Properties of Cement Mortar and Ultra-High Strength Concrete Incorporating Graphene Oxide Nanosheets
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Specimens
2.3. Test Methods
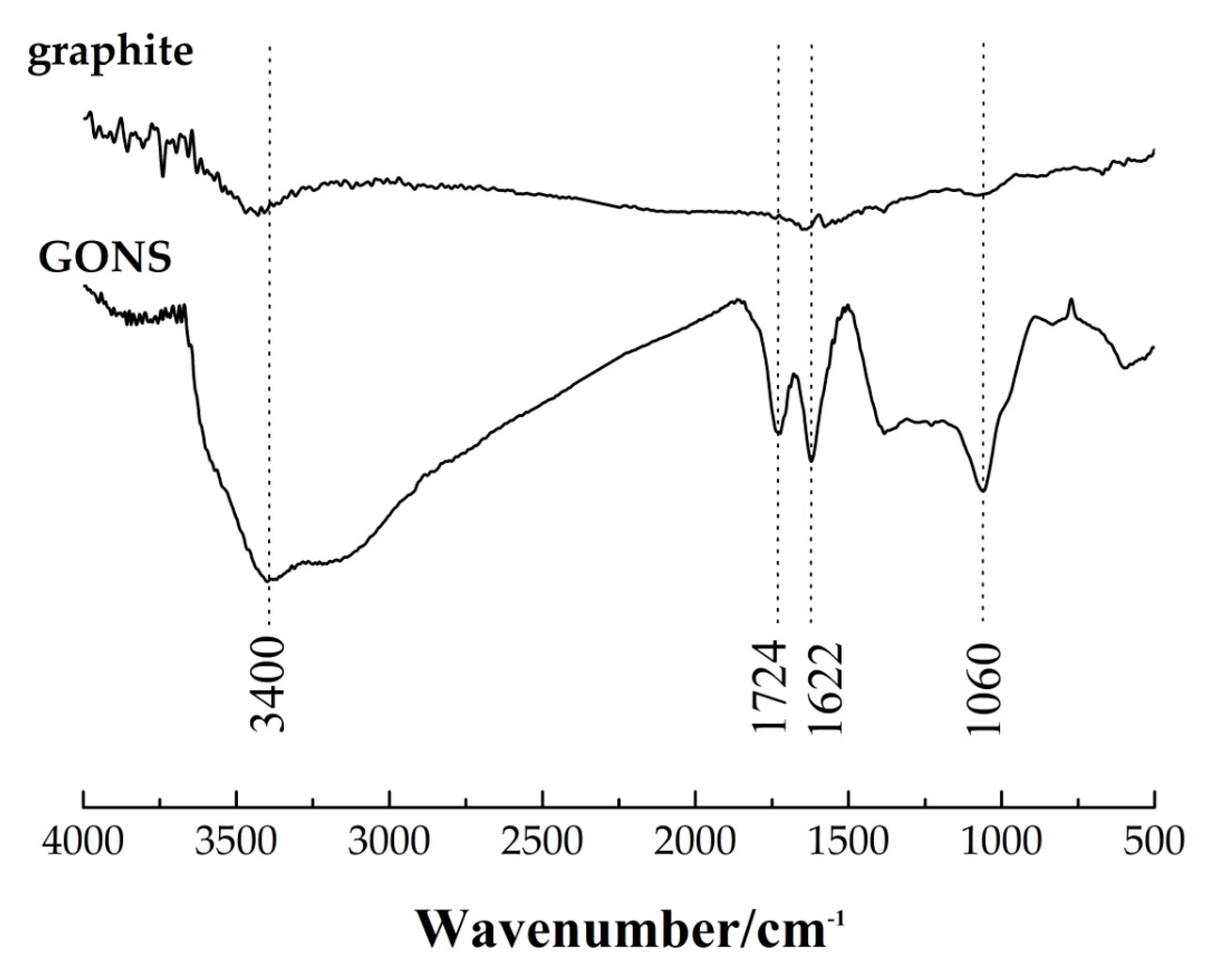
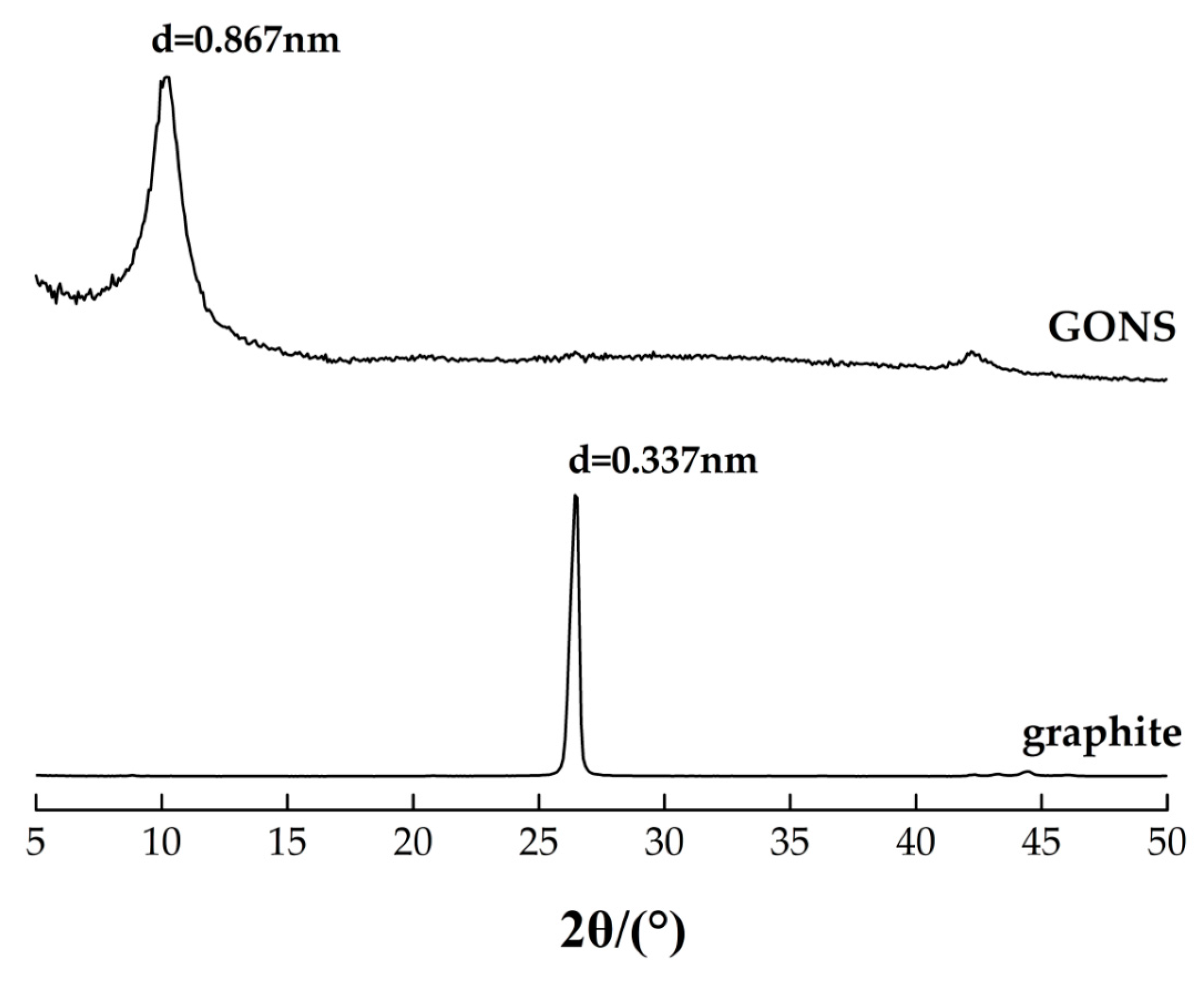
2.3.1. Characterization of GONSs
2.3.2. Fluidity Measurements
2.3.3. Flexural and Compressive Strength Tests
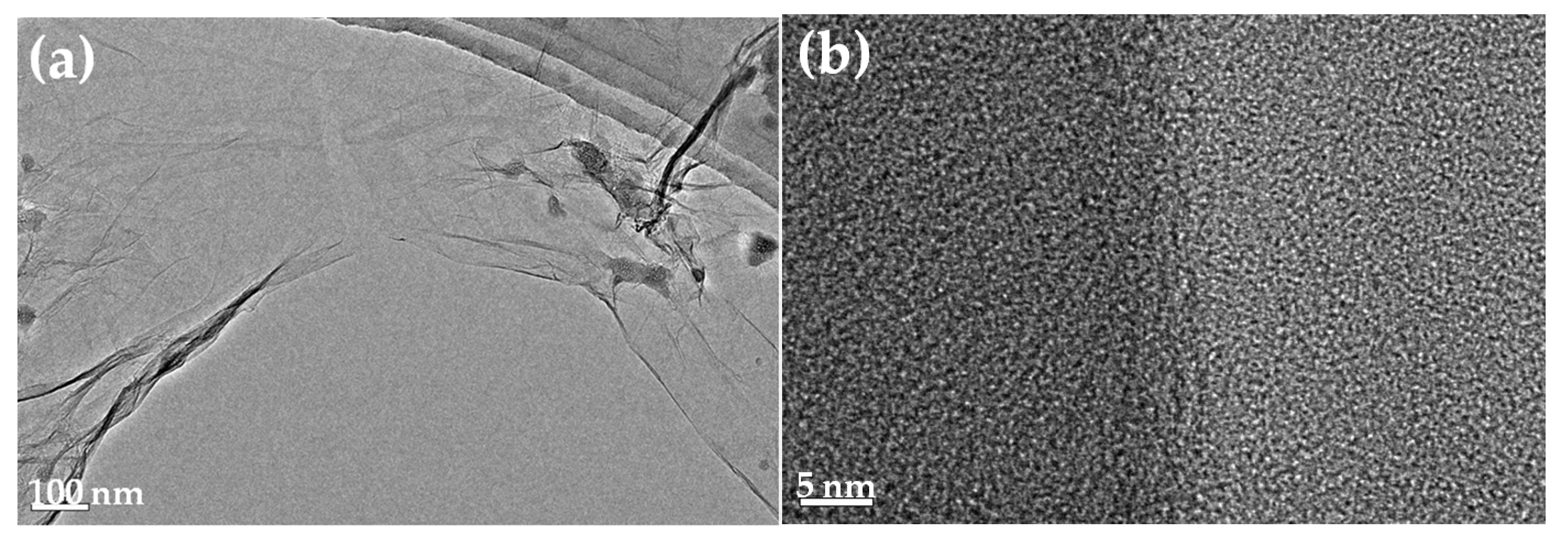
2.3.4. Morphology Observation
3. Results and Discussion
3.1. Characterization of Graphene Oxide Nanosheets
3.2. Fluidity
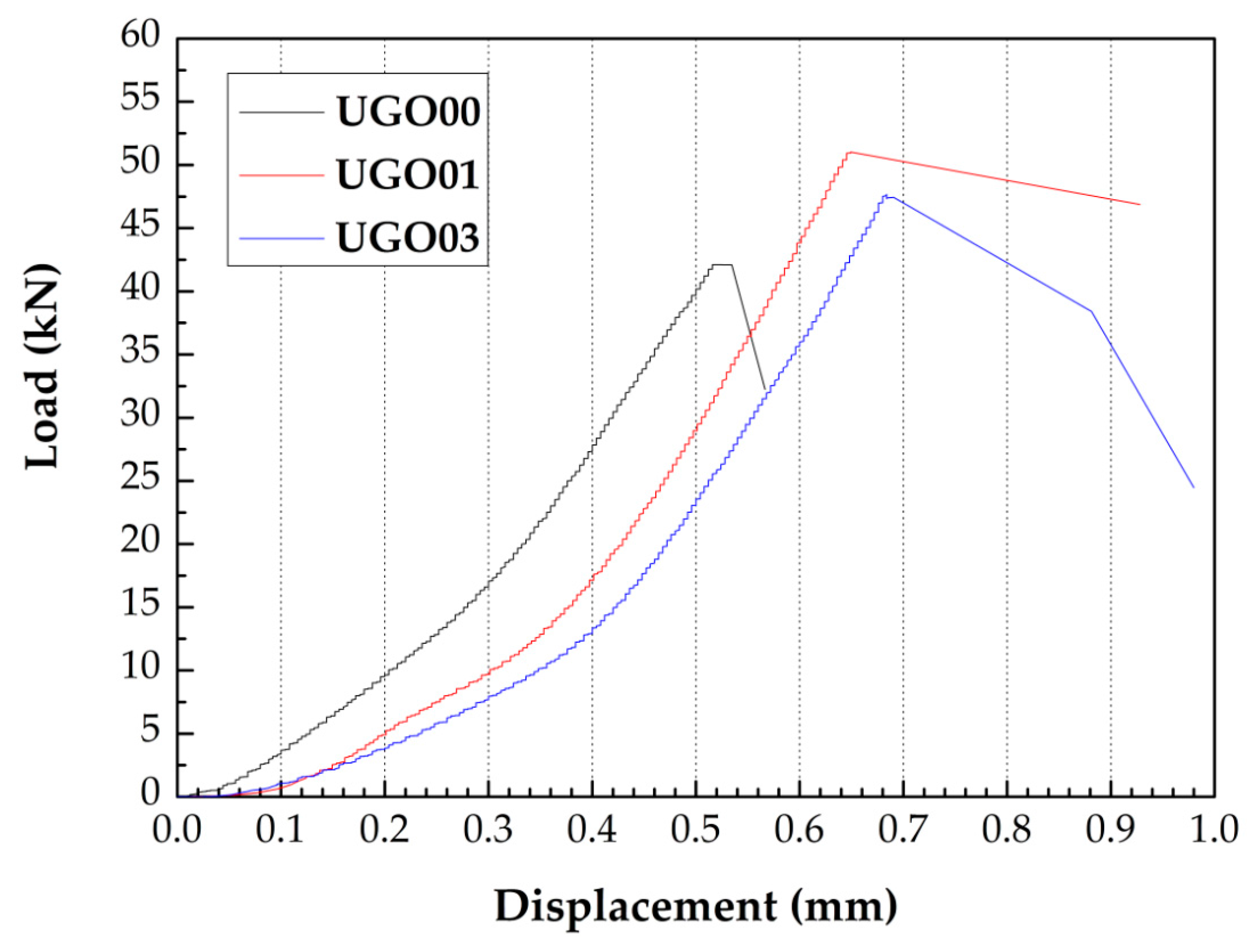
3.3. Mechanical Properties
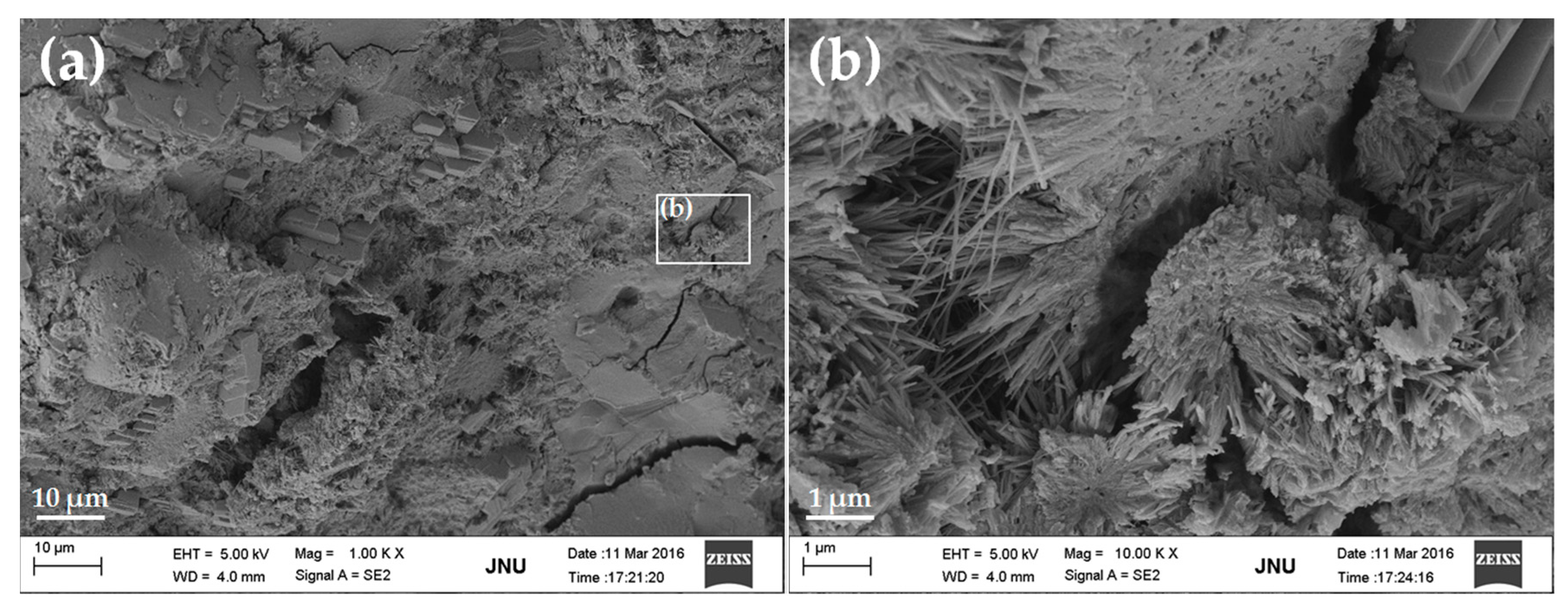
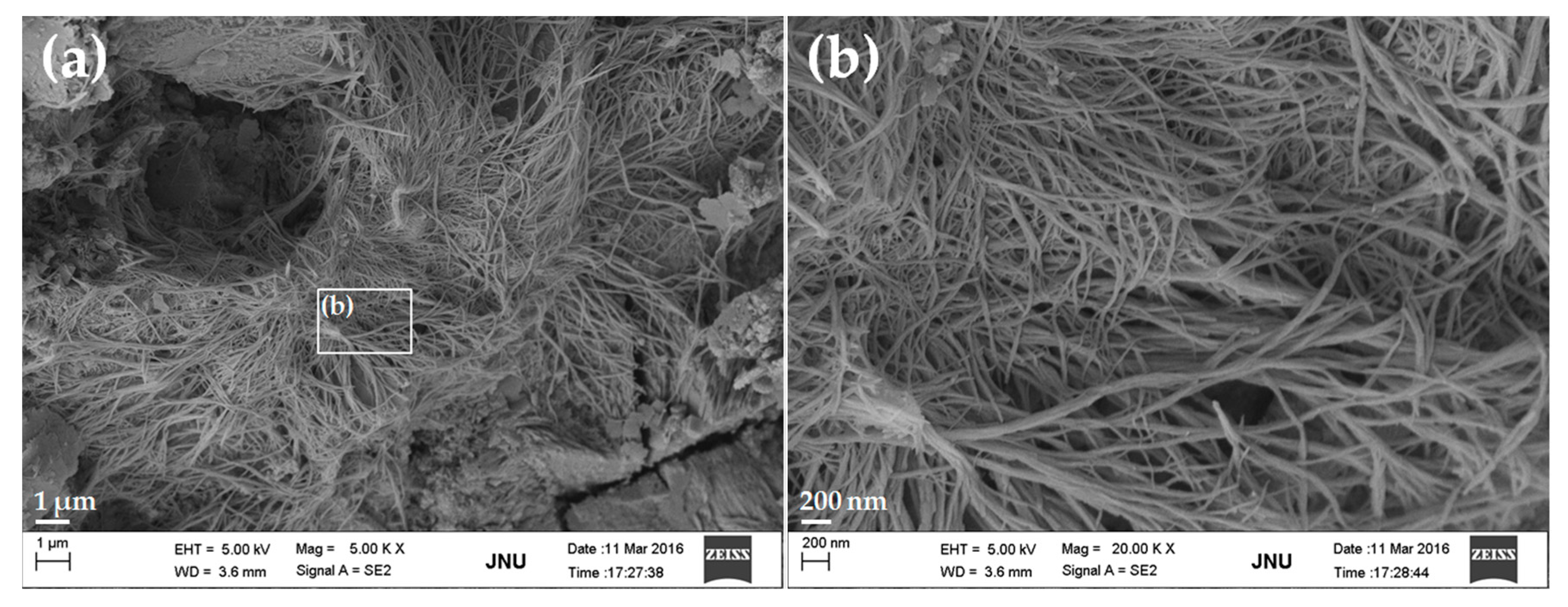
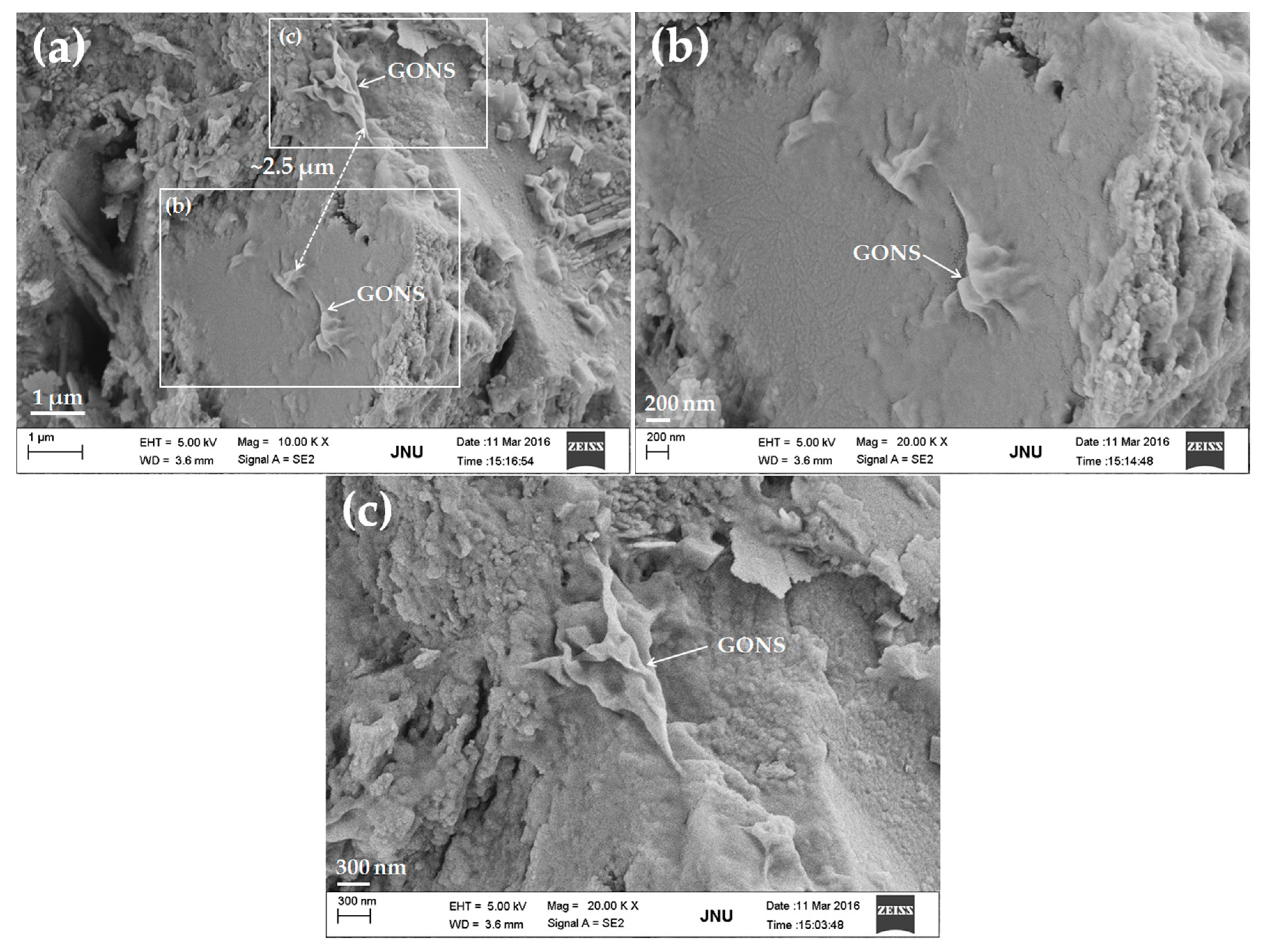
3.4. Micrograph
4. Conclusions
- The fluidity of cement mortar and ultra-high strength concrete (UHSC) decreased with the increasing addition of graphene oxide nanosheets (GONSs).
- Adding GONSs improved the flexural and compressive strengths of cement mortar and UHSC, with the increase in flexural strength more than that of compressive strength. Particularly, the compressive strength of UHSC incorporating 0.01% by weight of cement GONSs after curing for 28 days increased by 7.82% than that of UHSC without GONSs (117.34 MPa). Moreover, GONS additives significantly increased the deformation ability of UHSC.
- FE-SEM observations showed that GONSs were well dispersed in the cement matrix and the bonding of GONSs with the surrounding matrix was strong.
- The microstructural studies indicated that GONSs might have an effect on the shape of the cement hydration products. However, the growth space for hydrates also had an important effect on the morphology of hydrates. Therefore, further study is needed to collect more meaningful statistics about the effect of GONSs on cement hydration mechanisms.
- Research concerning about the crack resistance mechanism of GONSs on cement composites is still very inadequate, which also calls for further study in the future.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, L.L.; Ouyang, D.; Xu, W.T. Mechanical properties and durability of ultra high strength concrete incorporating multi-walled carbon nanotubes. Materials 2016, 9, 419. [Google Scholar] [CrossRef]
- Ravi-Chandar, K.; Yang, B. On the role of microcracks in the dynamic fracture of brittle materials. J. Mech. Phys. Solids 1997, 45, 535–563. [Google Scholar] [CrossRef]
- Sain, T.; Kishen, J.M.C. Energy-based equivalence between damage and fracture in concrete under fatigue. Eng. Fract. Mech. 2007, 74, 2320–2333. [Google Scholar] [CrossRef]
- Ning, J.G. A constitutive model based on the evolution and coalescence of elliptical micro-cracks for quasi-brittle materials. Chin. Sci. Bull. 2012, 57, 3773–3781. [Google Scholar] [CrossRef]
- Nili, M.; Afroughsabet, V. The long-term compressive strength and durability properties of silica fume fiber-reinforced concrete. Mater. Sci. Eng. A 2012, 531, 107–111. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Wang, P.M.; Zhao, X. Mechanical behavior and microstructure of cement composites incorporating surface-treated multi-walled carbon nanotubes. Carbon 2005, 43, 1239–1245. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H.; Li, G.; Zhu, J.W.; Collins, F.; Li, D.; Duan, W.H.; Wang, M.C. Mechanical properties and microstructure of a graphene oxide-cement composite. Cem. Concr. Compos. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Zhou, C.; Li, F.; Hu, J.; Yu, Q.J. Enhanced mechanical properties of cement paste by hybrid graphene oxide/carbon nanotubes. Constr. Build. Mater. 2017, 134, 336–345. [Google Scholar] [CrossRef]
- Bastos, G.; Patiño-Barbeito, F.; Patiño-Cambeiro, F.; Armesto, J. Nano-Inclusions Applied in Cement-Matrix Composites: A Review. Materials 2016, 9, 1015. [Google Scholar] [CrossRef]
- Wang, B.M.; Jiang, R.S.; Wu, Z.L. Investigation of the Mechanical Properties and Microstructure of Graphene Nanoplatelet-Cement Composite. Nanomaterials 2016, 6, 200. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.H.; Ma, Y.J.; Qiu, C.C.; Sun, T.; Liu, J.J.; Zhou, Q.F. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, D.; Yang, C.; Liu, Y.Y.; Liu, Y. Effect of graphene oxide on the rheological properties of cement pastes. Constr. Build. Mater. 2015, 96, 20–28. [Google Scholar] [CrossRef]
- Horszczaruk, E.; Mijowska, E.; Kalenczuk, R.J.; Aleksandrzak, M.; Mijowska, S. Nanocomposite of cement/graphene oxide-Impact on hydration kinetics and Young’s modulus. Constr. Build. Mater. 2015, 78, 234–242. [Google Scholar] [CrossRef]
- Babak, F.; Abolfazl, H.; Alimorad, R.; Parviz, G. Preparation and mechanical properties of graphene oxide: Cement nanocomposites. Sci. World J. 2014, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Pan, Z.; Korayem, A.H.; Duan, W.H. Reinforcing effects of graphene oxide on Portland cement paste. J. Mater. Civ. Eng. 2015, 27, A4014010. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Hou, D.S.; Ma, H.Y.; Fan, T.Y.; Li, Z.J. Effects of graphene oxide on the properties and microstructures of the magnesium potassium phosphate cement paste. Constr. Build. Mater. 2016, 119, 107–112. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.L.; Ge, C.; Li, Q.; Guo, L.P.; Shu, X.; Liu, J.P. Mechanical behavior and toughening mechanism of polycarboxylate superplasticizer modified graphene oxide reinforced cement composites. Compos. Part B Eng. 2017, 113, 308–316. [Google Scholar] [CrossRef]
- Shi, C.J.; Wang, D.H.; Wu, L.M.; Wu, Z.M. The hydration and microstructure of ultra high-strength concrete with cement-Silica fume-Slag binder. Cem. Concr. Compos. 2015, 61, 44–52. [Google Scholar] [CrossRef]
- Ouyang, D.; Yu, B. A study on mechanical properties of very high strength concrete. J. Chongqing Jianzhu Univ. 2003, 25, 38–42. (In Chinese) [Google Scholar]
- Shen, W.G.; Liu, Y.; Cao, L.H.; Huo, X.J.; Yang, Z.G.; Zhou, C.C.; He, P.T.; Lu, Z.L. Mixing design and microstructure of ultra high strength concrete with manufactured sand. Constr. Build. Mater. 2017, 143, 312–321. [Google Scholar] [CrossRef]
- Yi, N.H.; Kim, J.H.J.; Han, T.S.; Cho, Y.G.; Lee, J.H. Blast-resistant characteristics of ultra-high strength concrete and reactive powder concrete. Constr. Build. Mater. 2012, 28, 694–707. [Google Scholar] [CrossRef]
- Song, X.B.; Zhang, J.Y.; Shang, S.S. Mechanical properties of early-age concrete reinforced with multi-walled carbon nanotubes. Mag. Concr. Res. 2017, 69, 320–327. [Google Scholar] [CrossRef]
- Fan, X.B.; Peng, W.C.; Li, Y.; Li, X.Y.; Wang, S.L.; Zhang, G.L.; Zhang, F.B. Deoxygenation of exfoliated graphite oxide under alkaline conditions: A green route to graphene preparation. Adv. Mater. 2008, 23, 4490–4493. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Hanif, A.; Ning, C.; Shao, H.Y.; Yin, R.; Li, Z.Y. Steric stabilization of graphene oxide in alkaline cementitious solutions Mechanical enhancement of cement composite. Mater. Des. 2017, 127, 154–161. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, Z.N.; Kong, D.Y.; Chen, R.S. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar]
- Tamimi, A.; Hassan, N.M.; Fattah, K.; Talachi, A. Performance of cementitious materials produced by incorporating surface treated multiwall carbon nanotubes and silica fume. Constr. Build. Mater. 2016, 114, 934–945. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Lu, C.X.; Liu, B.W.; Zhang, K.; Li, C.Z. Influence of graphene oxide additions on the microstructure and mechanical strength of cement. New Carbon Mater. 2015, 30, 349–356. [Google Scholar] [CrossRef]
- GB/T 17671-1999. Method of Testing Cements-Determination of Strength; National Standard of the P.R. China: Beijing, China, 1999. (In Chinese)
- GB/T 2419-2005. Test Method for Fluidity of Cement Mortar; National Standard of the P.R. China: Beijing, China, 2005. (In Chinese)
- GB/T 50080-2002. Standard for Test Method of Performance on Ordinary Fresh Concrete; National Standard of the P.R. China: Beijing, China, 2002. (In Chinese)
- GB/T 50081-2002. Standard for Test Method of Mechanical Properties on Ordinary Concrete; National Standard of the P.R. China: Beijing, China, 2002. (In Chinese)
- Chen, W.; Yan, L. Preparation of graphene by a low-temperature thermal reduction at atmosphere pressure. Nanoscale 2010, 2, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, D.; Ge, H.Y.; Wang, J. Graphene oxide-deposited carbon fiber/cement composites for electromagnetic interference shielding application. Constr. Build. Mater. 2015, 84, 66–72. [Google Scholar] [CrossRef]
- Pan, D.Y.; Wang, S.; Zhao, B.; Wu, M.H.; Zhang, H.J.; Wang, Y.; Jiao, Z. Li Storage Properties of Disordered Graphene Nanosheets. Chem. Mater. 2009, 21, 3136–3142. [Google Scholar] [CrossRef]
- Lv, S.H.; Liu, J.J.; Sun, T.; Ma, Y.J.; Zhou, Q.F. Effect of graphene oxide nanosheets on shapes of cement hydration crystals and their formation process. Constr. Build. Mater. 2014, 64, 231–239. [Google Scholar] [CrossRef]
- Li, X.Y.; Korayem, A.H.; Li, C.Y.; Liu, Y.M.; He, H.S.; Sanjayan, J.G.; Duan, W.H. Incorporation of graphene oxide and silica fume into cement paste: A study of dispersion and compressive strength. Constr. Build. Mater. 2016, 123, 327–335. [Google Scholar] [CrossRef]
- Li, X.Y.; Lu, Z.Y.; Chuah, S.; Li, W.G.; Liu, Y.M.; Duan, W.H.; Li, Z.J. Effects of graphene oxide aggregates on hydration degree, sorptivity, and tensile splitting strength of cement paste. Composites Part A 2017, 100, 1–8. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions—Enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.M. Some emphases of scanning electron microscopy in research on original fracture surface of cement paste. J. Build. Mater. 1998, 2, 129–133. (In Chinese) [Google Scholar]
- Xia, H.Y.; Zhang, X.; Shi, Z.Q.; Zhao, C.J.; Li, Y.F.; Wang, J.P.; Qiao, G.J. Mechanical and thermal properties of reduced graphene oxide reinforced aluminum nitride ceramic composites. Mater. Sci. Eng. A 2015, 29–36. [Google Scholar] [CrossRef]
- Liu, J.; Yan, H.X.; Reece, M.J.; Jiang, K. Toughening of zirconia/alumina composites by the addition of graphene platelets. J. Eur. Ceram. Soc. 2012, 32, 4185–4193. [Google Scholar] [CrossRef]




| Material | Chemical Composition (wt %) | Physical Properties | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | LOI | SG | SSA (m2/kg) | |
| C | 20.13 | 4.53 | 4.11 | 63.88 | 1.35 | 2.28 | 2.82 | 3.10 | 331 |
| SF | 93.85 | 0.69 | 0.17 | 0.75 | 1.22 | 0.41 | 1.88 | 2.20 | ~20,000 |
| BS | 44.91 | 14.86 | – | 31.08 | 7.18 | 0.65 | 1.80 | 2.83 | 1228 |
| NO. | w/cm | GONS (wt %) | Quantities (kg/m3) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | SF | BS | GONS | FA | CA | W | PCs | |||
| UGO00 | 0.2 | 0.00 | 420 | 60 | 120 | 0.000 | 798 | 976 | 120 | 15 |
| UGO01 | 0.2 | 0.01 | 420 | 60 | 120 | 0.042 | 798 | 976 | 120 | 15 |
| UGO03 | 0.2 | 0.03 | 420 | 60 | 120 | 0.126 | 798 | 976 | 120 | 15 |
| No. | Fluidity (mm) | Flexural Strength (MPa) | Compressive Strength (MPa) | ||
|---|---|---|---|---|---|
| Slump | Slump Flow | 7 d | 7 d | 28 d | |
| UGO00 | 240 | 450 | 8.92 (0.00) | 90.60 (0.00) | 117.34 (0.00) |
| UGO01 | 235 | 420 | 9.98 (11.88) | 93.92 (3.66) | 126.52 (7.82) |
| UGO03 | 220 | 380 | 9.54 (6.95) | 94.73 (4.55) | 122.73 (4.59) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Ouyang, D. Properties of Cement Mortar and Ultra-High Strength Concrete Incorporating Graphene Oxide Nanosheets. Nanomaterials 2017, 7, 187. https://doi.org/10.3390/nano7070187
Lu L, Ouyang D. Properties of Cement Mortar and Ultra-High Strength Concrete Incorporating Graphene Oxide Nanosheets. Nanomaterials. 2017; 7(7):187. https://doi.org/10.3390/nano7070187
Chicago/Turabian StyleLu, Liulei, and Dong Ouyang. 2017. "Properties of Cement Mortar and Ultra-High Strength Concrete Incorporating Graphene Oxide Nanosheets" Nanomaterials 7, no. 7: 187. https://doi.org/10.3390/nano7070187





