Use of Polycaprolactone Electrospun Nanofibers as a Coating for Poly(methyl methacrylate) Bone Cement
Abstract
:1. Introduction
2. Results
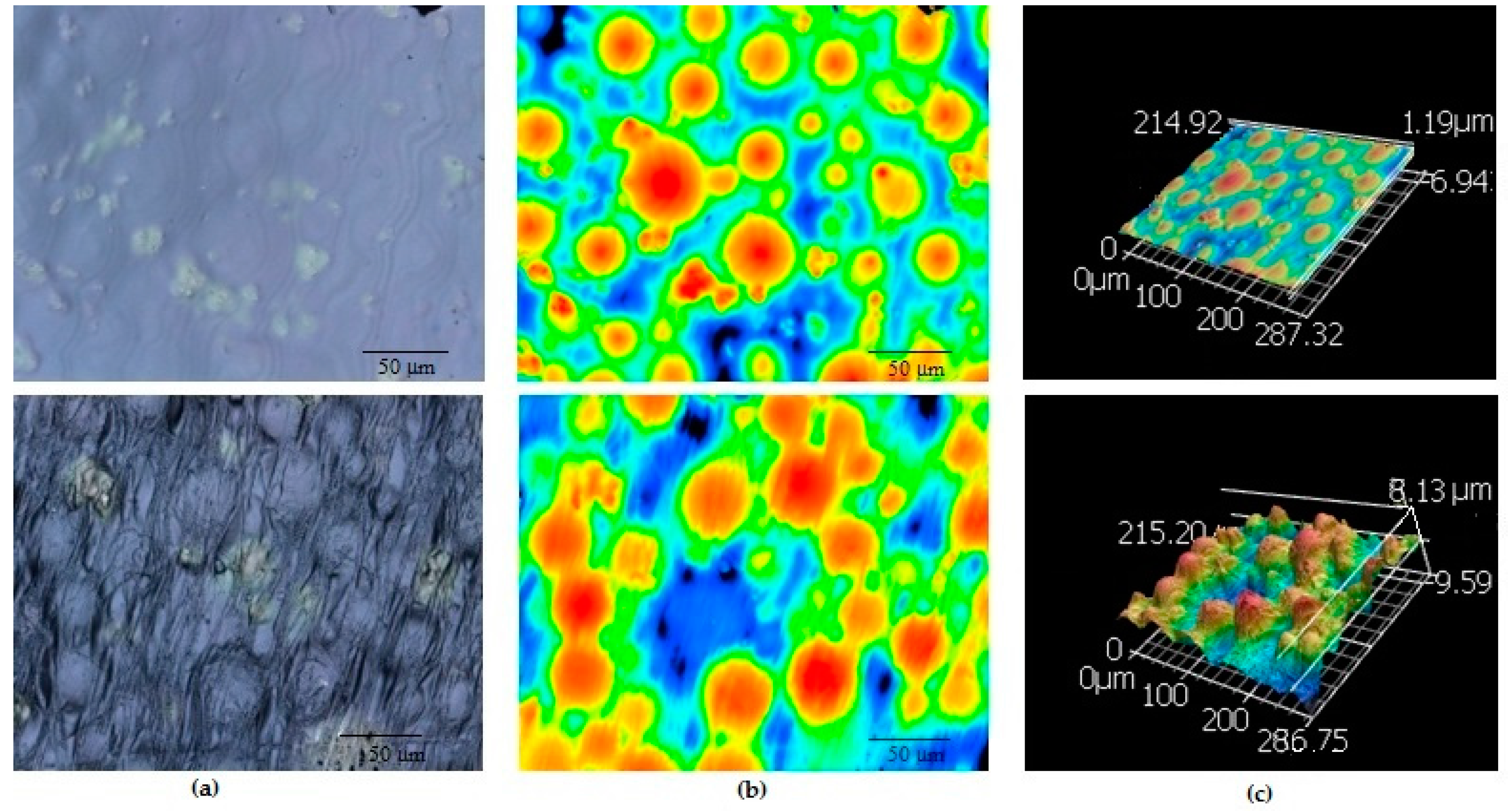
2.1. Surface Topography
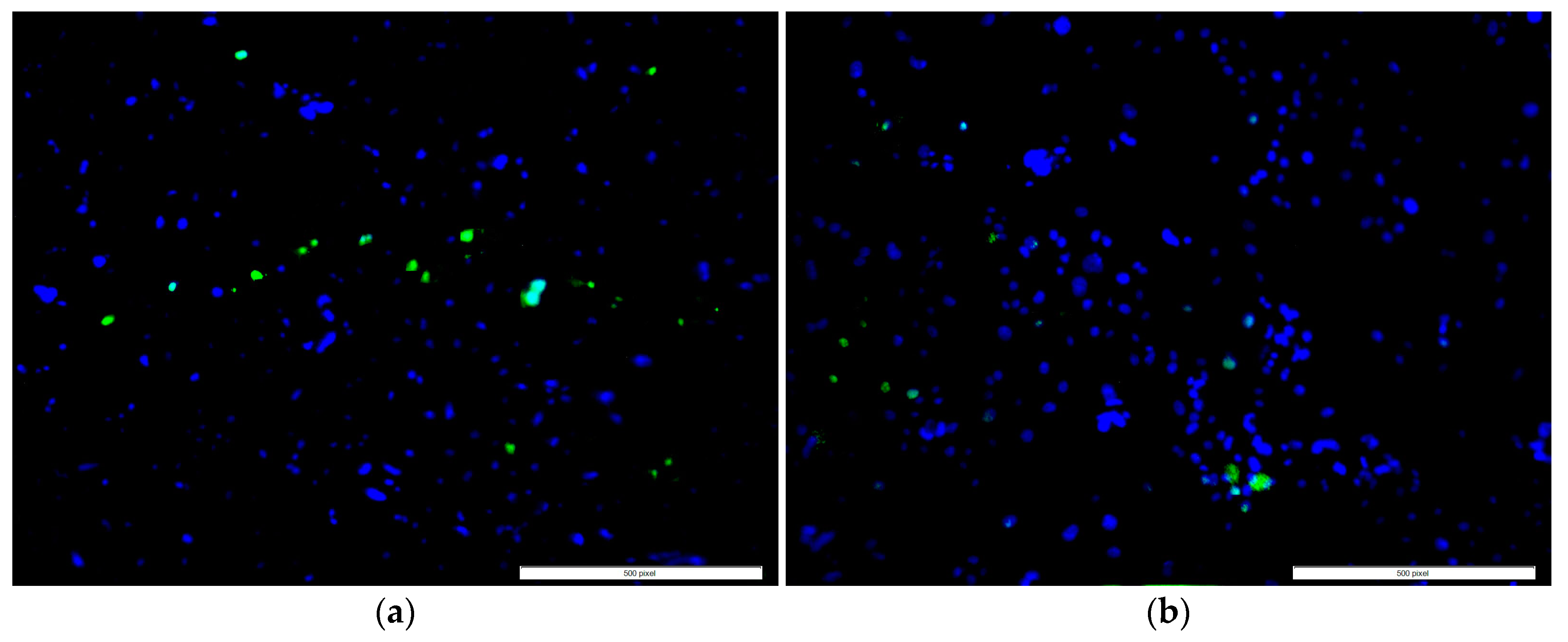
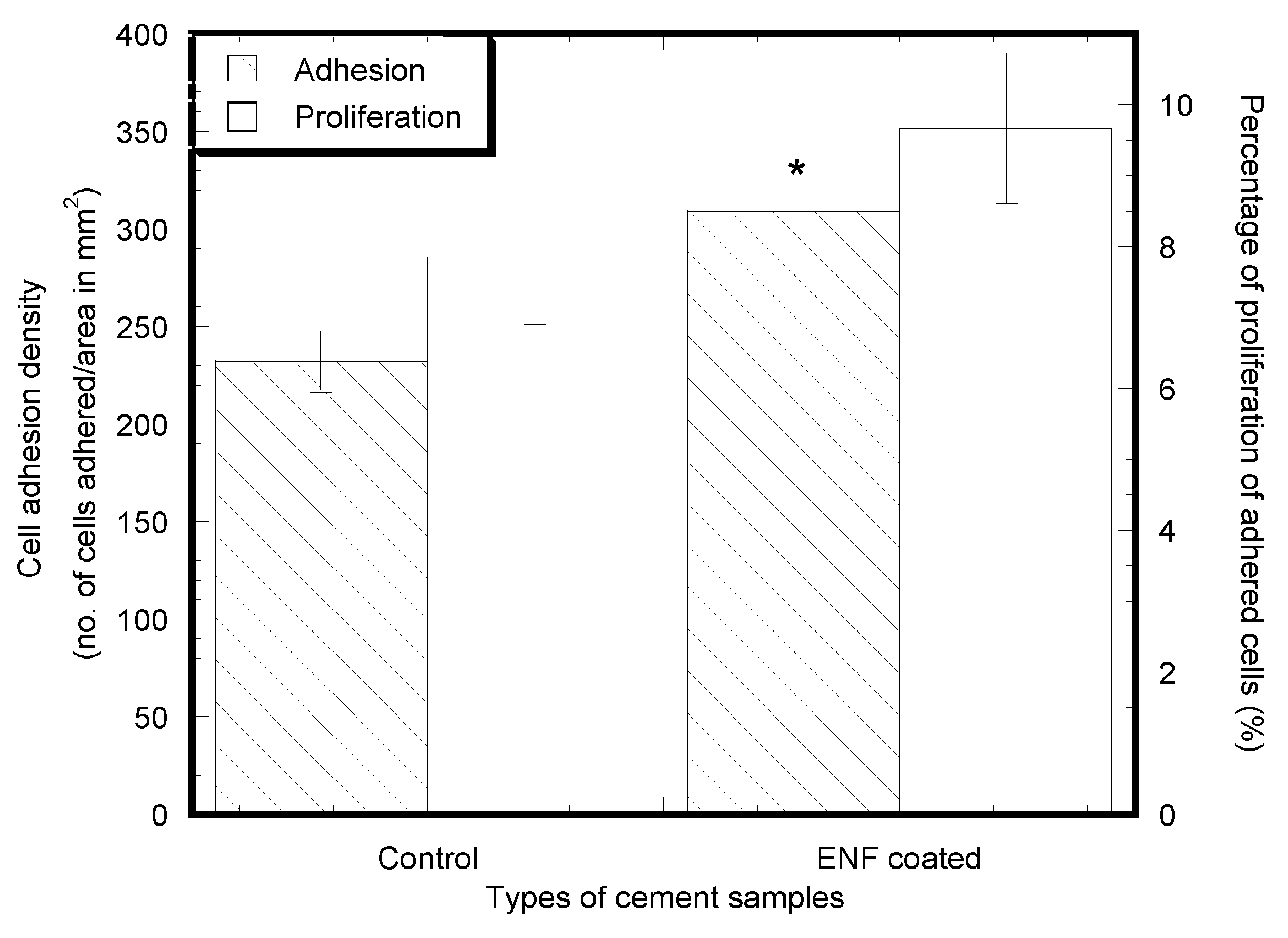
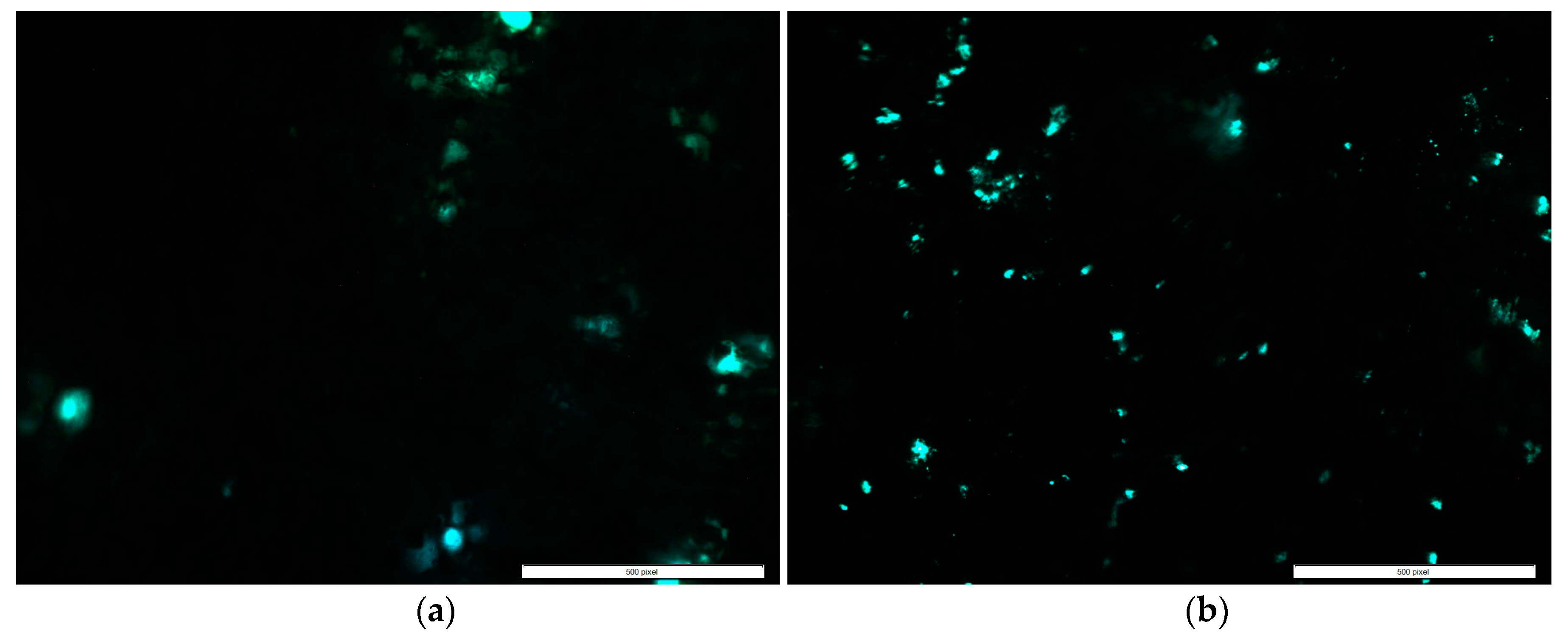
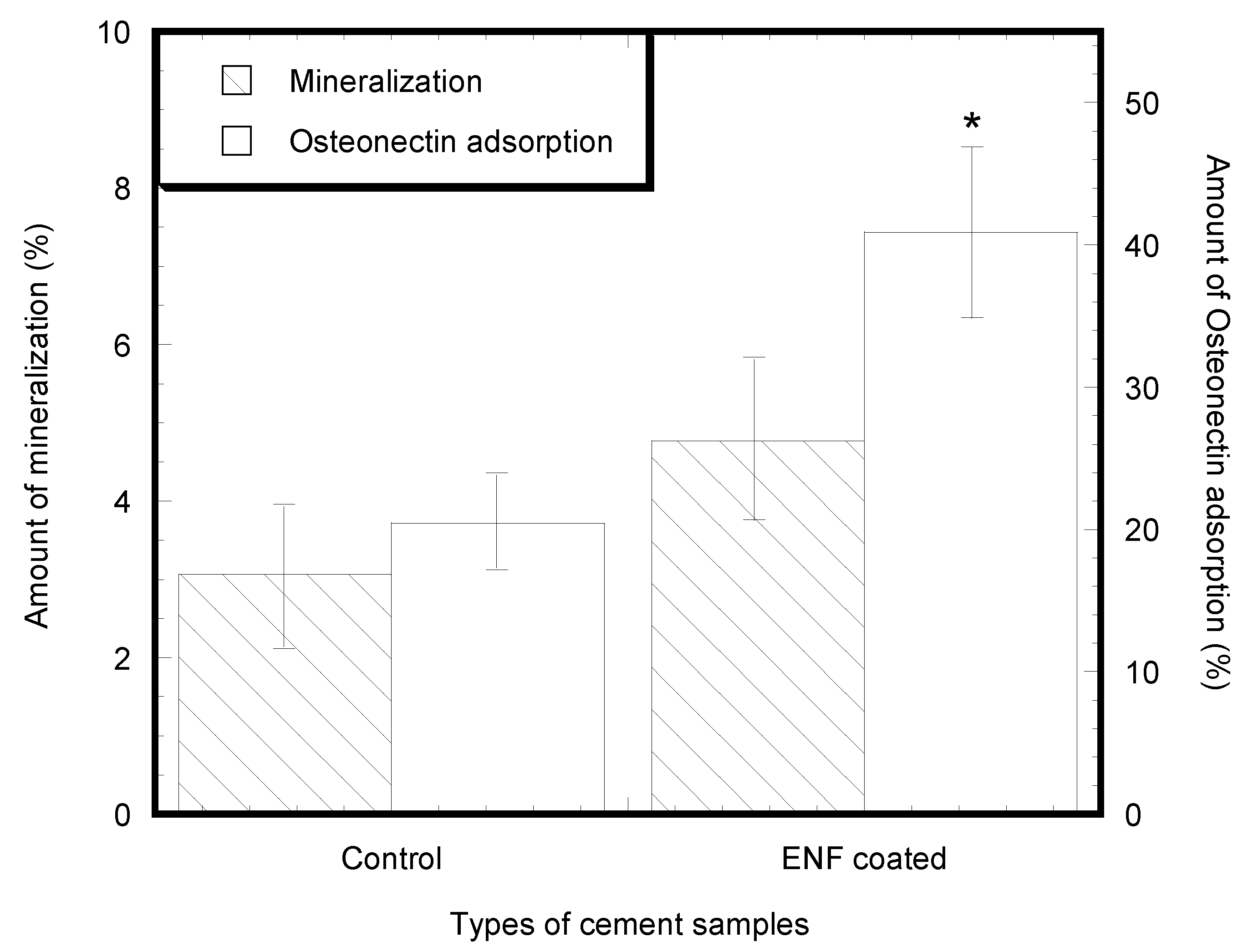
2.2. Cytocompatibility Properties
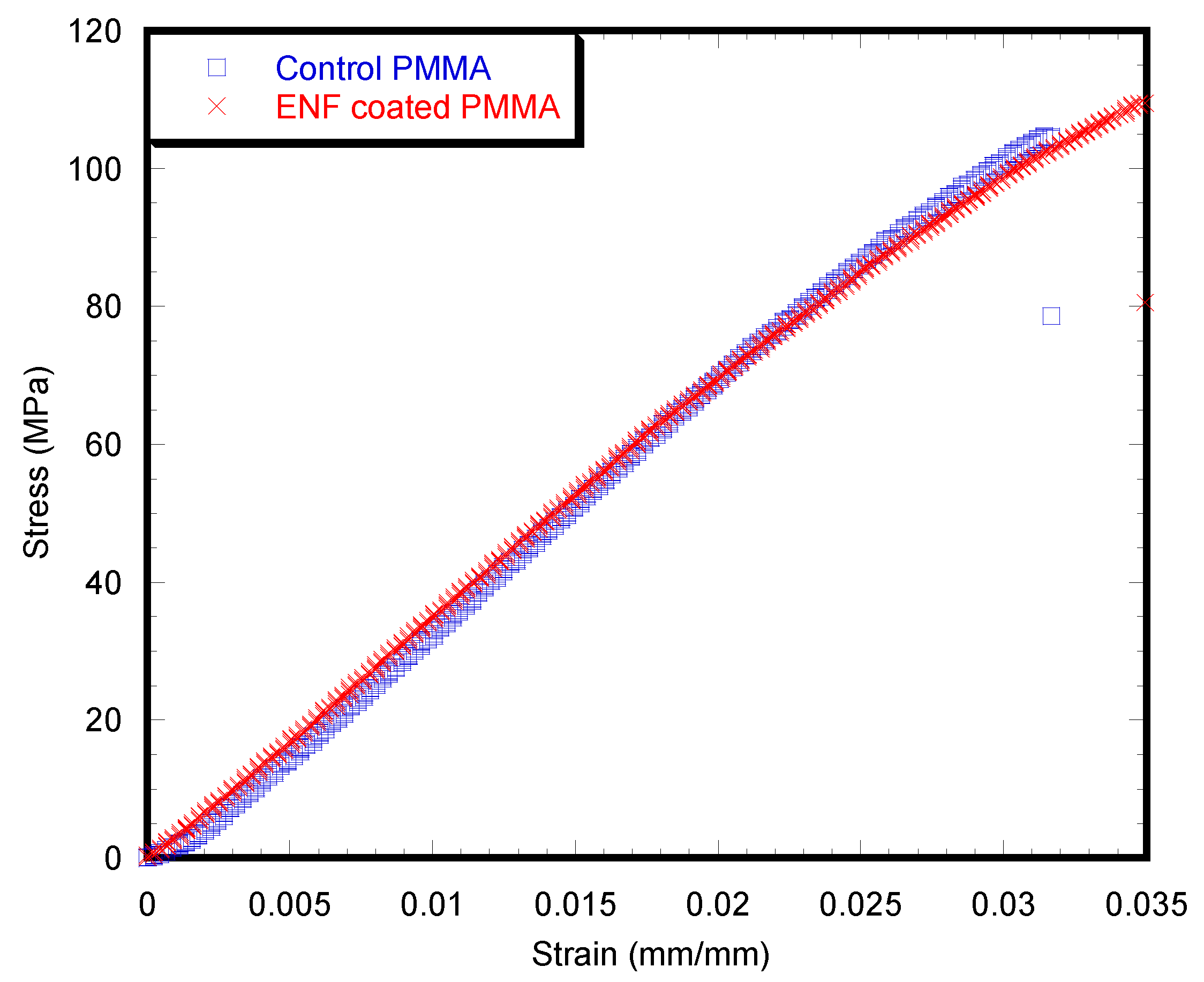
2.3. Mechanical Tests
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.2.1. Sample Design
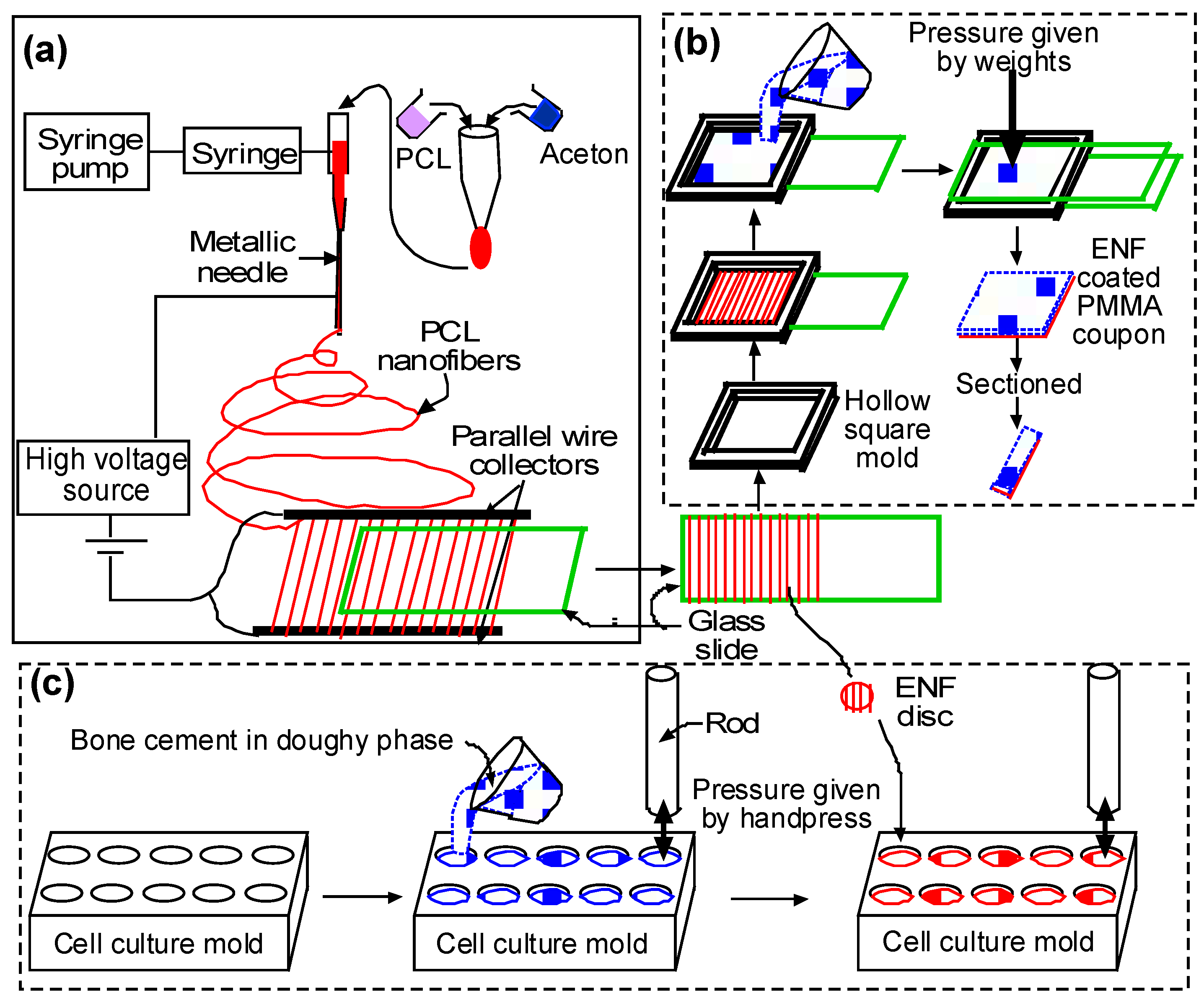
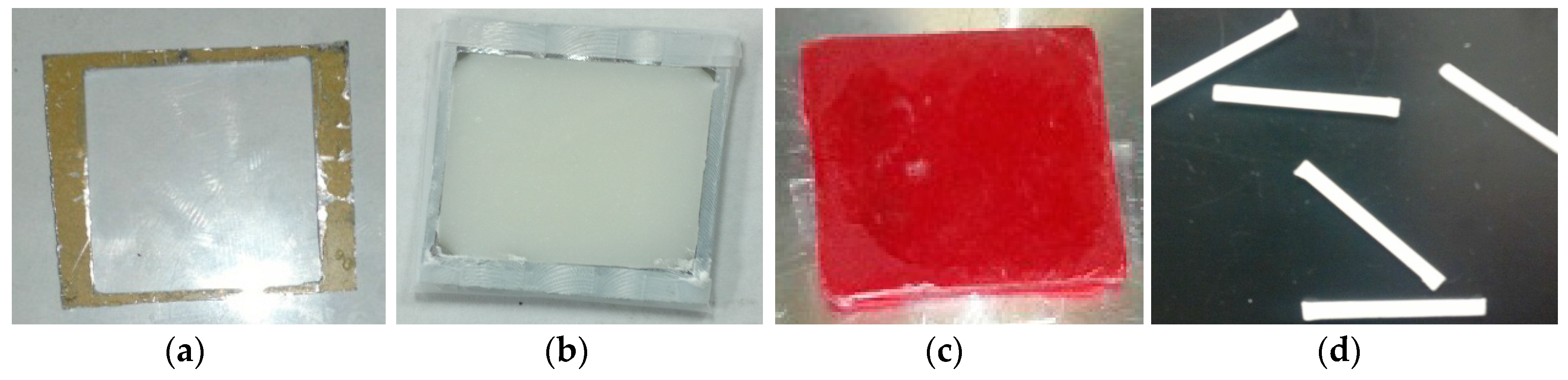
4.2.2. Sample Fabrication Process
(a) Fiber Production
(b) Surface Characterization and Mechanical Test Samples
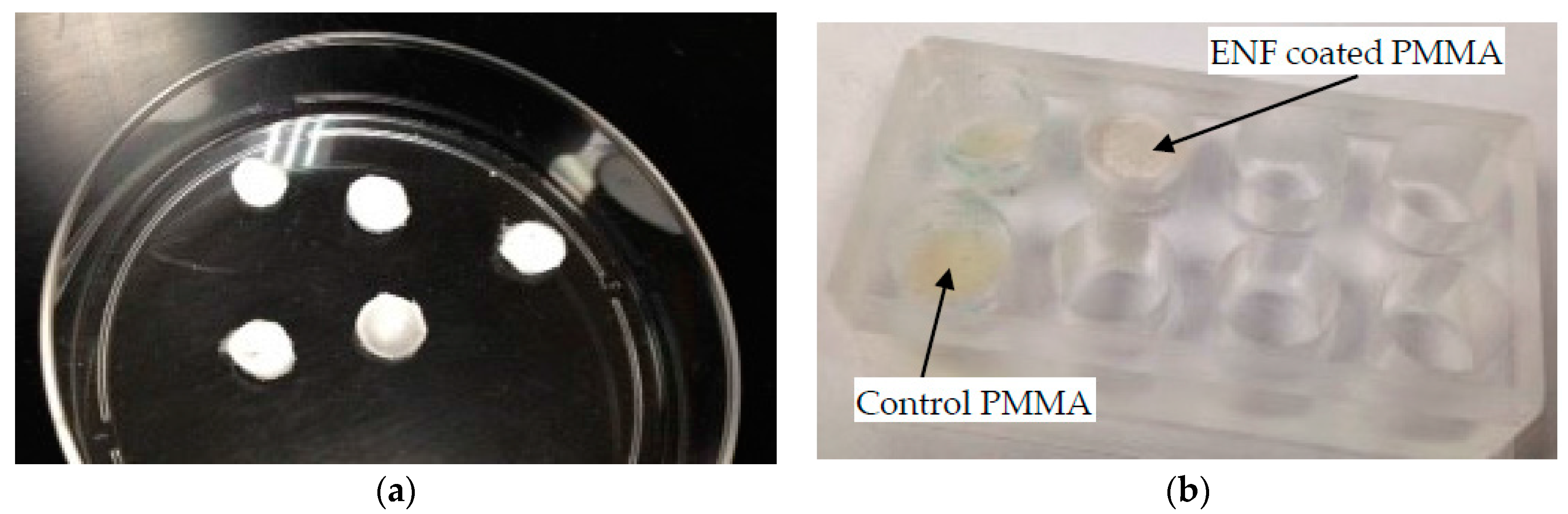
(c) Cytocompatibility Test Samples
4.3. Experiments and Analysis
4.3.1. Surface Topography
4.3.2. Cell Adhesion, Proliferation, Mineralization, and Protein Adsorption Tests on PMMA Samples
4.3.3. Mechanical Tests
4.3.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Biggs, M.; Dalby, M.; Wilkinson, C.; Gadegaard, N.; Richards, G. The influence of nanoscale biomimetic structures on osteoblast adhesion. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, S64. [Google Scholar] [CrossRef]
- Im, B.J.; Lee, S.W.; Oh, N.; Lee, M.H.; Kang, J.H.; Leesungbok, R.; Lee, S.C.; Ahn, S.J.; Park, J.S. Texture direction of combined microgrooves and submicroscale topographies of titanium substrata influence adhesion, proliferation, and differentiation in human primary cells. Arch. Oral Biol. 2012, 57, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Travan, A.; Marsich, E.; Donati, I.; Foulc, M.-P.; Moritz, N.; Aro, H.T.; Paoletti, S. Polysaccharide-coated thermosets for orthopedic applications: From material characterization to in vivo tests. Biomacromolecules 2012, 13, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Zankovych, S.; Diefenbeck, M.; Bossert, J.; Mückley, T.; Schrader, C.; Schmidt, J.; Schubert, H.; Bischoff, S.; Faucon, M.; Finger, U.; et al. The effect of polyelectrolyte multilayer coated titanium alloy surfaces on implant anchorage in rats. Acta Biomater. 2013, 9, 4926–4934. [Google Scholar] [CrossRef] [PubMed]
- Moffat, K.L.; Wang, I.N.; Rodeo, S.A.; Lu, H.H. Orthopedic interface tissue engineering for the biological fixation of soft tissue grafts. Clin. Sports Med. 2009, 28, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Pal, S. Improvement of mechanical properties of acrylic bone cement by fiber reinforcement. J. Biomech. 1984, 17, 467–478. [Google Scholar] [CrossRef]
- Wagner, H.D.; Cohn, D. Use of high-performance polyethylene fibres as a reinforcing phase in poly(methylmethacrylate) bone cement. Biomaterials 1989, 10, 139–141. [Google Scholar] [CrossRef]
- Zupančič, Š.; Baumgartner, S.; Lavrič, Z.; Petelin, M.; Kristl, J. Local delivery of resveratrol using polycaprolactone nanofibers for treatment of periodontal disease. J. Drug Deliv. Sci. Technol. 2015, 30 Pt B, 408–416. [Google Scholar] [CrossRef]
- Wu, X.; Mahalingam, S.; VanOosten, S.K.; Wisdom, C.; Tamerler, C.; Edirisinghe, M. New generation of tunable bioactive shape memory mats integrated with genetically engineered proteins. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; Hurtado, A.; Oudega, M.; Trombley, M.T.; Gilbert, R.J. Creation of highly aligned electrospun poly-l-lactic acid fibers for nerve regeneration applications. J. Neural Eng. 2009, 6, 016001. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.K.; Kidoaki, S.; Matsuda, T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: Structural characteristics, mechanical properties and cell adhesion potential. Biomaterials 2005, 26, 3929–3939. [Google Scholar] [CrossRef] [PubMed]
- Po-Yee Lui, P.; Zhang, P.; Chan, K.-M.; Qin, L. Biology and augmentation of tendon-bone insertion repair. J. Orthop. Res. Surg. Res. 2010, 5. [Google Scholar] [CrossRef]
- Kim, G.H. Electrospun pcl nanofibers with anisotropic mechanical properties as a biomedical scaffold. Biomed. Mater. 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.; Vaughan, M.; Coles, A.; Jamadagni, H.; Wolf, R.; Williams, W. Application of polycaprolactone nanofibers and mgo nanoparticles for a cemented implant surgery. In Proceedings of the 2017 Orthopaedic Research Society (ORS) Annual Meeting, San Diego, CA, USA, 19–22 March 2017. [Google Scholar]
- Khandaker, M.; Vaughan, M.; Morris, T.; White, J.; Meng, Z. Effect of additives particles on mechanical, thermal and cell functions properties of poly (methyl methacrylate) cement. Int. J. Nanomed. 2014, 9, 2699–2712. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Shaughnessy, M.C.; Zhou, Z.; Noh, H.; Vogler, E.A.; Donahue, H.J. Surface energy effects on osteoblast spatial growth and mineralization. Biomaterials 2008, 29, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, E.P.; Sa, J.C.; De Oliveira, P.T.; Alves, C., Jr.; Beloti, M.M.; Rosa, A.L. The effect of plasma-nitrided titanium surfaces on osteoblastic cell adhesion, proliferation, and differentiation. J. Biomed. Mater. Res. Part A 2014, 102, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.; Riahinezhad, S. Method and Apparatus to Control the Heterogeneous Flow of Bone Cement and Improve Osseointegration of Cemented Implant. U.S. Patent Application No. US62373786, 11 August 2016. [Google Scholar]
- Moursi, A.M.; Winnard, A.V.; Winnard, P.L.; Lannutti, J.J.; Seghi, R.R. Enhanced osteoblast response to a polymethylmethacrylate-hydroxyapatite composite. Biomaterials 2002, 23, 133–144. [Google Scholar] [CrossRef]
- Mahalingam, S.; Edirisinghe, M. Forming of polymer nanofibers by a pressurised gyration process. Macromol. Rapid Commun. 2013, 34, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Deravi, L.F.; Sinatra, N.R.; Chantre, C.O.; Nesmith, A.P.; Yuan, H.; Deravi, S.K.; Goss, J.A.; MacQueen, L.A.; Badrossamy, M.R.; Gonzalez, G.M.; et al. Design and fabrication of fibrous nanomaterials using pull spinning. Macromol. Mater. Eng. 2017, 302. [Google Scholar] [CrossRef]
- Khandaker, M.; Snow, P. Method and Apparatus for Controlled Alignment and Deposition of Branched Electrospun Fiber. U.S. Patent US9359694 B2, 7 June 2016. [Google Scholar]
- Kanungo, I.; Fathima, N.N.; Rao, J.R.; Nair, B.U. Influence of pcl on the material properties of collagen based biocomposites and in vitro evaluation of drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Sultanova, Z.; Kaleli, G.; Kabay, G.; Mutlu, M. Controlled release of a hydrophilic drug from coaxially electrospun polycaprolactone nanofibers. Int. J. Pharm. 2016, 505, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.H.; Hassan, M.I.; Sultana, N. Electrospun polycaprolactone (pcl) and pcl/nano-hydroxyapatite (pcl/nha)-based nanofibers for bone tissue engineering application. In Proceedings of the 10th Asian Control Conference (ASCC), Kota Kinabalu, Malaysia, 31 May–3 June 2015; pp. 1–4. [Google Scholar]
- Ries, M.D.; Rauscher, L.A.; Hoskins, S.; Lott, D.; Richman, J.A.; Lynch, F. Intramedullary pressure and pulmonary function during total knee arthroplasty. Clin. Orthop. Relat. Res. 1998, 356, 154–160. [Google Scholar] [CrossRef]
- Graham, J.; Ries, M.; Pruitt, L. Effect of bone porosity on the mechanical integrity of the bone-cement interface. J. Bone Jt. Surg. Am. Vol. 2003, 85A, 1901–1908. [Google Scholar] [CrossRef]
- ASTM. D790-03: Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials, 8th ed.; ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Apedo, K.L.; Munzer, C.; He, H.; Montgomery, P.; Serres, N.; Fond, C.; Feugeas, F. Cement paste surface roughness analysis using coherence scanning interferometry and confocal microscopy. Mater. Charact. 2015, 100, 108–119. [Google Scholar] [CrossRef]
- Invitrogen. Click-it® Edu Imaging Kits. Available online: https://tools.thermofisher.com/content/sfs/manuals/mp10338.pdf (accessed on 23 June 2017).



| Descriptions | Control | ENF Coated | t | p-Value |
|---|---|---|---|---|
| Ra (μm) | 0.26 ± 0.03 | 2.75 ± 0.17 ** | 14.82 | 0.003 |
| Rz (μm) | 1.45 ± 0.25 | 14.13 ± 1.24 ** | 10.02 | 0.008 |
| Rsum (μm) | 75.47 ± 3.57 | 102.00 ± 6.30 * | 3.66 | 0.032 |
| Parameters Descriptions | Control | ENF Coated | F1,56 | p-Value |
|---|---|---|---|---|
| No. of adhered cells after 2 days of cell culture | 232 ± 16 | 309 ± 12 * | 6.01 | 0.018 |
| No. of adhered cells after 14 days of cell culture | 428 ± 53 | 615 ± 65 ** | 10.48 | 0.002 |
| Parameters Descriptions | Control | ENF Coated | t | p-Value |
|---|---|---|---|---|
| Width of the sample | 1.97 ± 0.01 | 1.98 ± 0.03 | 0.23 | 0.83 |
| Height of the sample | 1.49 ± 0.01 | 1.51 ± 0.01 | 1.31 | 0.20 |
| Bending modulus (GPa) | 3.32 ± 0.11 | 3.30 ± 0.07 | 0.15 | 0.88 |
| Bending strength (MPa) | 106.97 ± 4.94 | 108.12 ± 3.05 | 0.20 | 0.85 |
| Maximum deflection (mm) | 1.56 ± 0.07 | 1.58 ± 0.04 | 0.15 | 0.88 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandaker, M.; Riahinezhad, S.; Jamadagni, H.G.; Morris, T.L.; Coles, A.V.; Vaughan, M.B. Use of Polycaprolactone Electrospun Nanofibers as a Coating for Poly(methyl methacrylate) Bone Cement. Nanomaterials 2017, 7, 175. https://doi.org/10.3390/nano7070175
Khandaker M, Riahinezhad S, Jamadagni HG, Morris TL, Coles AV, Vaughan MB. Use of Polycaprolactone Electrospun Nanofibers as a Coating for Poly(methyl methacrylate) Bone Cement. Nanomaterials. 2017; 7(7):175. https://doi.org/10.3390/nano7070175
Chicago/Turabian StyleKhandaker, Morshed, Shahram Riahinezhad, Harsha G. Jamadagni, Tracy L. Morris, Alexis V. Coles, and Melville B. Vaughan. 2017. "Use of Polycaprolactone Electrospun Nanofibers as a Coating for Poly(methyl methacrylate) Bone Cement" Nanomaterials 7, no. 7: 175. https://doi.org/10.3390/nano7070175






