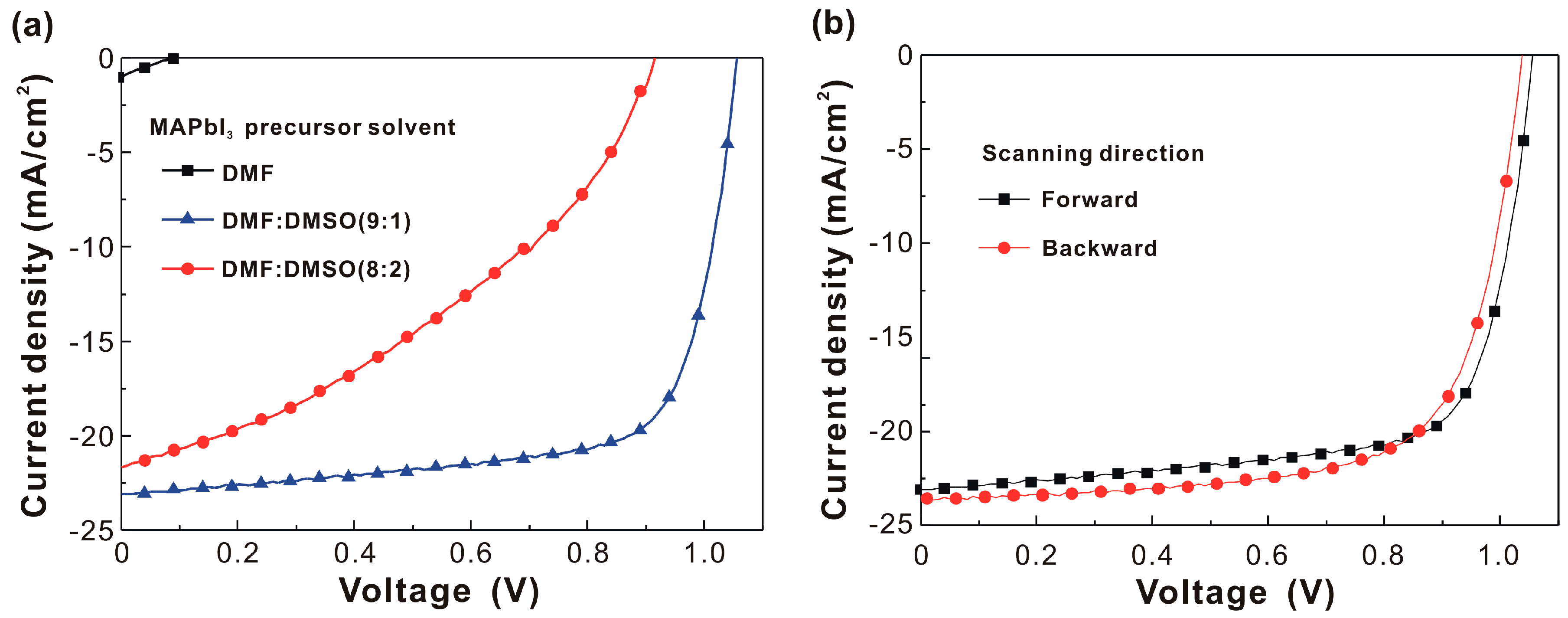
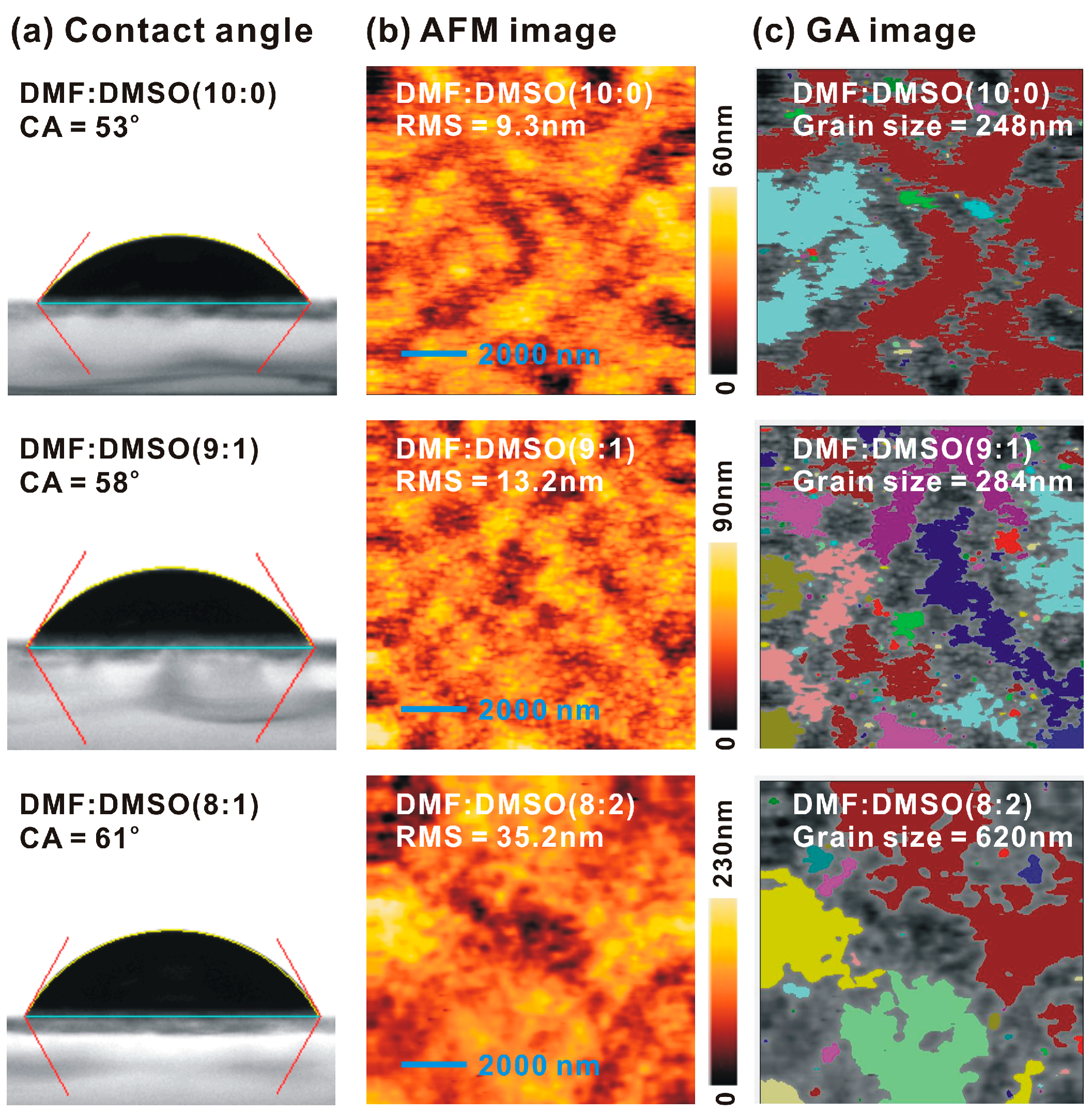
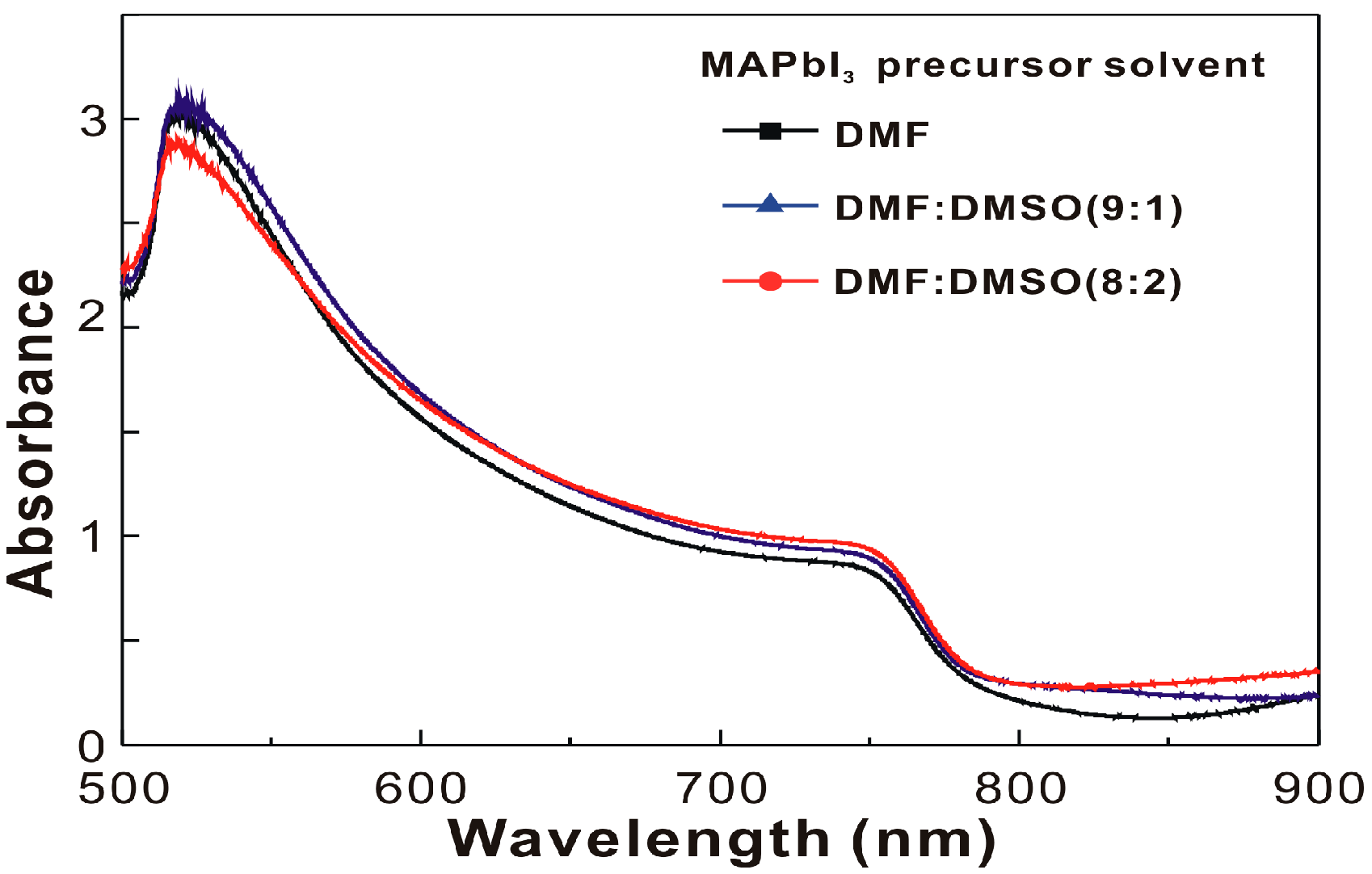
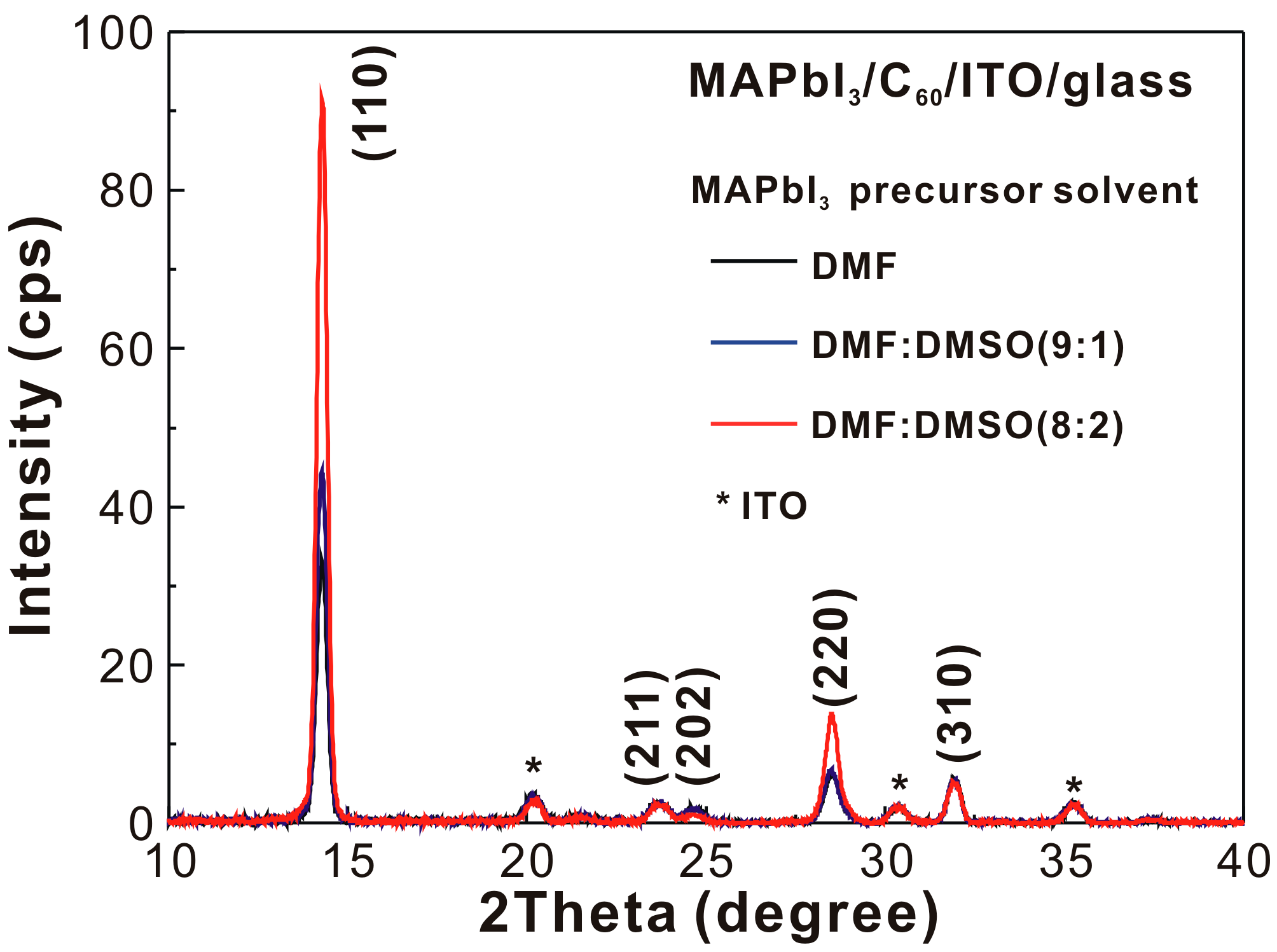
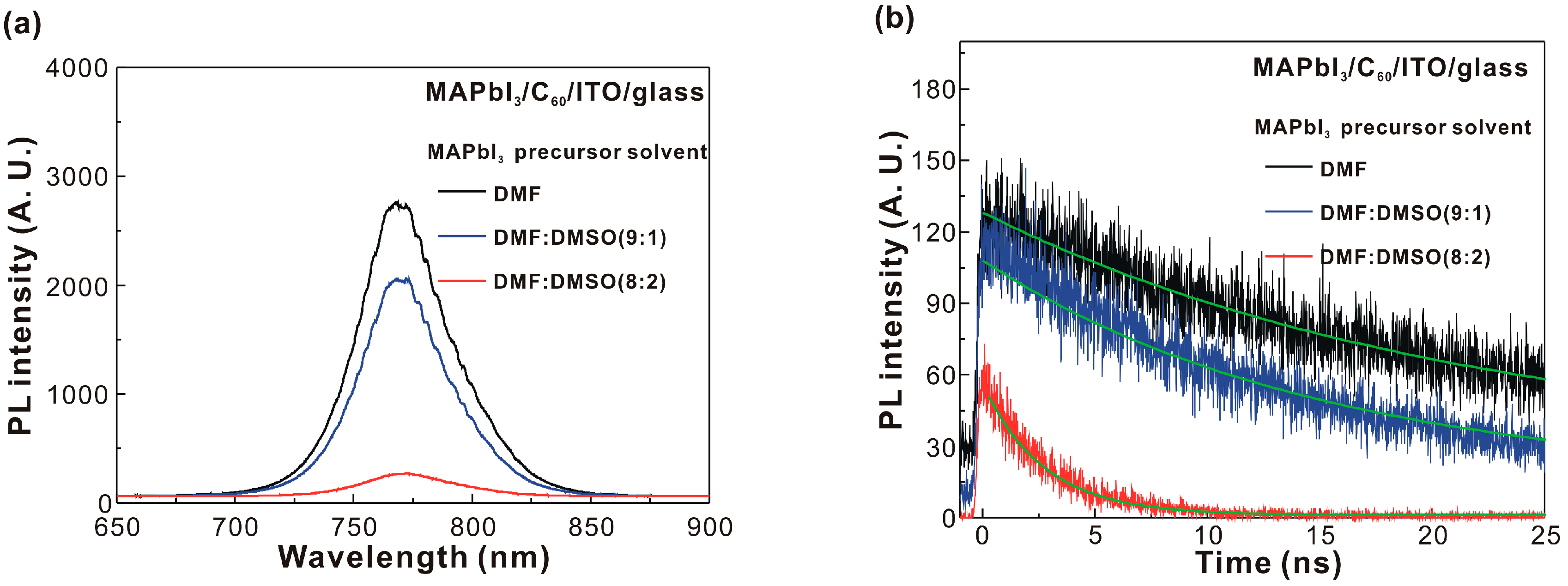
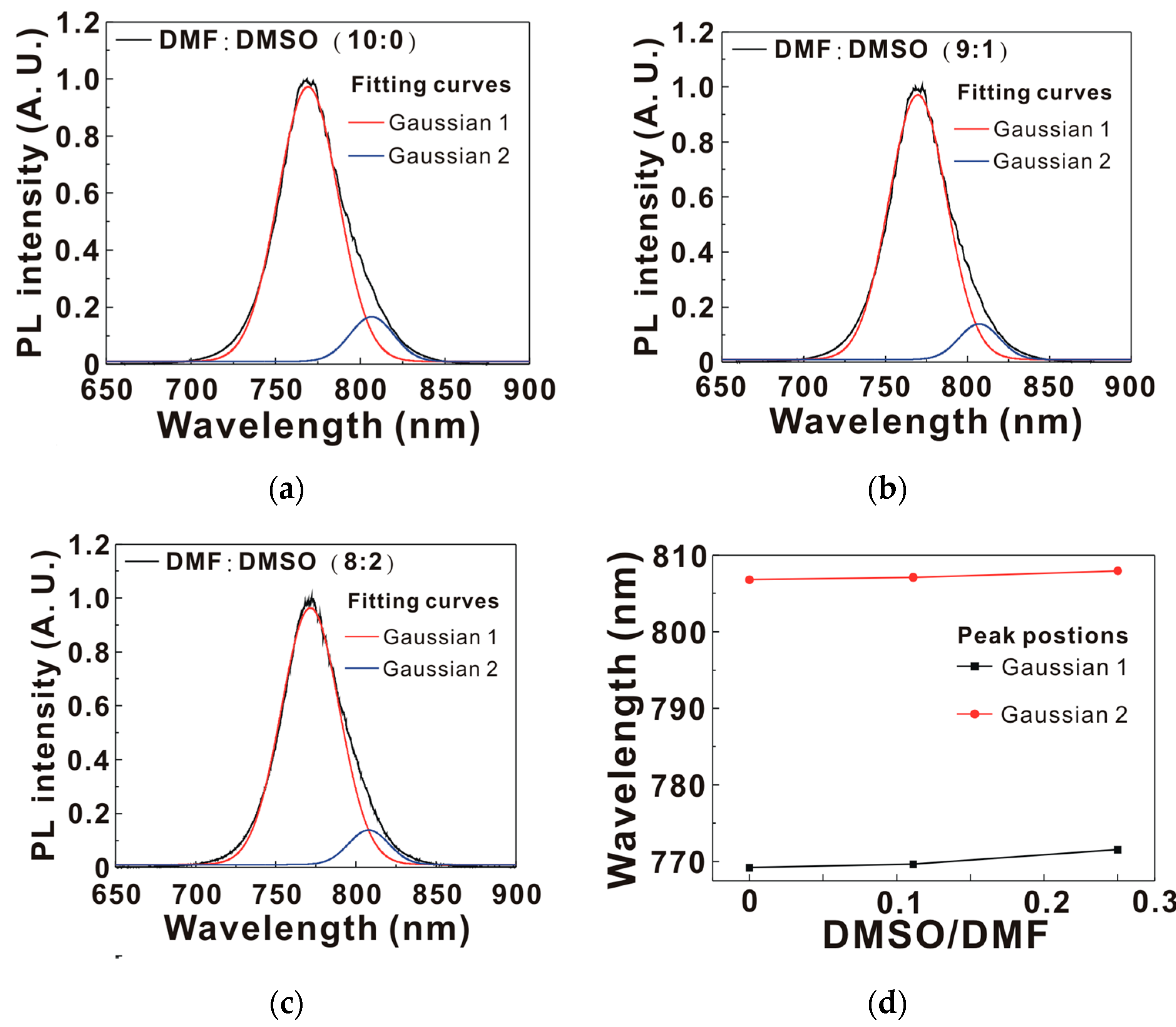
| MAPbI3 Precursor Solvent | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| DMF | 0.106 ± 0.028 | 0.84 ± 0.64 | 22.9 ± 0.5 | 0.02 ± 0.01 |
| DMF:DMSO (9:1) | 1.045 ± 0.011 | 22.10 ± 1.17 | 70.1 ± 3.2 | 16.21 ± 1.29 |
| DMF:DMSO (9:1) a | 1.056 | 23.11 | 71.9 | 17.55 |
| DMF:DMSO (9:1) b | 1.040 | 23.69 | 69.6 | 17.20 |
| DMF:DMSO (8:2) | 0.929 ± 0.032 | 21.58 ± 0.88 | 39.2 ± 5.2 | 7.80 ± 1.40 |
| MAPbI3 Precursor Solvent | PVH (nm) | D (nm) | IXRD | PG1 (nm) | PG2 (nm) | Ratio | τ (ns) |
|---|---|---|---|---|---|---|---|
| DMF | 68 | 22.3 | 33 | 769.2 | 806.8 | 8.47 | 21.55 |
| DMF:DMSO (9:1) | 144 | 22.7 | 44 | 769.6 | 807.1 | 10.71 | 15.13 |
| DMF:DMSO (8:2) | 268 | 24.0 | 91 | 771.6 | 808.0 | 10.76 | 2.71 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-C.; Lin, Y.-S.; Tseng, Z.-L.; Wu, C.; Kao, F.-S.; Chen, S.-H. Overcoming the Intrinsic Difference between Hydrophilic CH3NH3PbI3 and Hydrophobic C60 Thin Films to Improve the Photovoltaic Performance. Nanomaterials 2017, 7, 166. https://doi.org/10.3390/nano7070166
Chen L-C, Lin Y-S, Tseng Z-L, Wu C, Kao F-S, Chen S-H. Overcoming the Intrinsic Difference between Hydrophilic CH3NH3PbI3 and Hydrophobic C60 Thin Films to Improve the Photovoltaic Performance. Nanomaterials. 2017; 7(7):166. https://doi.org/10.3390/nano7070166
Chicago/Turabian StyleChen, Lung-Chien, Yu-Shiang Lin, Zong-Liang Tseng, Chiale Wu, Feng-Sheng Kao, and Sheng-Hui Chen. 2017. "Overcoming the Intrinsic Difference between Hydrophilic CH3NH3PbI3 and Hydrophobic C60 Thin Films to Improve the Photovoltaic Performance" Nanomaterials 7, no. 7: 166. https://doi.org/10.3390/nano7070166






