Eco-Friendly Acaricidal Effects of Nylon 66 Nanofibers via Grafted Clove Bud Oil-Loaded Capsules on House Dust Mites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
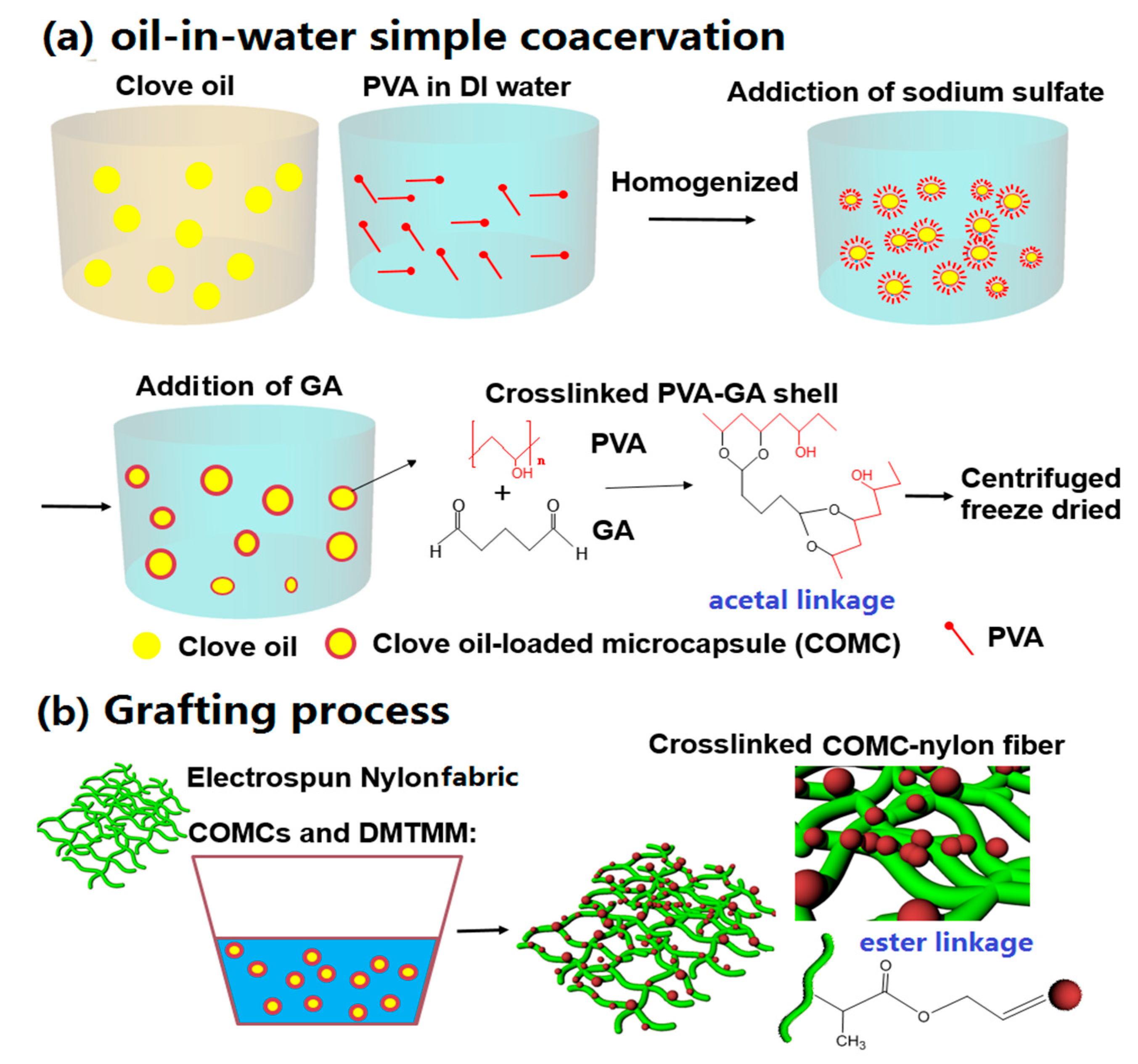
2.2. Microencapsulation of COMCs by Oil-in-Water Simple Coacervation
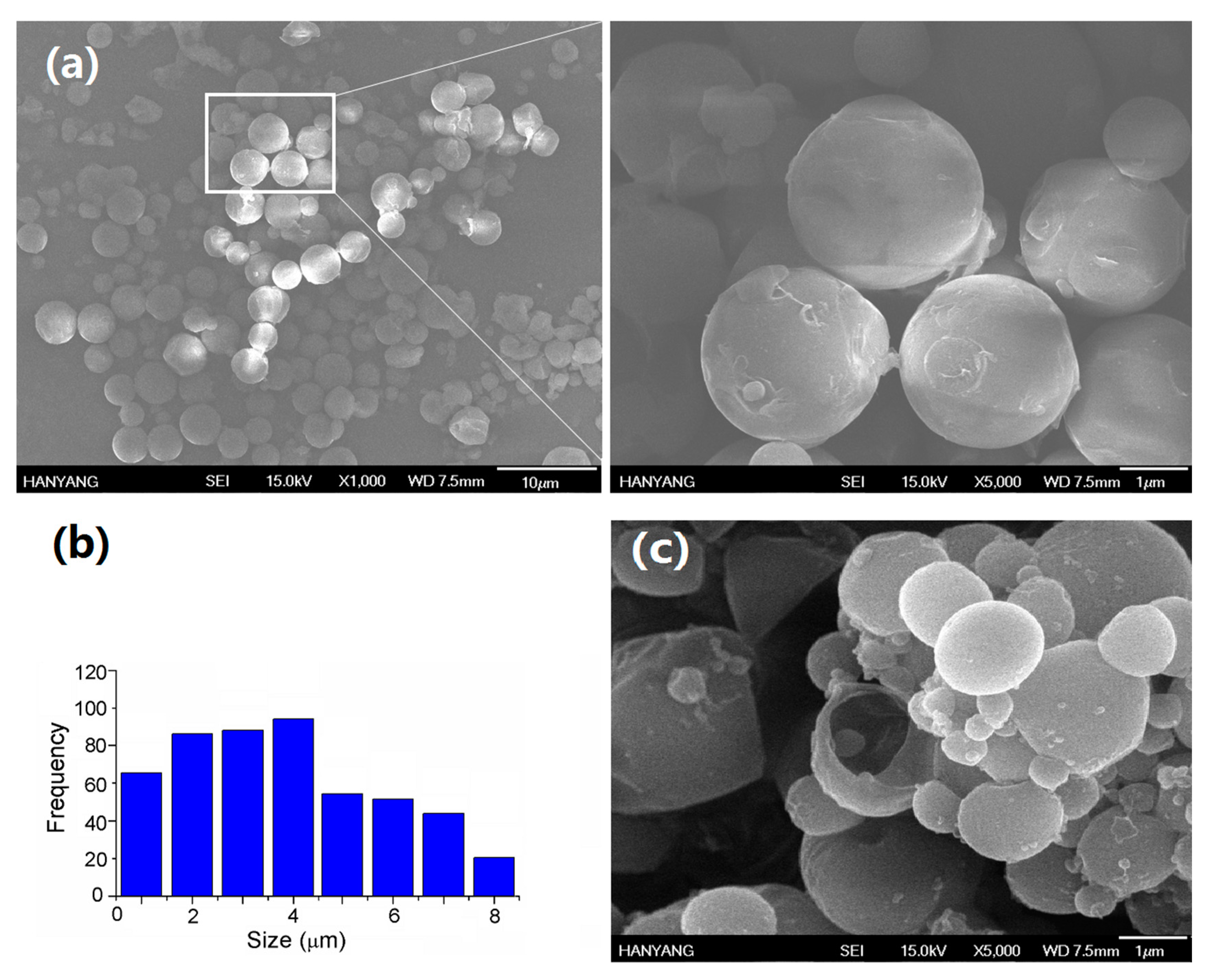
2.3. Analysis of the Core Loading of COMCs
2.4. The Internal Structure of COMCs
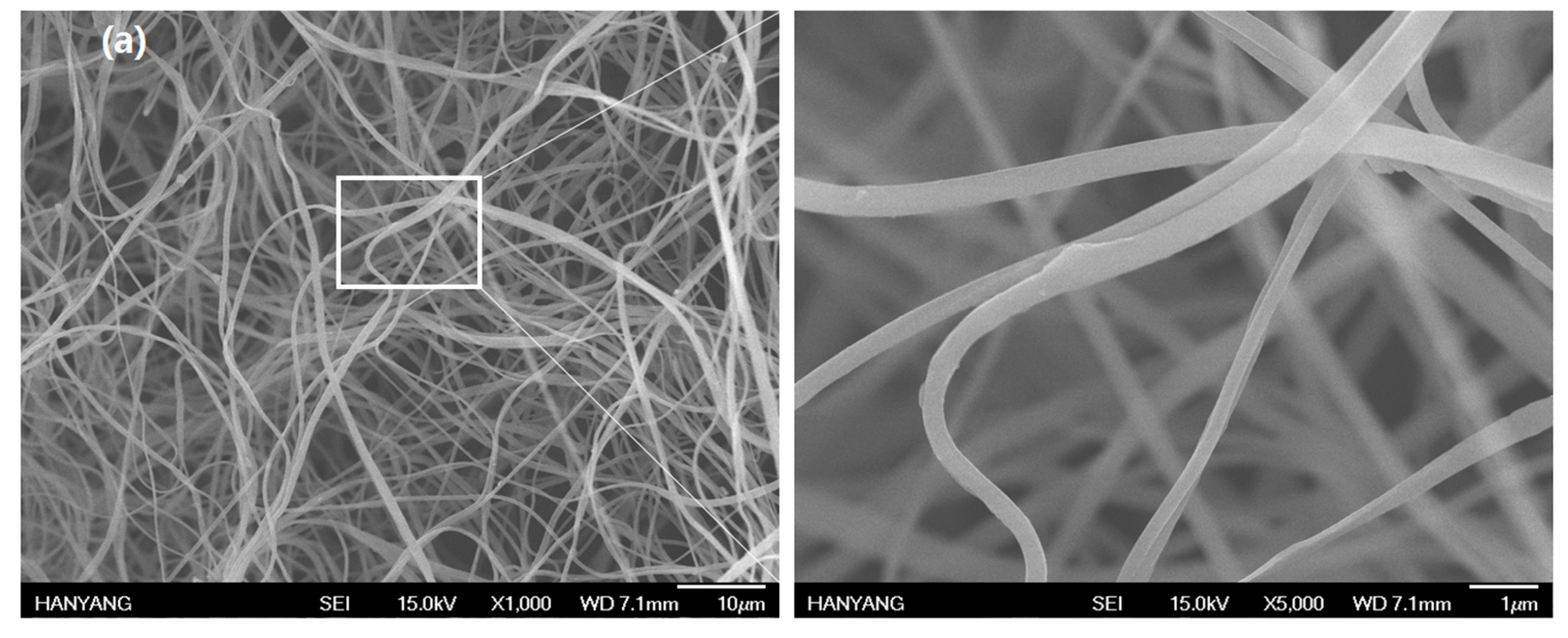
2.5. Nylon 66 Nanofiber-Based Fabrics (Nylon Nanofabrics) through Electrospinning
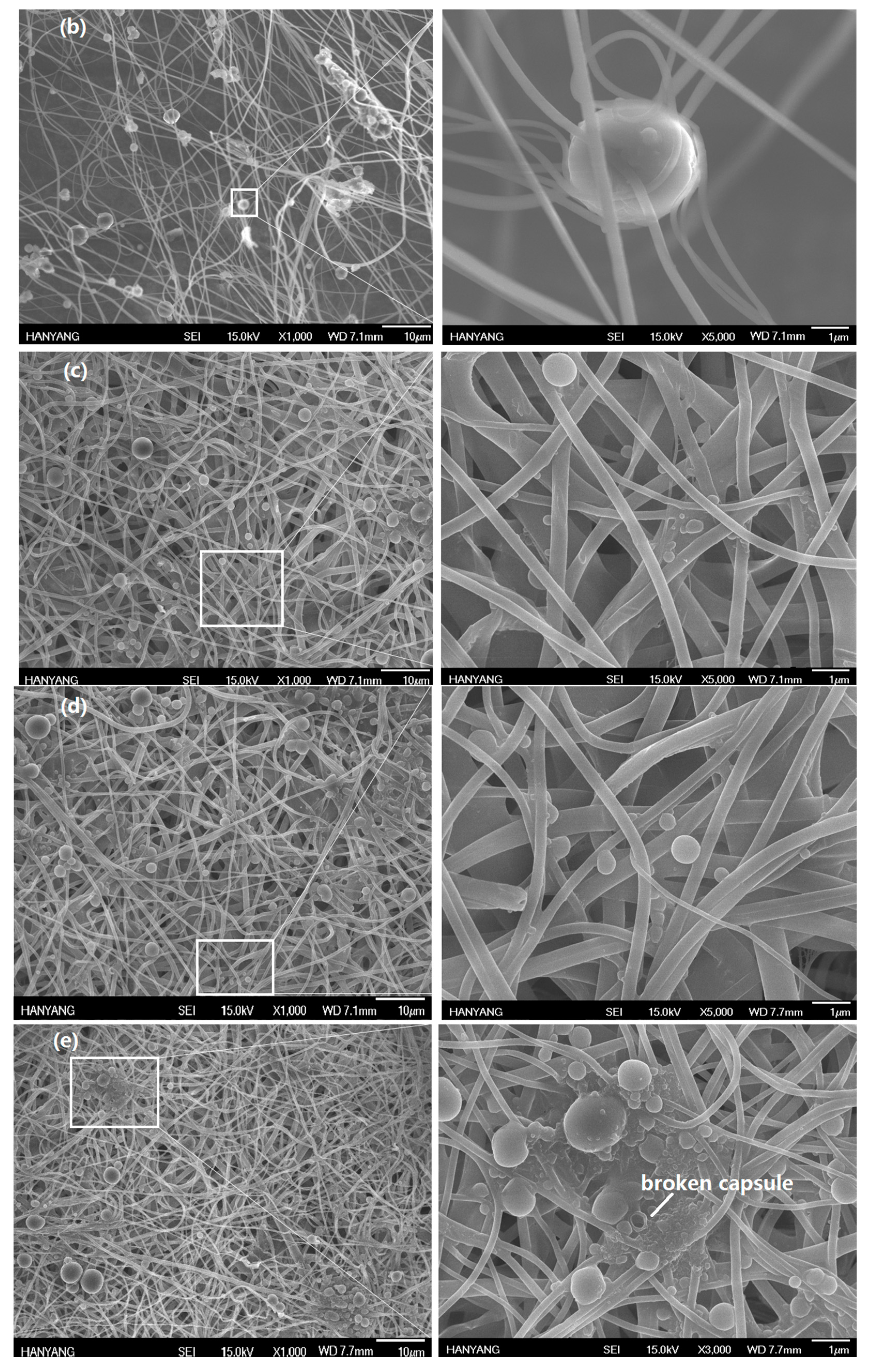
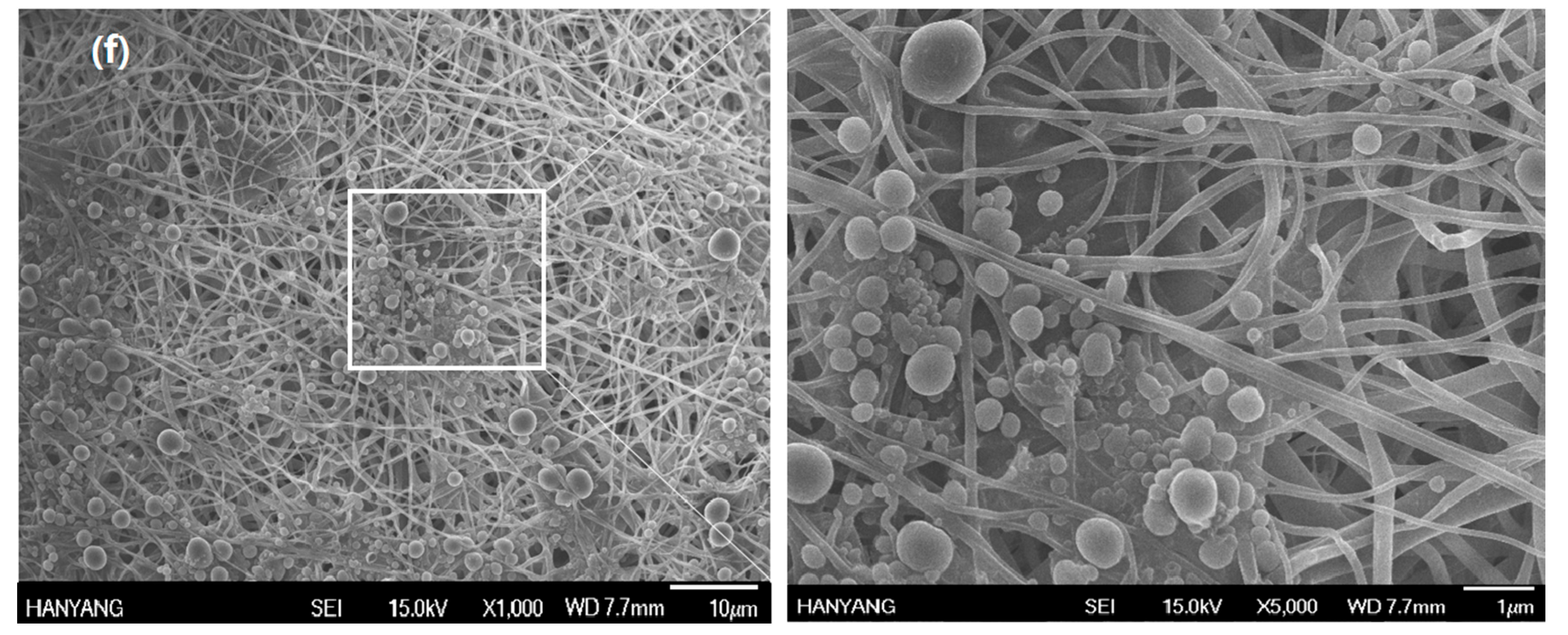
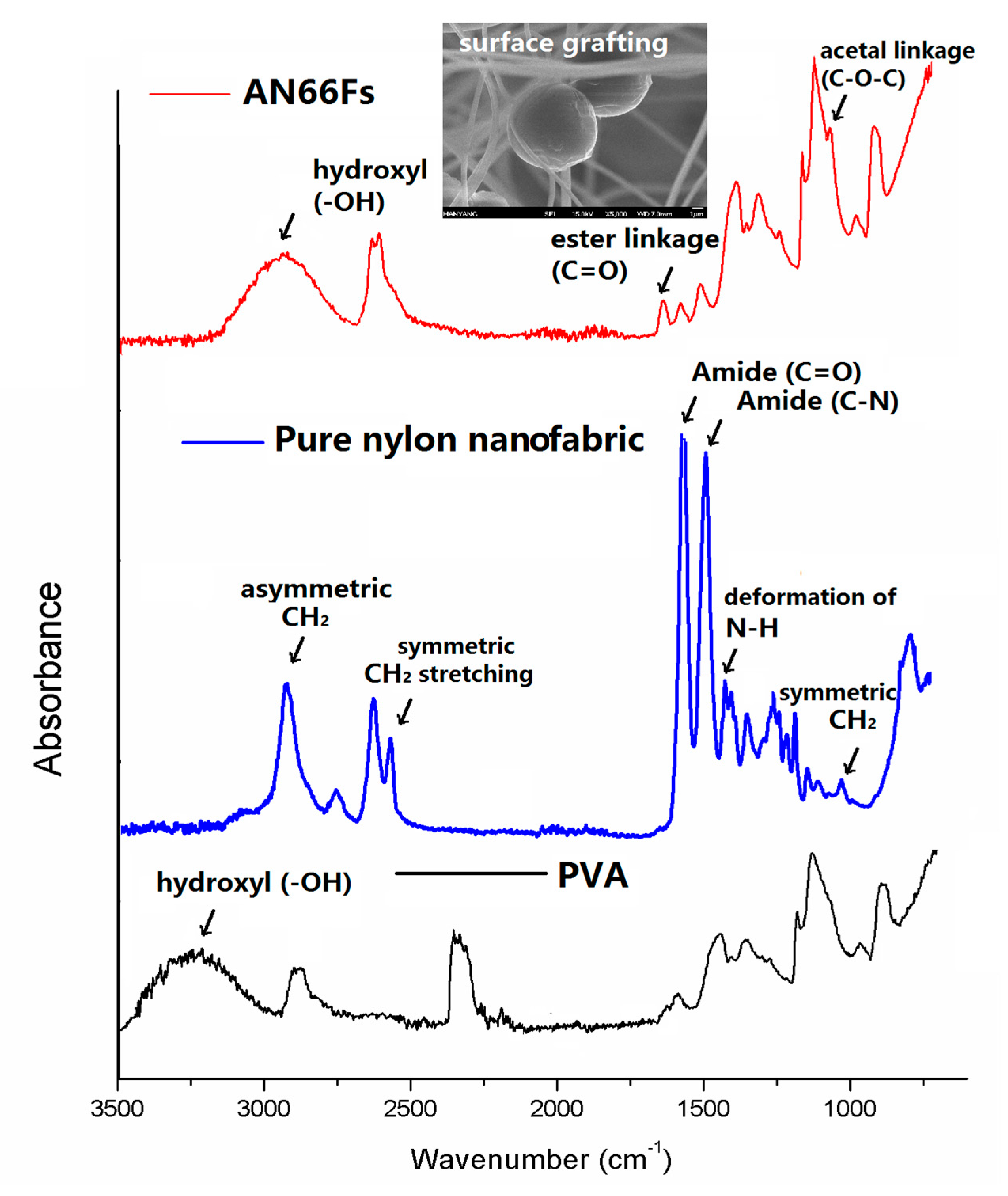
2.6. Grafting of COMCs onto Nylon 66 Nanofibers
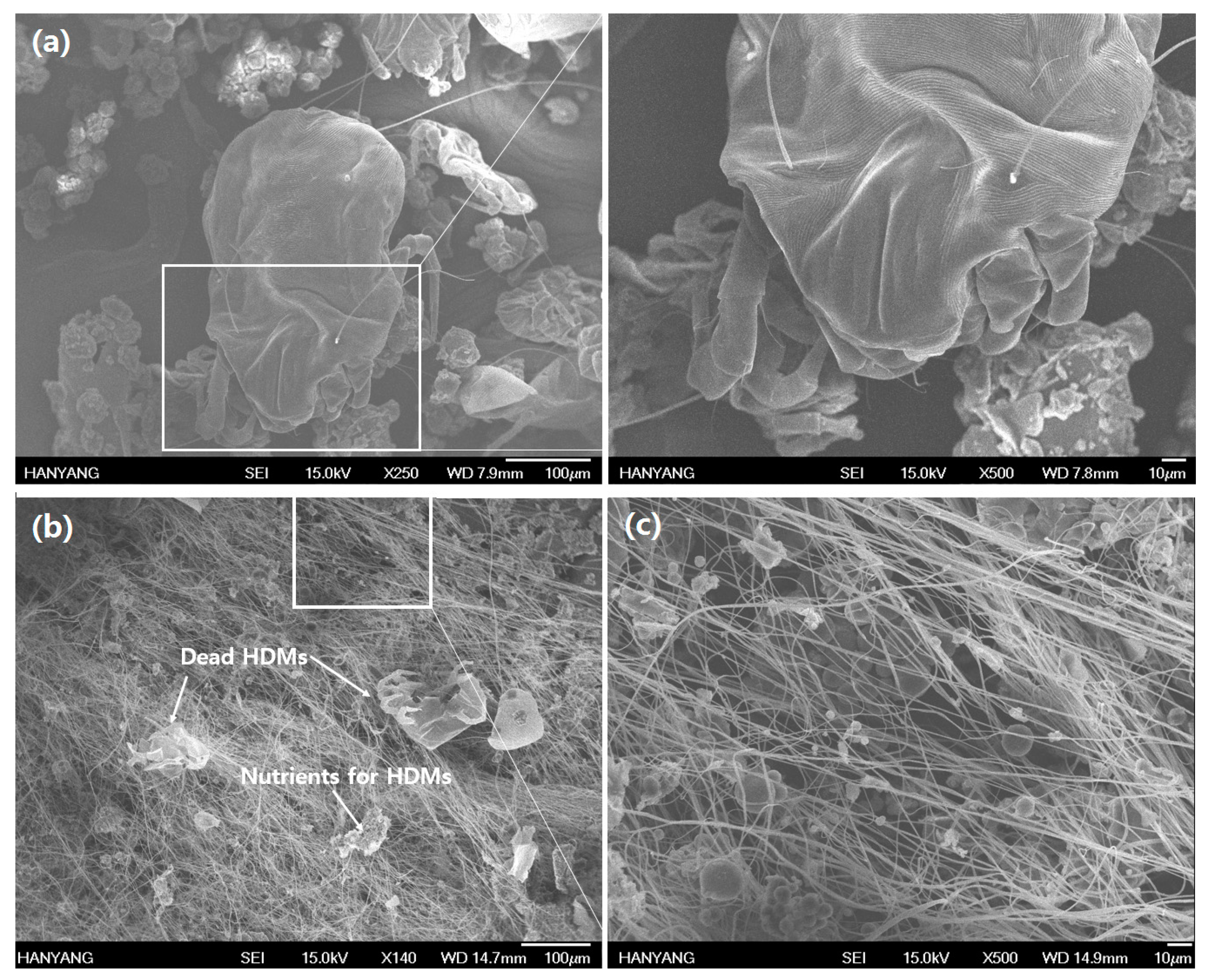
2.7. The Acaricidal Effect of AN66Fs on D. farinae
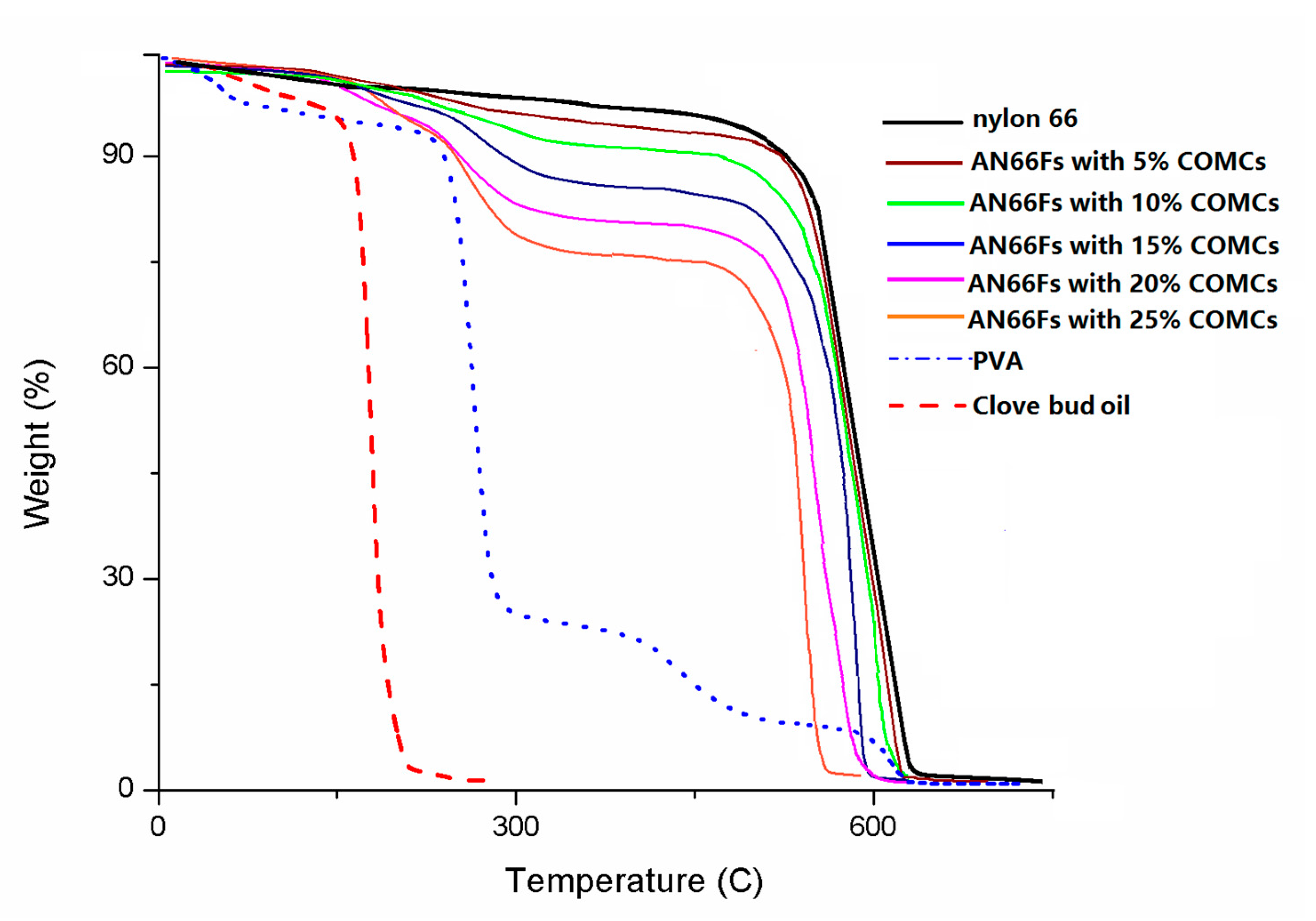
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wahida, L.; Aribi, N.; Soltani, N. Evaluation of secondary effects of some acaricides on apis mellifera intermissa (hymenoptera, apidae): Acetylcholinesterase and glutathione S-transferase activities. Eur. J. Sci. Res. 2008, 21, 642–649. [Google Scholar]
- Romi, R.; Lo Nostro, P.; Bocci, E.; Ridi, F.; Baglioni, P. Bioengineering of a cellulosic fabric for insecticide delivery via grafted cyclodextrin. Biotechnol. Prog. 2005, 21, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Huang, P.K.; Lin, S.X.; Tsai, M.J.; Leong, M.; Lin, S.R.; Kankala, R.; Lee, C.H.; Weng, C.F. Encapsulation of 16-Hydroxycleroda-3,13-Dine-16,15-Olide in mesoporous silica nanoparticles as a natural dipeptidyl peptidase-4 inhibitor potentiated hypoglycemia in diabetic mice. Nanomaterials 2017, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.-Z.; Hussien, R.; Saher, F.; Ahmed, Z. Acaricidal activities of some essential oils and their monoterpenoidal constituents against house dust mite, Dermatophagoides pteronyssinus (acari: Pyroglyphidae). J. Zhejiang Univ. Sci. B 2006, 7, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Kung, M.L.; Lin, P.Y.; Hsieh, C.W.; Tai, M.H.; Wu, D.C.; Kuo, C.H.; Hsieh, S.L.; Chen, H.T.; Hsieh, S. Bifunctional peppermint oil nanoparticles for antibacterial activity and fluorescence imaging. ACS Sustain. Chem. Eng. 2014, 2, 1769–1775. [Google Scholar] [CrossRef]
- Wei, M.C.; Lin, P.H.; Hong, S.J.; Chen, J.M.; Yang, Y.C. Development of a green alternative procedure for the simultaneous separation and quantification of clove oil and its major bioactive constituents. ACS Sustain. Chem. Eng. 2016, 4, 6491–6499. [Google Scholar] [CrossRef]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun nanofibres containing antimicrobial plant extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Tovey, E.R.; McDonald, L.G. A simple washing procedure with eucalyptus oil for controlling house dust mites and their allergens in clothing and bedding. J. Allergy Clin. Immunol. 1997, 100, 464–466. [Google Scholar] [CrossRef]
- Liakos, I.L.; Holban, A.M.; Carzino, R.; Lauciello, S.; Grumezescu, A. Electrospun fiber pads of cellulose acetate and essential oils with antimicrobial activity. Nanomaterials 2017, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R. Eucalyptus oil-loaded microcapsules grafted to cotton fabrics for acaricidal effect against dermatophagoides farina. J. Microencapsul. 2017, 34, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.J.; Coats, J.R. Insecticidal properties of several monoterpenoids to the house fly (diptera: Muscidae), red flour beetle (coleoptera: Tenebrionidae), and southern corn rootworm (coleoptera: Chrysomelidae). J. Econ. Entomol. 1994, 87, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Ahn, Y.J. Acaricidal activity of butylidenephthalide identified in cnidium officinale rhizome against dermatophagoides farinae and dermatophagoides pteronyssinus (acari: Pyroglyphidae). J. Agric. Food Chem. 2002, 50, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Yi, J.H.; Tak, J.H.; Ahn, Y.J. Acaricidal activity of plant essential oils against dermanyssus gallinae (acari: Dermanyssidae). Vet. Parasitol. 2004, 120, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ariana, A.; Ebadi, R.; Tahmasebi, G. Laboratory evaluation of some plant essences to control varroa destructor (acari: Varroidae). Exp. Appl. Acarol. 2002, 27, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; George, D.R.; Sparagano, O.A.; Seal, C.; Shiel, R.S.; Guy, J.H. A pilot study into the chemical and sensorial effect of thyme and pennyroyal essential oil on hens eggs. Int. J. Food Sci. Technol. 2009, 44, 1836–1842. [Google Scholar] [CrossRef]
- Furuno, T.; Terada, Y.; Yano, S.; Uehara, T.; Jodai, S. Activities of leaf oils and their components from lauraceae trees against house dust mites. J. Jpn. Wood Res. Soc. 1994, 40, 78–87. [Google Scholar]
- Jang, Y.S.; Lee, C.H.; Kim, M.K.; Kim, J.H.; Lee, S.H.; Lee, H.S. Acaricidal activity of active constituent isolated in Chamaecyparis obtusa leaves against Dermatophagoides spp. J. Agric. Food Chem. 2005, 53, 1934–1937. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Yang, J.Y.; Chung, N.; Lee, H.S. Contact and fumigant toxicities of 3-methylphenol isolated from Ostericum koreanum and its derivatives against house dust mites. J. Agric. Food Chem. 2012, 60, 12349–12354. [Google Scholar] [CrossRef] [PubMed]
- Sanbongi, C.; Takano, H.; Osakabe, N.; Sasa, N.; Natsume, M.; Yanagisawa, R.; Inoue, K.-I.; Sadakane, K.; Ichinose, T.; Yoshikawa, T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin. Exp. Allergy 2004, 34, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Rim, I.S.; Jee, C.H. Acaricidal effects of herb essential oils against Dermatophagoides farinae and D. pteronyssinus (acari: Pyroglyphidae) and qualitative analysis of a herb Mentha pulegium (pennyroyal). Korean J. Parasitol. 2006, 44, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, H.K.; Ahn, Y.J. Acaricidal activity of clove bud oil compounds against Dermatophagoides farinae and Dermatophagoides pteronyssinus (acari: Pyroglyphidae). J. Agric. Food Chem. 2003, 51, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Myint, S.; Wan Daud, W.R.; Mohamad, A.B.; Kadhum, A.A.H. Gas chromatographic determination of eugenol in ethanol extract of cloves. J. Chromatogr. B Biomed. Sci. Appl. 1996, 679, 193–195. [Google Scholar] [CrossRef]
- Deans, S.; Ritchie, G. Antibacterial properties of plant essential oils. Int. J. Food Microbiol. 1987, 5, 165–180. [Google Scholar] [CrossRef]
- Sánchez-Ramos, I.; Castañera, P. Acaricidal activity of natural monoterpenes on Tyrophagus putrescentiae (schrank), a mite of stored food. J. Stored Prod. Res. 2000, 37, 93–101. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, M.; Fan, C.; Dong, H.; Ding, G.; Zhang, W.; Tang, G.; Yang, J.; Kong, D.; Cao, Y. Development of novel urease-responsive pendimethalin microcapsules using silica-IPTS-PEI as controlled release carrier materials. ACS Sustain. Chem. Eng. 2017, 5. [Google Scholar] [CrossRef]
- Fong, J. Microencapsulation by solvent evaporation and organic phase separation processes. Control. Release Syst. 1988, 1, 81–108. [Google Scholar]
- Li, Y.; Zhou, M.; Pang, Y.; Qiu, X. Lignin-based microsphere: Preparation and performance on encapsulating the pesticide avermectin. ACS Sustain. Chem. Eng. 2017, 5, 3321–3328. [Google Scholar] [CrossRef]
- Thomas, W.; Smith, W. House-dust-mite allergens. Allergy 1998, 53, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, E.F.; Campos, F.S.; Lage, A.P.; Leite, R.C.; Heneine, L.G.; Vasconcelos, W.L.; Lobato, Z.I.P.; Mansur, H.S. Synthesis and characterization of poly (vinyl alcohol) hydrogels and hybrids for rMPB70 protein adsorption. Mater. Res. 2006, 9, 185–191. [Google Scholar] [CrossRef]
- Leimann, F.V.; Gonçalves, O.H.; Machado, R.A.; Bolzan, A. Antimicrobial activity of microencapsulated lemongrass essential oil and the effect of experimental parameters on microcapsules size and morphology. Mater. Sci. Eng. C 2009, 29, 430–436. [Google Scholar] [CrossRef]
- Fang, D.; Zhou, X.L.; Ye, Z.W.; Liu, Z.L. Brønsted acidic ionic liquids and their use as dual solvent-catalysts for fischer esterifications. Ind. Eng. Chem. Res. 2006, 45, 7982–7984. [Google Scholar] [CrossRef]
- Kim, J.R.; Michielsen, S. Photodynamic activity of nanostructured fabrics grafted with xanthene and thiazine dyes against opportunistic fungi. J. Photochem. Photobiol. B 2015, 150, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Michielsen, S. Photodynamic antifungal activities of nanostructured fabrics grafted with rose bengal and phloxine b against Aspergillus fumigatus. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Boury, F.; Ivanova, T.; Panaieotov, I.; Proust, J.; Bois, A.; Richou, J. Dilatational properties of adsorbed poly (D, L-lactide) and bovine serum albumin monolayers at the dichloromethane/water interface. Langmuir 1995, 11, 1636–1644. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Du, Y.; George, S.M. Molecular layer deposition of nylon 66 films examined using in situ ftir spectroscopy. J. Phys. Chem. C 2007, 111, 8509–8517. [Google Scholar] [CrossRef]
- Kim, J.R.; Michielsen, S. Synthesis of antifungal agents from xanthene and thiazine dyes and analysis of their effects. Nanomaterials 2016, 6, 243. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Sharma, S. Acaricidal activities of clove bud oil and red thyme oil using microencapsulation against HDMs. J. Microencapsul. 2011, 28, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Pasay, C.; Mounsey, K.; Stevenson, G.; Davis, R.; Arlian, L.; Morgan, M.; Vyszenski-Moher, D.; Andrews, K.; McCarthy, J. Acaricidal activity of eugenol based compounds against scabies mites. PLoS ONE 2010, 5, e12079. [Google Scholar] [CrossRef] [PubMed]
- Varel, V.H.; Miller, D.L. Eugenol stimulates lactate accumulation yet inhibits volatile fatty acid production and eliminates coliform bacteria in cattle and swine waste. J. Appl. Microbiol. 2004, 97, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Ignatowicz, S.; Brzostek, G. Use of irradiation as quarantine treatment for agricultural products infested by mites and insects. Int. J. Radiat. Appl. Instrum. C Radiat. Phys. Chem. 1990, 35, 263–267. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, J.R.; Ahn, Y.J. Acaricidal activity of cinnamaldehyde and its congeners against Tyrophagus putrescentiae (acari: Acaridae). J. Stored Prod. Res. 2004, 40, 55–63. [Google Scholar] [CrossRef]

| COMCs Loading to Nylon Nanofabrics | 0 wt % | 5 wt % | 10 wt % | 15 wt % | 20 wt % | 25 wt % |
|---|---|---|---|---|---|---|
| Surviving Number of D. farinae after 72 h | 50 (0.2) | 39 (1.6) | 18 (2.2) | 4 (0.8) | 1 (0.5) | 0 (0) |
| Mortality (%) | 0.1 (0) | 22 (1.8) | 64 (2.5) | 93 (2.4) | 100 (0.5) | 100 (0) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.R.; Kim, S.H. Eco-Friendly Acaricidal Effects of Nylon 66 Nanofibers via Grafted Clove Bud Oil-Loaded Capsules on House Dust Mites. Nanomaterials 2017, 7, 179. https://doi.org/10.3390/nano7070179
Kim JR, Kim SH. Eco-Friendly Acaricidal Effects of Nylon 66 Nanofibers via Grafted Clove Bud Oil-Loaded Capsules on House Dust Mites. Nanomaterials. 2017; 7(7):179. https://doi.org/10.3390/nano7070179
Chicago/Turabian StyleKim, Joo Ran, and Seong Hun Kim. 2017. "Eco-Friendly Acaricidal Effects of Nylon 66 Nanofibers via Grafted Clove Bud Oil-Loaded Capsules on House Dust Mites" Nanomaterials 7, no. 7: 179. https://doi.org/10.3390/nano7070179






