Synthesis and Self-Assembled Behavior of pH-Responsive Chiral Liquid Crystal Amphiphilic Copolymers Based on Diosgenyl-Functionalized Aliphatic Polycarbonate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structural Characterization of mPEG43-b-PMBC40
2.2. Synthesis and Structural Characterization of mPEG43-b-PMCC40
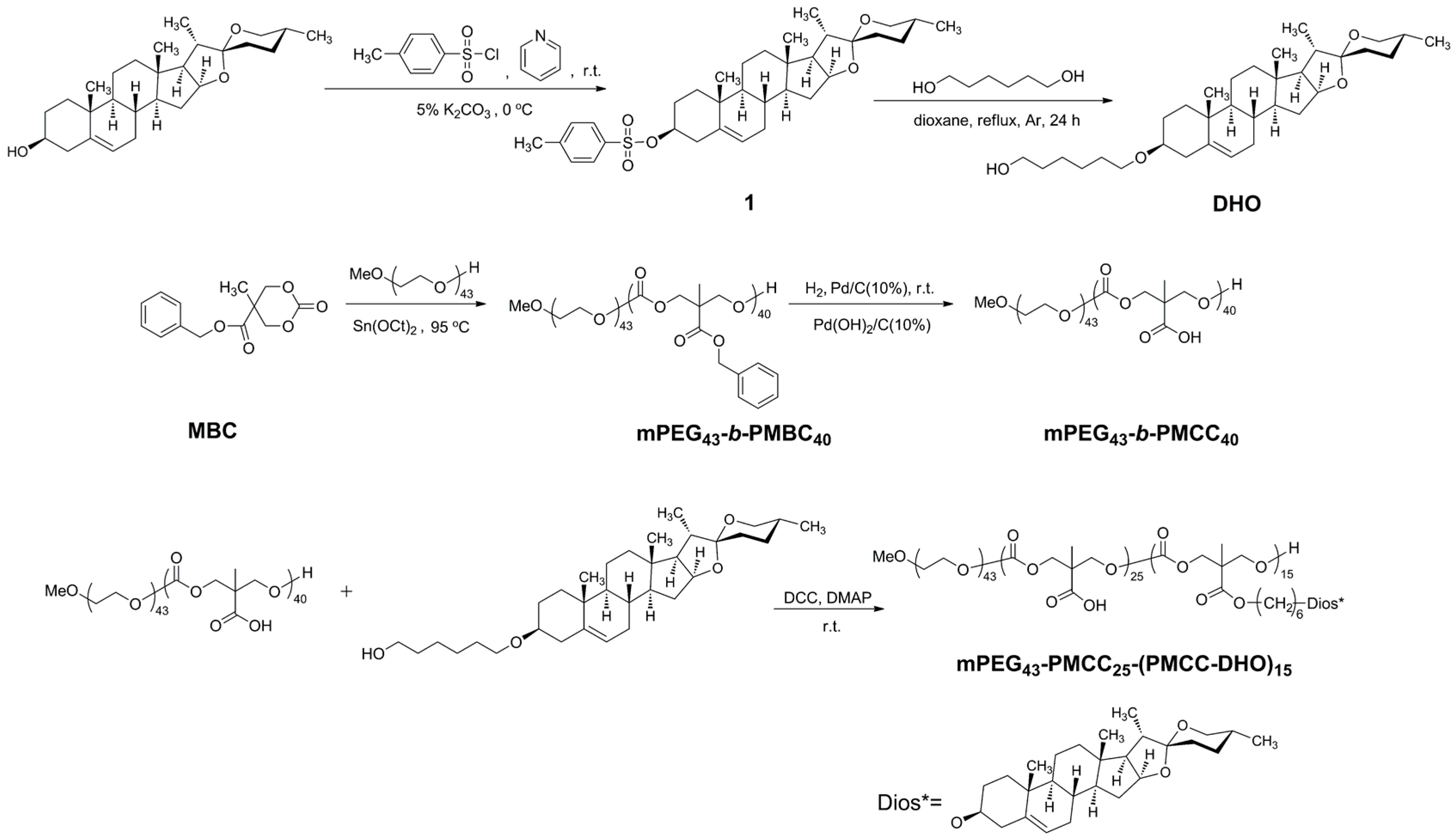
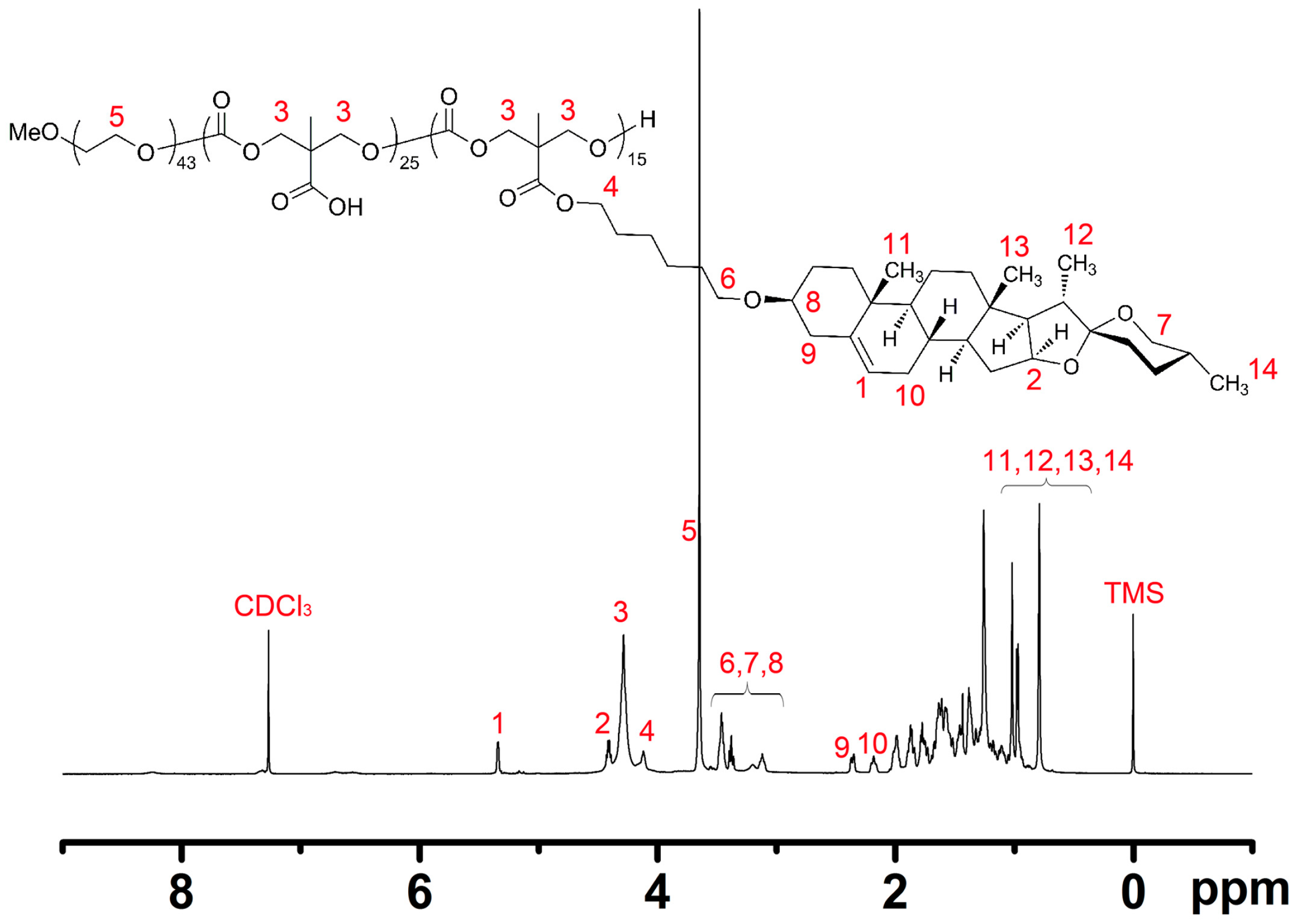
2.3. Synthesis and Structural Characterization of mPEG43-PMCC25-P(MCC-DHO)15
2.4. Liquid Crystal Behaviour of mPEG43-PMCC25-P(MCC-DHO)15
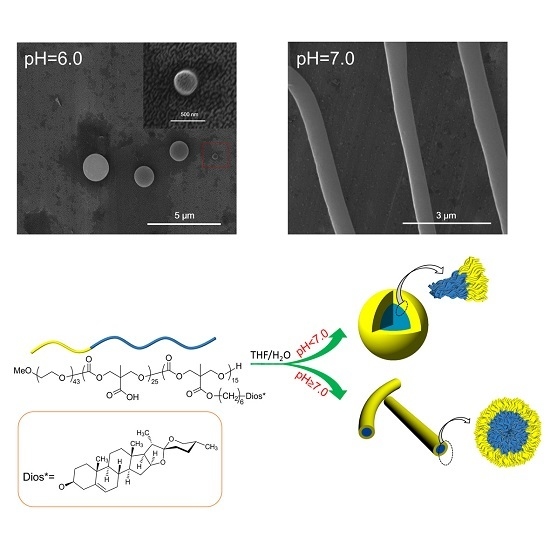
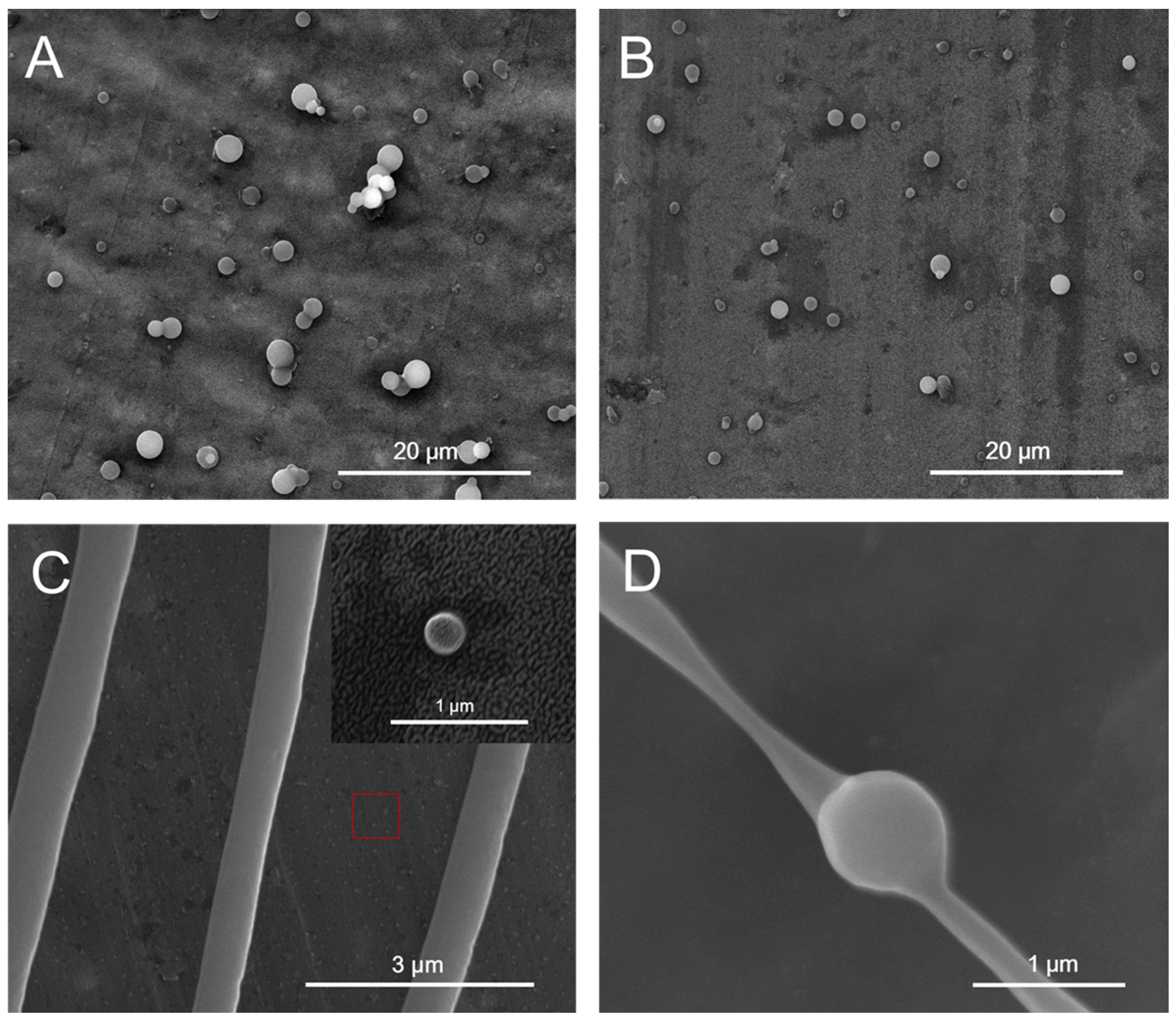
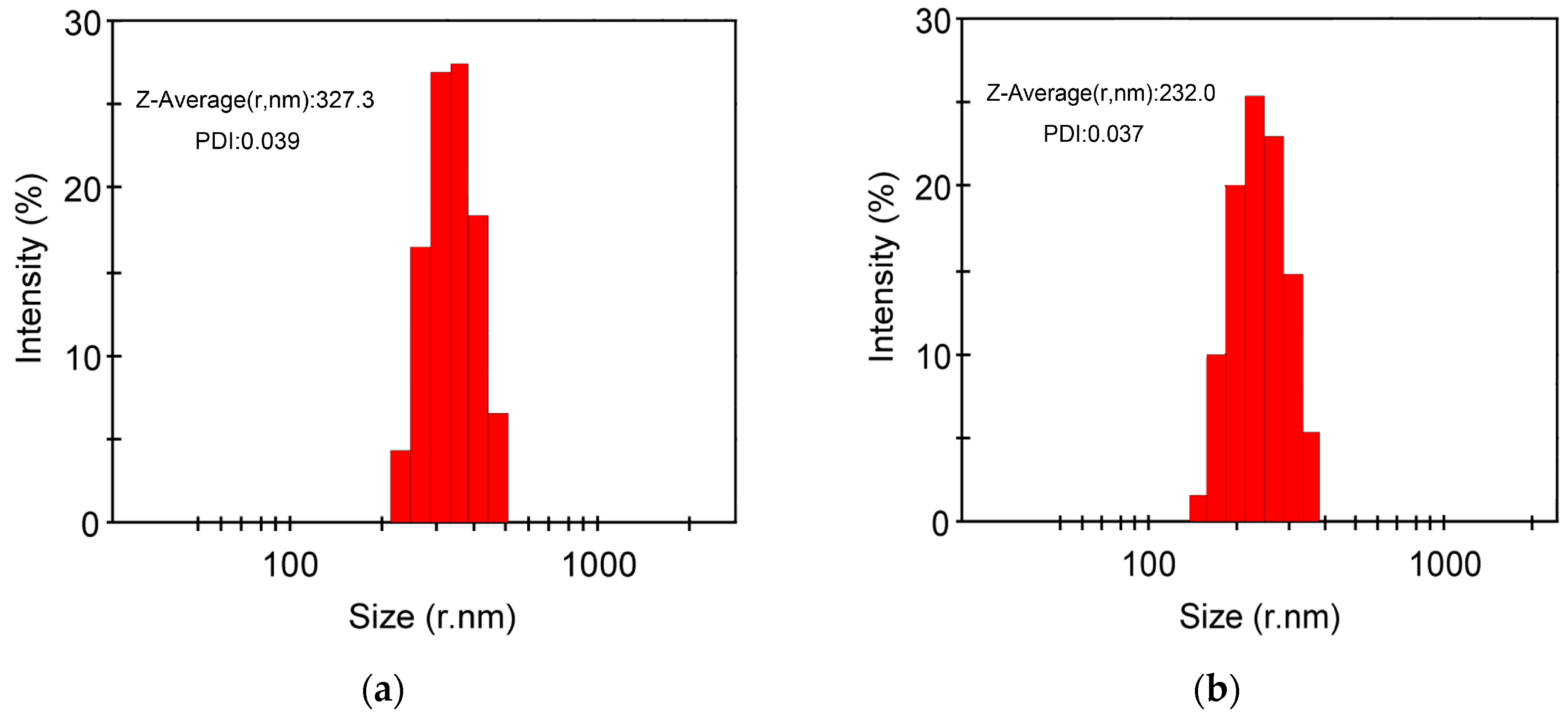
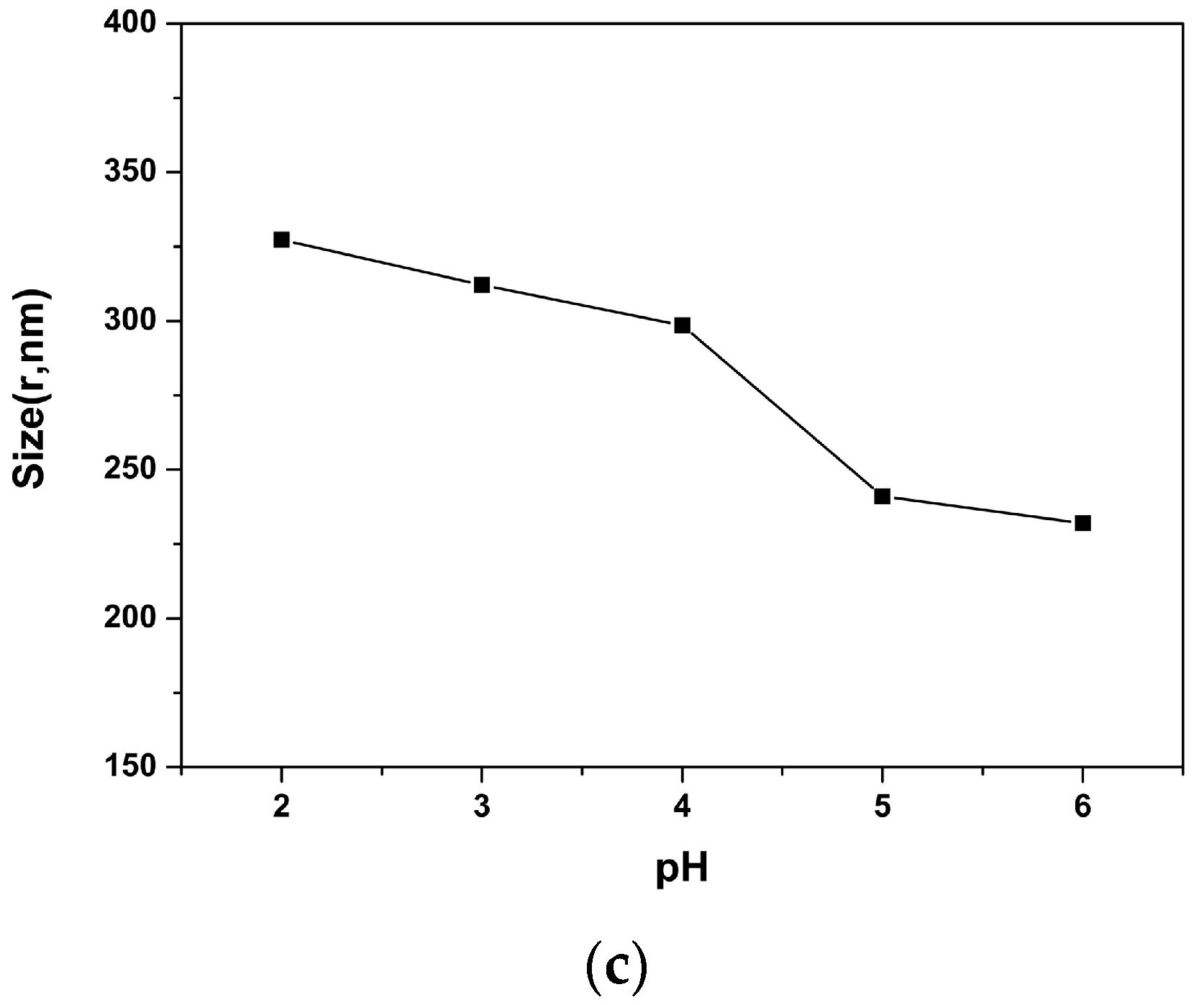
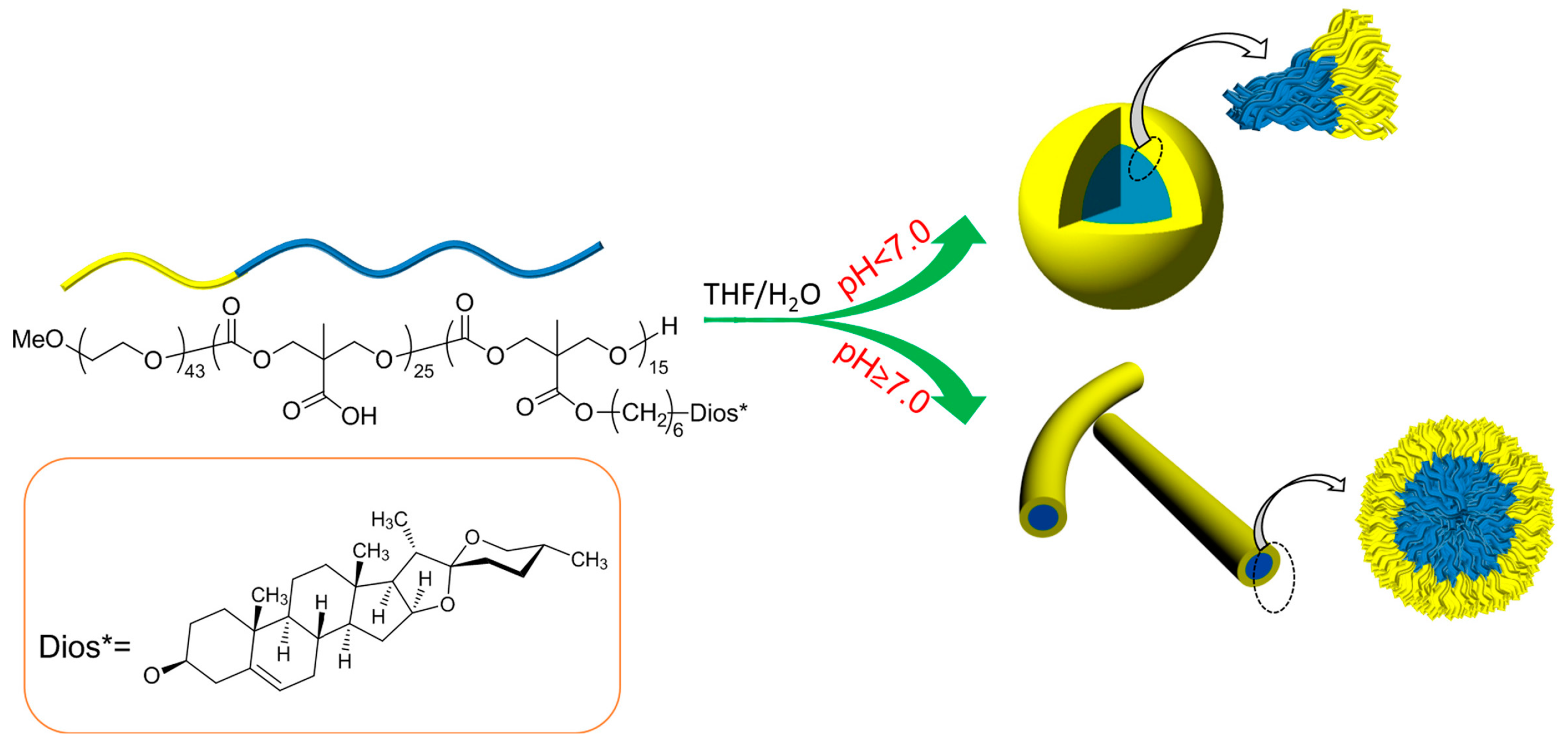
2.5. pH Responsive Self-Assembly of mPEG43-PMCC25-P(MCC-DHO)15
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Surnar, B.; Jayakannan, M. Stimuli-responsive poly (caprolactone) vesicles for dual drug delivery under the gastrointestinal tract. Biomacromolecules 2013, 14, 4377–4387. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Lee, A.L.; Maune, H.T. Formation of disk-and stacked-disk-like self-assembled morphologies from cholesterol-functionalized amphiphilic polycarbonate diblock copolymers. Macromolecules 2013, 46, 4839–4846. [Google Scholar] [CrossRef]
- Piñol, R.; Jia, L.; Gubellini, F. Self-assembly of PEG-b-liquid crystal polymer: The role of smectic order in the formation of nanofibers. Macromolecules 2007, 40, 5625–5627. [Google Scholar] [CrossRef]
- Jia, L.; Cao, A.; Lévy, D. Smectic polymer vesicles. Soft Matter 2009, 5, 3446–3451. [Google Scholar] [CrossRef]
- McKenzie, B.E.; Nudelman, F.; Bomans, P.H. Temperature-responsive nanospheres with bicontinuous internal structures from a semicrystalline amphiphilic block copolymer. J. Am. Chem. Soc. 2010, 132, 10256–10259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Dong, C.M. Bioreducible and core-crosslinked hybrid micelles from trimethoxysilyl-ended poly (ε-caprolactone)-S-S-poly (ethylene oxide) block copolymers: Thiol-ene click synthesis and properties. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1645–1656. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, C.; Jin, H. A supramolecular Janus hyperbranched polymer and its photoresponsive self-assembly of vesicles with narrow size distribution. J. Am. Chem. Soc. 2013, 135, 4765–4770. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhao, L.; Akinc, M. Novel amphiphilic multi-arm, star-like block copolymers as unimolecular micelles. Macromolecules 2011, 44, 3746–3752. [Google Scholar] [CrossRef]
- Piyapakorn, P.; Akagi, T.; Hachisuka, M. Structural Analysis of Unimer Nanoparticles Composed of Hydrophobized Poly (amino acid) s. Macromolecules 2013, 46, 6187–6194. [Google Scholar] [CrossRef]
- He, J.; Huang, X.; Li, Y.C. Self-assembly of amphiphilic plasmonic micelle-like nanoparticles in selective solvents. J. Am. Chem. Soc. 2013, 135, 7974–7984. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Cheng, Y.; Ranatunga, R.U. A combined experimental and computational study of the substituent effect on micellar behavior of γ-substituted thermoresponsive amphiphilic poly (ε-caprolactone) s. Macromolecules 2013, 46, 4829–4838. [Google Scholar] [CrossRef]
- Sun, L.; Petzetakis, N.; Pitto-Barry, A. Tuning the size of cylindrical micelles from poly (L-lactide)-b-poly (acrylic acid) diblock copolymers based on crystallization-driven self-assembly. Macromolecules 2013, 46, 9074–9082. [Google Scholar] [CrossRef]
- Glavas, L.; Olsén, P.; Odelius, K. Achieving micelle control through core crystallinity. Biomacromolecules 2013, 14, 4150–4156. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Akiba, I.; Harrisson, S. Facile Formation of Uniform Shell-Crosslinked Nanoparticles with Built-in Functionalities from N-Hydroxysuccinimide-Activated Amphiphilic Block Copolymers. Adv. Funct. Mater. 2008, 18, 551–559. [Google Scholar] [CrossRef]
- Chang, H.Y.; Lin, Y.L.; Sheng, Y.J. Structural Characteristics and Fusion Pathways of Onion-Like Multilayered Polymersome Formed by Amphiphilic Comb-Like Graft Copolymers. Macromolecules 2013, 46, 5644–5656. [Google Scholar] [CrossRef]
- Ray, J.G.; Naik, S.S.; Hoff, E.A. Stimuli-Responsive Peptide-Based ABA-Triblock Copolymers: Unique Morphology Transitions With pH. Macromol. Rapid Commun. 2012, 33, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Luo, Z.; Li, N. Amphiphilic polymeric nanocarriers with luminescent gold nanoclusters for concurrent bioimaging and controlled drug release. Adv. Funct. Mater. 2013, 23, 4324–4331. [Google Scholar] [CrossRef]
- Shen, J.; Chen, C.; Fu, W. Conformation-specific self-assembly of thermo-responsive poly (ethylene glycol)-b-polypeptide diblock copolymer. Langmuir 2013, 29, 6271–6278. [Google Scholar] [CrossRef] [PubMed]
- Korchagina, E.V.; Qiu, X.P.; Winnik, F.M. Effect of heating rate on the pathway for vesicle formation in salt-free aqueous solutions of thermosensitive cationic diblock copolymers. Macromolecules 2013, 46, 2341–2351. [Google Scholar] [CrossRef]
- Zhao, J.; Jeromenok, J.; Weber, J. Thermoresponsive aggregation behavior of triterpene-poly (ethylene oxide) conjugates in water. Macromol. Biosci. 2012, 12, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Stuart, M.; Vorenkamp, J. One-Pot Synthesis for Biocompatible Amphiphilic Hyperbranched Polyurea Micelles. Macromolecules 2013, 46, 4418–4425. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, S. Luminescent invertible polymersome by remarkably stable supramolecular assembly of naphthalene diimide (NDI) π-system. Macromolecules 2013, 46, 3939–3949. [Google Scholar] [CrossRef]
- Lee, C.H.; Wong, C.H.; Ouhab, D. Synthesis and characterization of solvent-invertible amphiphilic hollow particles. Langmuir 2013, 29, 7583–7590. [Google Scholar] [CrossRef] [PubMed]
- Hevus, I.; Modgil, A.; Daniels, J. Invertible micellar polymer assemblies for delivery of poorly water-soluble drugs. Biomacromolecules 2012, 13, 2537–2545. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S.; Stayton, P.S.; El-Sayed, M.E.H. Design of “Smart” Nano-Scale Delivery Systems for Biomolecular Therapeutics. J. Biomed. Nanotechnol. 2007, 3, 213–217. [Google Scholar] [CrossRef]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Zhang, X.N.; Song, Z.W. Study on new chiral liquid crystalline monomers and polymers containing menthyl groups. Liq. Cryst. 2014, 41, 986–999. [Google Scholar] [CrossRef]
- Hwang, J.J.; Iyer, S.N.; Li, L.S. Self-assembling biomaterials: liquid crystal phases of cholesteryl oligo (L-lactic acid) and their interactions with cells. Proc. Natl. Acad. Sci. USA 2002, 99, 9662–9667. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sun, J.; Cao, H. Synthesis and characterization of functionalized triblock polymer: The prepared polymer is cholesteryl terminated and chain-extended PCL. J. Appl. Polym. Sci. 2007, 105, 3505–3512. [Google Scholar] [CrossRef]
- Wan, T.; Zou, T.; Cheng, S.X. Synthesis and characterization of biodegradable cholesteryl end-capped polycarbonates. Biomacromolecules 2005, 6, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Ercole, F.; Whittaker, M.R.; Quinn, J.F. Cholesterol modified self-assemblies and their application to nanomedicine. Biomacromolecules 2015, 16, 1886–1914. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Fujita, S.; Iemitsu, M. Acute administration of diosgenin or dioscorea improves hyperglycemia with increases muscular steroidogenesis in STZ-induced type 1 diabetic rats. J. Steroid Biochem. 2014, 143, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Arteaga, M.A.; Sandoval-Ramı́rez, J.; Mata-Esma, M.Y. Abnormal Beckmann rearrangement in 23-hydroxyiminodiosgenin acetate. Tetrahedron Lett. 2004, 45, 4921–4926. [Google Scholar] [CrossRef]
- Man, S.; Gao, W.; Zhang, Y. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.H. Naringinases: Occurrence, characteristics, and applications. Appl. Microbiol. Biot. 2011, 90, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Pan, K.L.; Hsieh, T.C. Diosgenin, a plant-derived sapogenin, exhibits antiviral activity in vitro against hepatitis C virus. J. Nat. Prod. 2011, 74, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.M.; Lin, J.Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Qin, Y.; Huang, W. Anti-thrombosis effect of diosgenin extract from dioscorea zingiberensis CH Wright in vitro and in vivo. Phytomedicine 2011, 18, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Jan, T.R.; Wey, S.P.; Kuan, C.C. Diosgenin, a steroidal sapogenin, enhances antigen-specific IgG2a and interferon-γ expression in ovalbumin-sensitized BALB/c mice. Planta Med. 2007, 53, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Wu, C.C.; Kuo, C.L. Solanum lyratum Extracts Induce Extrinsic and Intrinsic Pathways of Apoptosis in WEHI-3 Murine Leukemia Cells and Inhibit Allograft Tumor. Evid. Based Complement. Altern. Med. 2012, 2012, 254960. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.L.; Venkataraman, S.; Sirat, S.B. The use of cholesterol-containing biodegradable block copolymers to exploit hydrophobic interactions for the delivery of anticancer drugs. Biomaterials 2012, 33, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Pangeni, R.; Sahni, J.K.; Ali, J. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hu, J.; Dou, Q. Synthesis and mesomorphism of new aliphatic polycarbonates containing side cholesteryl groups. Liq. Cryst. 2016, 43, 1486–1494. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q. Light-Driven Chiral Molecular Switches or Motors in Liquid Crystals. Adv. Mater. 2012, 24, 1926–1945. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Q. Stimuli-Directing Self-Organized 3D Liquid-Crystalline Nanostructures: From Materials Design to Photonic Applications. Adv. Funct. Mater. 2016, 26, 10–28. [Google Scholar] [CrossRef]
- Tangso, K.J.; Patel, H.; Lindberg, S. Controlling the Mesostructure Formation within the Shell of Novel Cubic/Hexagonal Phase Cetyltrimethylammonium Bromide–Poly (acrylamide-acrylic acid) Capsules for pH Stimulated Release. ACS Appl. Mater. Interfaces 2015, 7, 24501–24509. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Mezzenga, R. pH-responsive lyotropic liquid crystals for controlled drug delivery. Langmuir 2011, 27, 5296–5303. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.-H.; Liu, X.-F.; Hu, J.-S.; Yang, L.-Q.; Chen, Z.-P. Synthesis and Self-Assembled Behavior of pH-Responsive Chiral Liquid Crystal Amphiphilic Copolymers Based on Diosgenyl-Functionalized Aliphatic Polycarbonate. Nanomaterials 2017, 7, 169. https://doi.org/10.3390/nano7070169
Guo Z-H, Liu X-F, Hu J-S, Yang L-Q, Chen Z-P. Synthesis and Self-Assembled Behavior of pH-Responsive Chiral Liquid Crystal Amphiphilic Copolymers Based on Diosgenyl-Functionalized Aliphatic Polycarbonate. Nanomaterials. 2017; 7(7):169. https://doi.org/10.3390/nano7070169
Chicago/Turabian StyleGuo, Zhi-Hao, Xiao-Feng Liu, Jian-She Hu, Li-Qun Yang, and Zhang-Pei Chen. 2017. "Synthesis and Self-Assembled Behavior of pH-Responsive Chiral Liquid Crystal Amphiphilic Copolymers Based on Diosgenyl-Functionalized Aliphatic Polycarbonate" Nanomaterials 7, no. 7: 169. https://doi.org/10.3390/nano7070169