The Influence of Copolymer Composition on PLGA/nHA Scaffolds’ Cytotoxicity and In Vitro Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Fabrication of Porous Scaffolds
2.3. Cytotoxicity Assay
2.4. In Vitro Degradation
2.5. Characterization
2.5.1. Water Absorption and Weight Loss
2.5.2. SEM Analysis
2.5.3. DSC Analysis
3. Results and Discussion
3.1. Cytotoxicity
3.2. In Vitro Degradation
3.2.1. Mass Loss and Molecular Weight
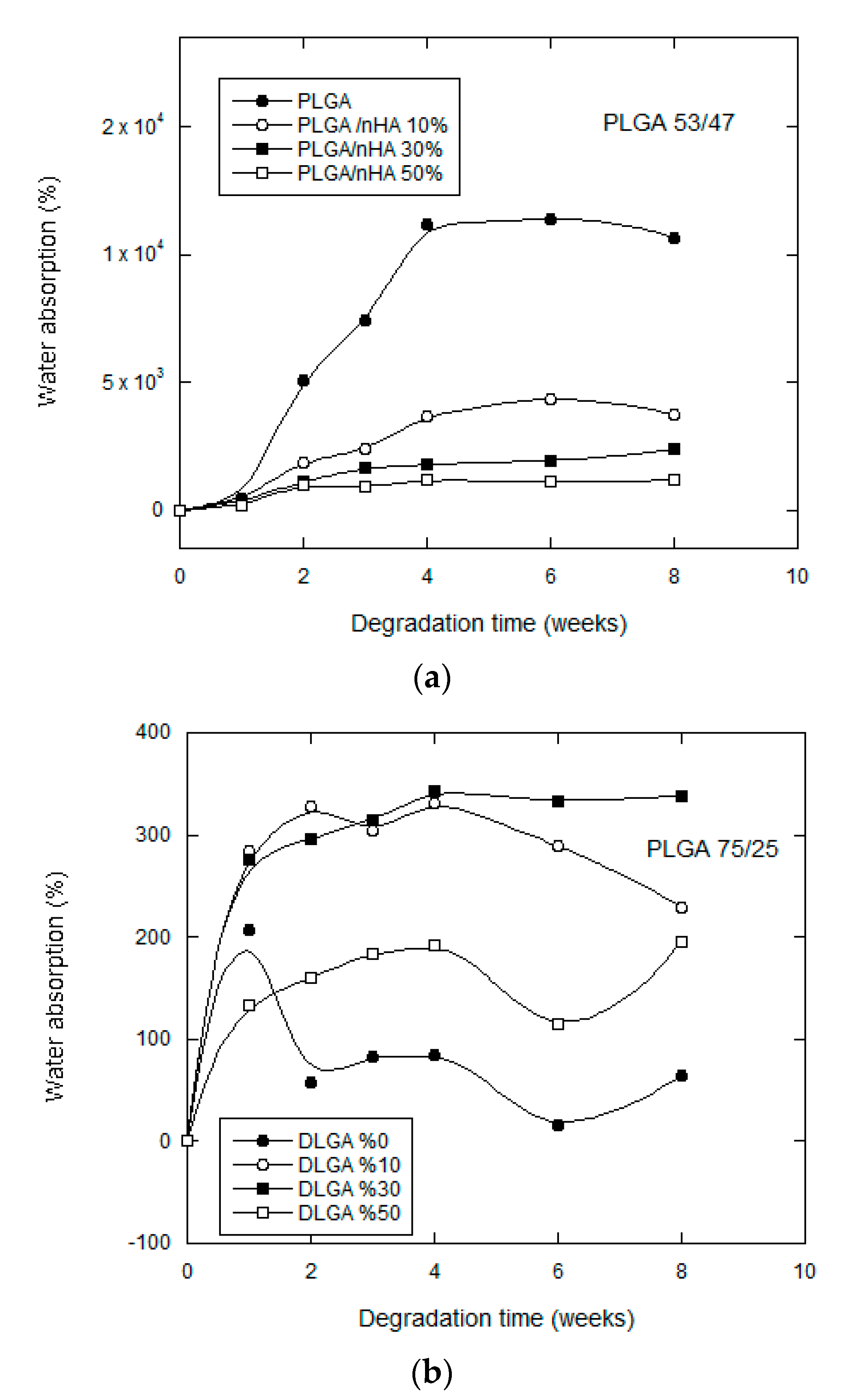
3.2.2. Water Uptake
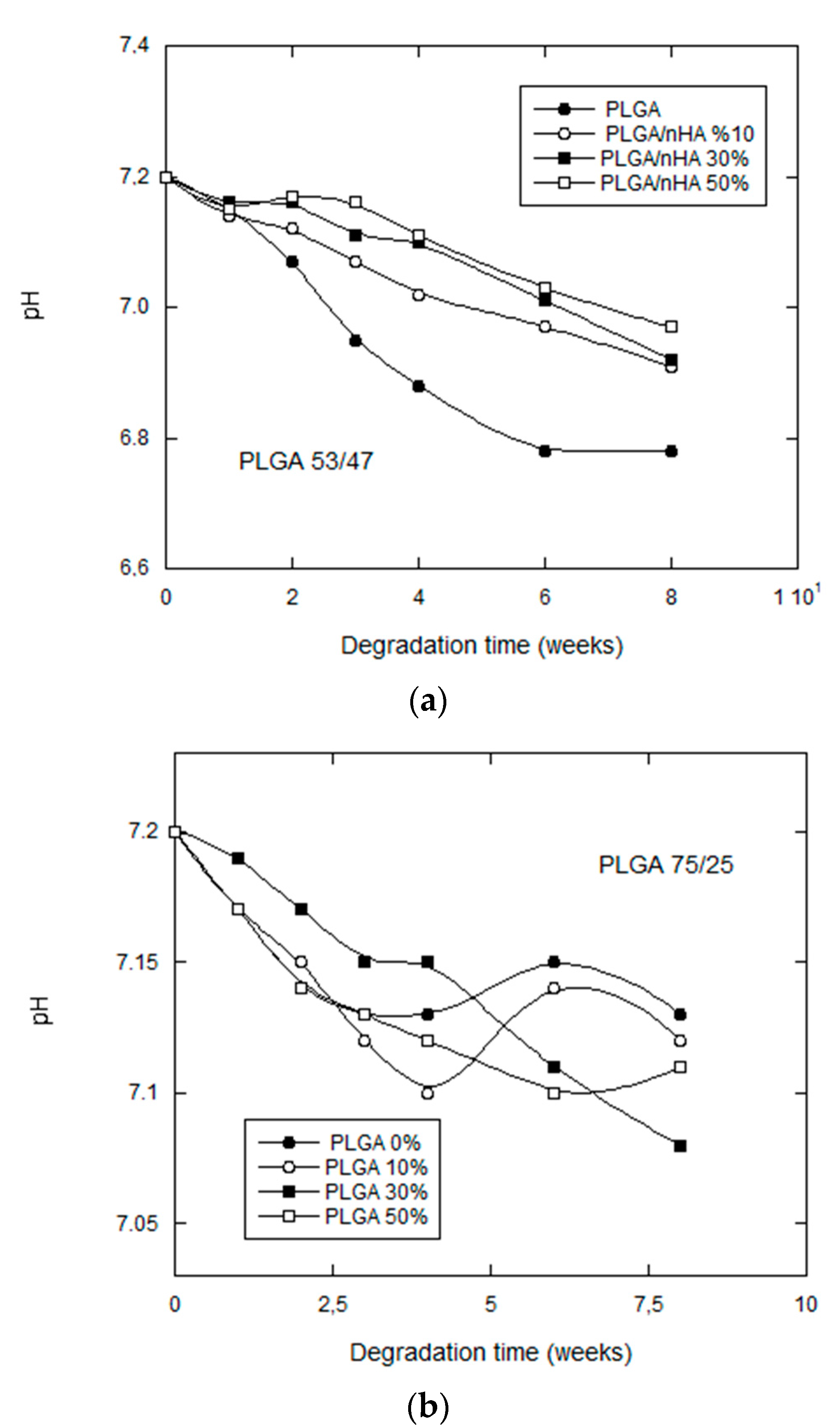
3.2.3. pH
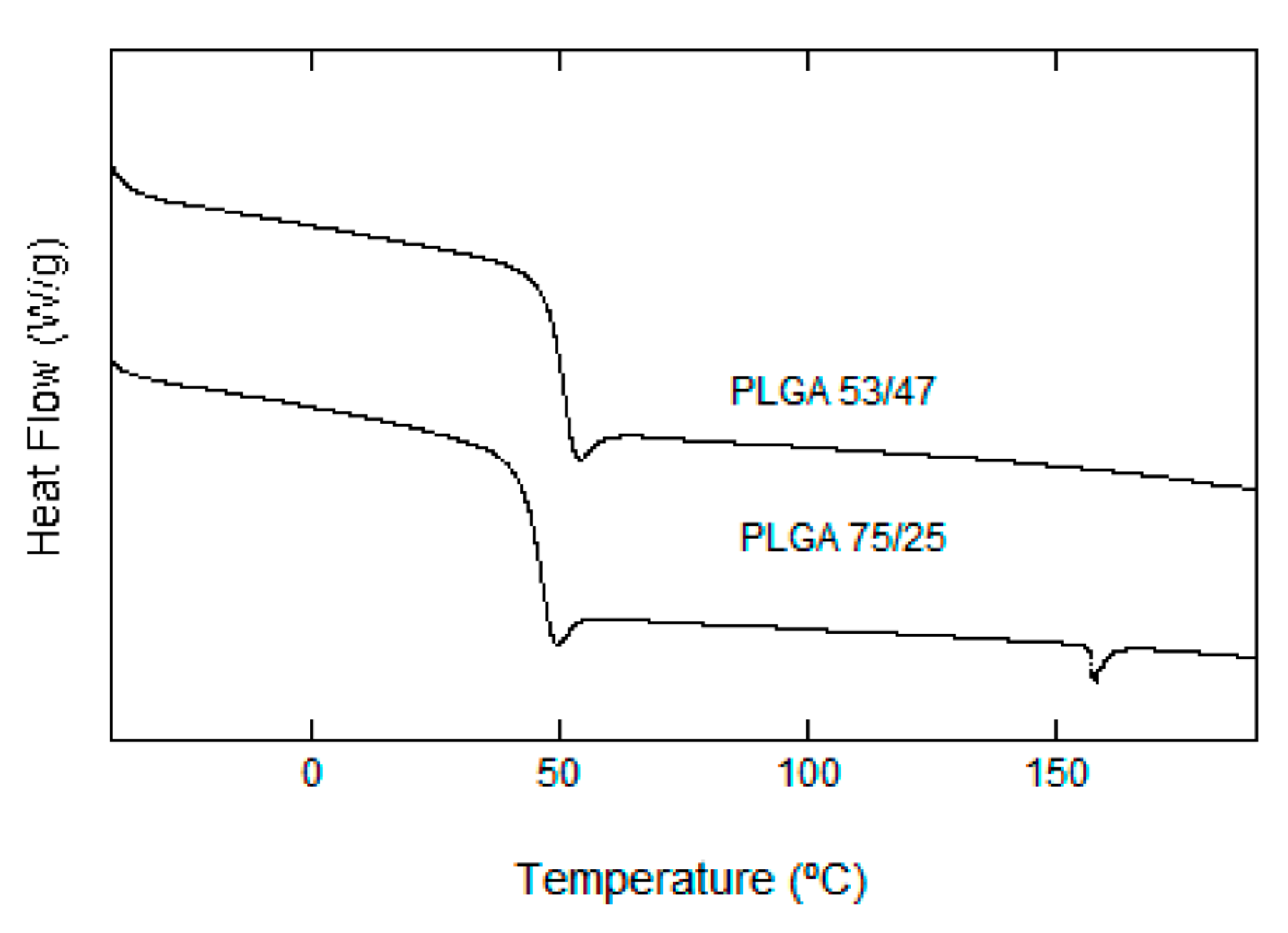
3.2.4. Thermal Analysis (DSC)
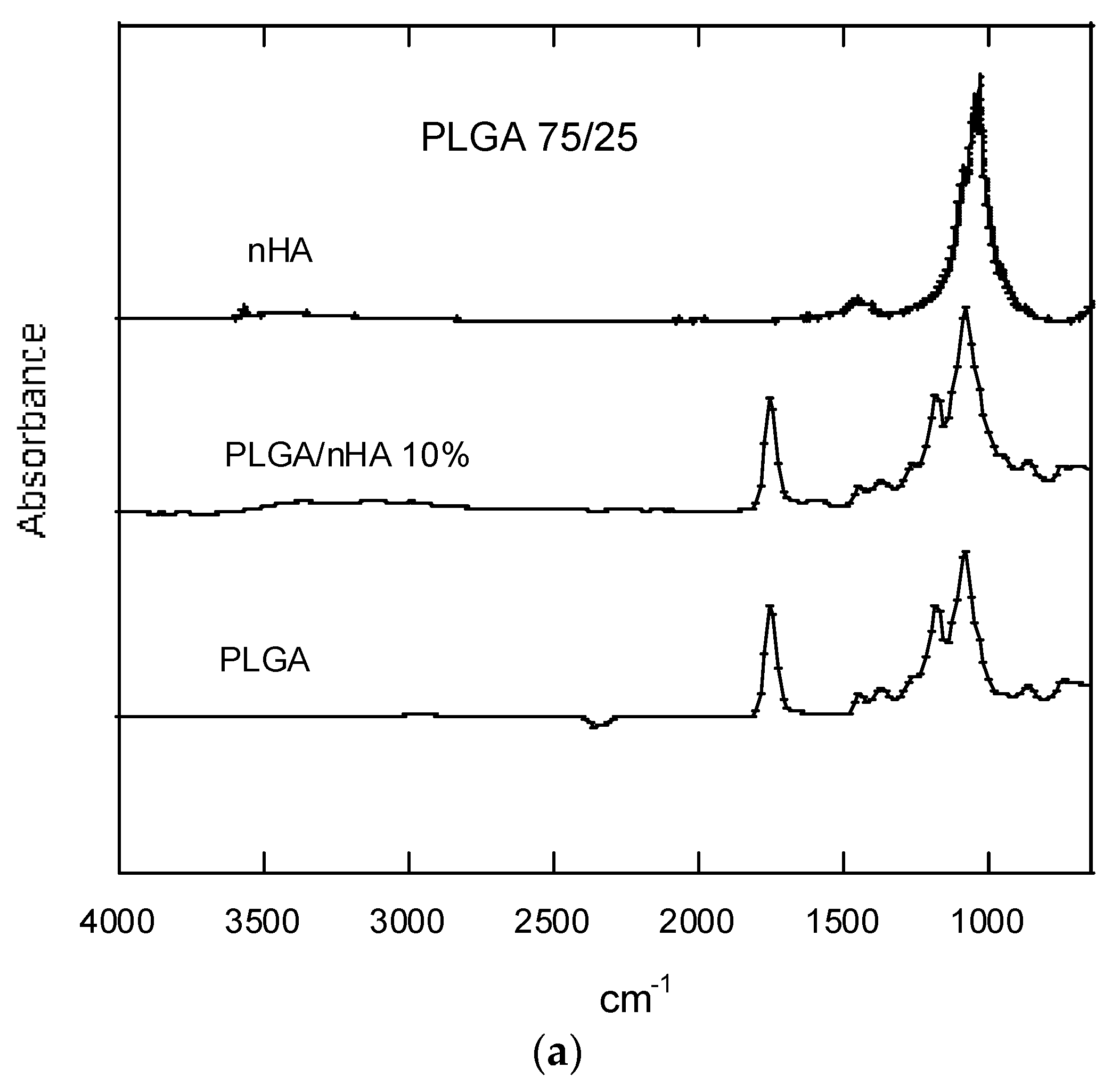
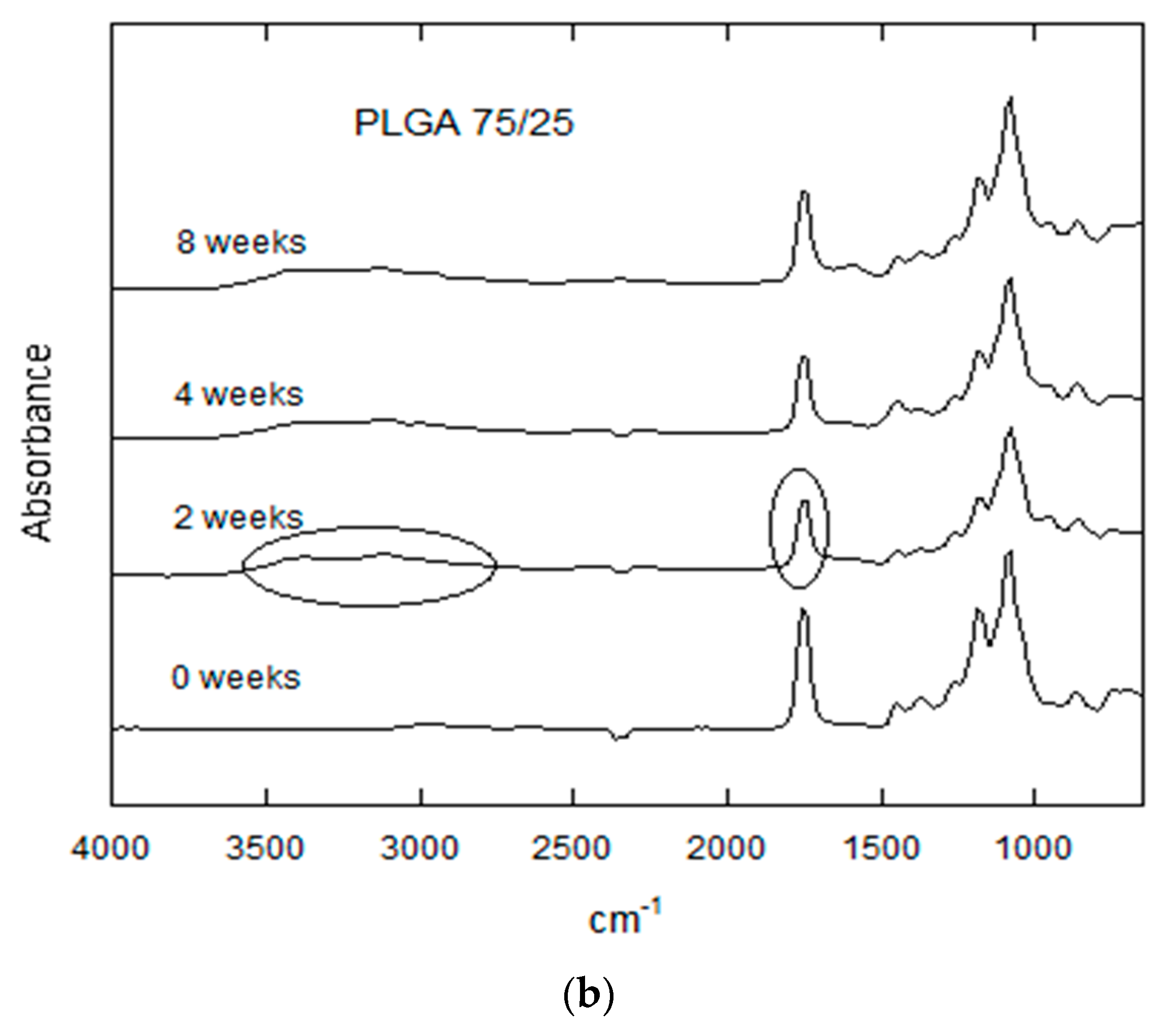
3.2.5. FTIR
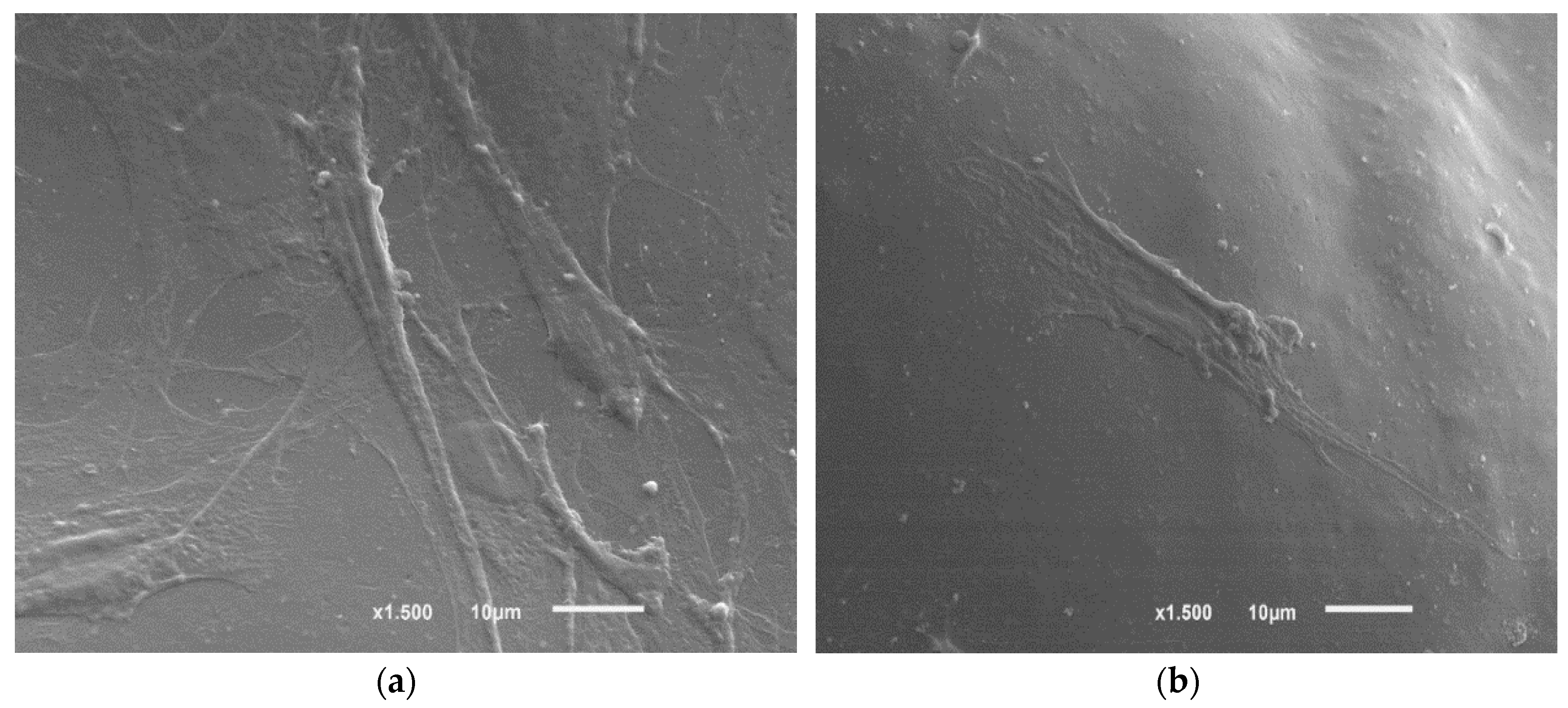
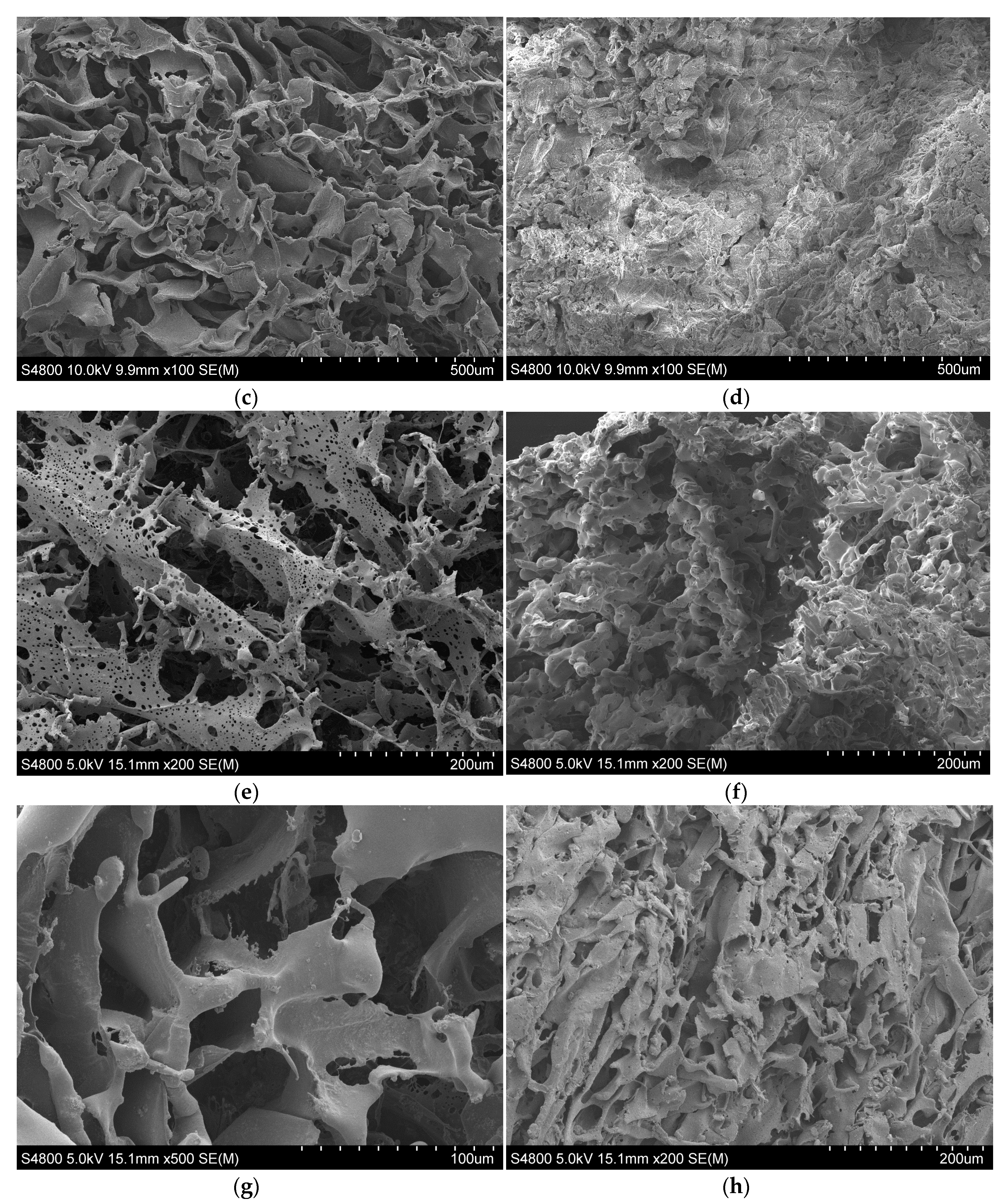
3.2.6. SEM
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yoshikawa, H.; Myoui, A. Bone tissue engineering with porous hydroxyapatite ceramics. J. Artif. Organs 2005, 8, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Belluci, D.; Sola, A.; Gazzarri, M.; Chiellini, F.; Cannillo, V. A new hydroxyapatite based biocomposite for bone replacement. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Pistone, A.; Iannazzo, D.; Panseri, S.; Montesi, M.; Tampieri, A.; Galvagno, S. Hydroxyapatite-magnetite-MWCNT nanocomposite as a biocompatible multifunctional drug delivery system for bone tissue engineering. Nanotechnology 2014, 25, 425701. [Google Scholar] [CrossRef] [PubMed]
- Pistone, A.; Iannazzo, D.; Espro, C.; Galvagno, S.; Tampieri, A.; Montesi, M.; Panseri, S.; Sandri, M. Tethering of Gly-Arg-Gly-Asp-Ser-Pro-Lys Peptides on Mg-Doped Hydroxyapatite. Engineering 2017, 3, 55–59. [Google Scholar] [CrossRef]
- O’Brien, F.J. Review paper: Biomaterials & Scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Naderi, H.; Matin, M.M.; Bahrami, A.R. Review paper: Critical issues in tissue engineering: Biomaterials, cell sources, angiogenesis, and drug delivery systems. J. Biomater. Appl. 2011, 26, 383–417. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Sandonis, I.; Puerto, I.; Ibañez, I. In vitro degradation of PLLA/nHA composite scaffolds. Polym. Eng. Sci. 2014, 54, 2571–2578. [Google Scholar] [CrossRef]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Gunatillake, P.A.; Adhikari, R. Biodegradable Synthetic Polymers for Tissue Engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Loo, S.C.J.; Ooi, C.P.; Wee, S.H.E.; Boey, Y.C.F. Effect of isothermal annealing on the hydrolytic degradation rate of poly(lactide-co-glycolide) (PLGA). Biomaterials 2005, 26, 2827–2833. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kawazoe, N.; Tateishi, T.; Chen, G. In vitro evaluation of biodegradation of poly(lactic-co –glycolic acid) sponges. Biomaterials 2008, 29, 3438–3443. [Google Scholar] [CrossRef] [PubMed]
- Kofron, M.D.; Griswold, A.; Kumbar, S.G.; Martin, K.; Wen, X.; Laurencin, C.T. The implications of polymer selection in regenerative medicine: A comparison of amorphous and semi-crystalline polymer for tissue regeneration. Adv. Funct. Mater. 2009, 19, 1351–1359. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Hajiali, F. A comprehensive study on the fabrication and properties of biocomposites of poly(lactic acid)/ceramics for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.O.; McCabe, L.R.; Baumann, M.J. MC3T3-EI osteoblast attachmente and proliferation on porous hydroxyapatite scaffolds fabricated with nanophase powder. Int. J. Nanomed. 2006, 1, 189–194. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.-I.; No, Y.J.; Lu, Z.; Ng, P.Y.; Chen, Y.; Shi, J.; Pavlos, N.J.; Zreiqat, H. A bioceramic withenhanced osteogenic properties to regulate the function of osteoblastic and osteocalastic cells for bone tissue regeneration. Biomed. Mater. 2016, 11, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Messias, A.D.; Aragones, A.; Duek, E.A.D. PLGA-Hydroxyapatite Composite Scaffolds for Osteoblastic-Like Cells. In Bioceramics 21; Prado, M., Zavaglia, C., Eds.; Trans Tech Publications Ltd.: Durnten-Zurich, Switzerland, 2009; pp. 461–464. [Google Scholar]
- Sheikh, F.A.; Ju, H.W.; Moon, B.M.; Lee, O.J.; Kim, J.H.; Park, H.J.; Kim, D.W.; Kim, D.K.; Janq, J.E.; Khanq, G. Hybrid scaffolds based on PLGA and silk for bone tissue engineering. J. Tissue Eng. Regen. Med. 2016, 10, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Puerto, I. In Vitro Degradation of PLCL/nHA Biodegradable Scaffolds. Polym. Plast. Technol. Eng. 2015, 54, 556–564. [Google Scholar] [CrossRef]
- Linbo, W.; Jiandong, D. In vitro degradation of three-dimensional porous poly(d,l-lactide-coglycolide) scaffolds for tissue engineering. Biomaterials 2004, 25, 5821–5830. [Google Scholar] [CrossRef]
- Park, T.G. Degradation of poly(lactic-co-glycolic acid) microspheres: Effect of copolymer composition. Biomaterials 1995, 16, 1123–1130. [Google Scholar] [CrossRef]
- Saha, S.K.; Tsuji, H. Hydrolitic Degradation of Amorphous Films of L-Lactide Copolymers with Glycolide and D-Lactide. Macromol. Mater. Eng. 2006, 291, 357–368. [Google Scholar] [CrossRef]
- Lee, C.T.; Lee, Y.D. Preparation of porous biodegradable poly(lactide-co-glycolide)/hyaluronic acid blend scaffolds: Characterization, in vitro cells culture and degradation behaviours. J. Mater. Sci. Mater. Med. 2006, 17, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.C.; Claase, M.B.; Grijpma, D.W.; Feijen, A. Development and properties of polycaprolactone/hydroxyapatite composite biomaterials. J. Mater. Sci. 2003, 14, 103–107. [Google Scholar] [CrossRef]
- Díaz, E.; Puerto, I.; Sandonis, I. The Effects of Bioactive Nanoparticles on the Degradation of DLGA. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 38–46. [Google Scholar] [CrossRef]
- Agrawal, C.M.; Best, J.; Heckman, J.D.; Boya, B.D. Protein release kinetics of a biodegradable implant for fracture non-unions. Biomaterials 1995, 16, 1255–1260. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Tang, G.; Li, H.; Yuan, X.; Fan, Y. In vitro degradation of porous poly(L-lactide-co-glycolide)/b-tricalcium phosphate (PLGA/b-TCP) scaffolds under dynamic and static conditions. Polym. Degrad. Stab. 2008, 93, 1838–1845. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Jing, D.; Ding, J. “Wet-state” mechanical properties of three-dimensional polyester porous scaffolds. J. Biomed. Mater. Res. A 2006, 76, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Sandonis, I.; Valle, M.B. In vitro degradation of poly(caprolactone)/nHA composites. J. Nanomater. 2014. [Google Scholar] [CrossRef]



| Degradation Time (Weeks) | PLGA 53/47 | |
|---|---|---|
| 0% nHA | 30% nHA | |
| Weight-Average Molecular Weight (Mw) | ||
| 0 | 94,800 | 94,800 |
| 1 | 29,916 | 58,670 |
| 2 | - | 35,425 |
| 3 | 890.7 | 23,106 |
| 4 | - | 23,811 |
| 6 | - | 22,141 |
| 8 | - | 22,936 |
| Number-Average Molecular Weight (Mn) | ||
| 0 | 65,600 | 65,600 |
| 1 | 16,688 | 33,744 |
| 2 | - | 10,708 |
| 3 | 430 | 10,831 |
| 4 | - | 11,690 |
| 6 | - | 15,644 |
| 8 | - | 10,923 |
| Polydispersity (I) | ||
| 0 | 1.445 | 1.445 |
| 2 | 1.792 | 1.738 |
| 3 | - | 3.308 |
| 4 | 2.071 | 2.133 |
| 6 | - | 1.415 |
| 8 | - | 2.099 |
| Degradation Time (Weeks) | PLGA 75/25 | |||
|---|---|---|---|---|
| 0% nHA | 10% nHA | 30% nHA | 50% nHA | |
| Weight-Average Molecular Weight (Mw) | ||||
| 0 | 86,985 | 86,985 | 86,985 | 86,985 |
| 2 | 73,311 | 79,604 | 78,758 | 79,455 |
| 3 | 72,334 | 75,392 | 73,433 | 78,577 |
| 4 | 66,296 | 75,368 | 69,166 | 72,973 |
| 6 | 56,212 | 68,749 | 57,371 | 66,178 |
| 8 | 39,979 | 56,101 | 49,760 | 58,429 |
| Number-Average Molecular Weight (Mn) | ||||
| 0 | 53,533 | 53,533 | 53,533 | 53,533 |
| 2 | 46,131 | 48,388 | 48,979 | 43,404 |
| 3 | 42,983 | 43,884 | 44,238 | 45,835 |
| 4 | 39,782 | 45,490 | 41,345 | 41,808 |
| 6 | 33,430 | 40,419 | 32,253 | 38,109 |
| 8 | 19,524 | 31,412 | 29,689 | 33,255 |
| Polydispersity (I) | ||||
| 0 | 1.650 | 1.625 | 1.625 | 1.625 |
| 2 | 1.589 | 1.645 | 1.608 | 1.830 |
| 3 | 1.682 | 1.718 | 1.659 | 1.714 |
| 4 | 1.666 | 1.656 | 1.672 | 1.745 |
| 6 | 1.681 | 1.701 | 1.778 | 1.736 |
| 8 | 2.047 | 1.786 | 1.676 | 1.757 |
| Degradation Time (Weeks) | Tg (°C) | |||
|---|---|---|---|---|
| nHA 0% | nHA 10% | nHA 30% | nHA 50% | |
| 0 | 18.2 | 30.2 | 32.8 | 28.9 |
| 1 | 44.2 | 33.7 | 34.4 | 30.7 |
| 2 | N/A | 35.9 | 36.3 | 33.5 |
| 3 | N/A | 38.4 | 39.4 | 38.8 |
| 4 | N/A | 35.9 | 43.7 | 43.5 |
| 6 | N/A | N/A | 40.2 | 40.6 |
| 8 | N/A | N/A | 40.1 | 40.9 |
| Degradation Time (Week) | Tg (°C) | |||
|---|---|---|---|---|
| nHA 0% | nHA 10% | nHA 30% | nHA 50% | |
| 0 | 51.5 | 50.4 | 52.9 | 51.0 |
| 1 | 52.2 | 52.8 | 51.9 | 51.8 |
| 2 | 51.8 | 52.1 | 51.5 | 53.1 |
| 3 | 52.6 | 52.2 | 51.9 | 52.5 |
| 4 | 52.1 | 52.4 | 51.8 | 52.8 |
| 6 | 52.1 | 52.1 | 51.9 | 52.9 |
| 8 | 51.0 | 51.9 | 52.1 | 52.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, E.; Puerto, I.; Ribeiro, S.; Lanceros-Mendez, S.; Barandiarán, J.M. The Influence of Copolymer Composition on PLGA/nHA Scaffolds’ Cytotoxicity and In Vitro Degradation. Nanomaterials 2017, 7, 173. https://doi.org/10.3390/nano7070173
Díaz E, Puerto I, Ribeiro S, Lanceros-Mendez S, Barandiarán JM. The Influence of Copolymer Composition on PLGA/nHA Scaffolds’ Cytotoxicity and In Vitro Degradation. Nanomaterials. 2017; 7(7):173. https://doi.org/10.3390/nano7070173
Chicago/Turabian StyleDíaz, Esperanza, Igor Puerto, Silvie Ribeiro, Senentxu Lanceros-Mendez, and José Manuel Barandiarán. 2017. "The Influence of Copolymer Composition on PLGA/nHA Scaffolds’ Cytotoxicity and In Vitro Degradation" Nanomaterials 7, no. 7: 173. https://doi.org/10.3390/nano7070173






