Magnetic Nanoemulsions: Comparison between Nanoemulsions Formed by Ultrasonication and by Spontaneous Emulsification
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Nanoemulsions
2.2. In Vitro Release Assay
2.3. Release Kinetics Modelling
2.4. Long-Term Stability
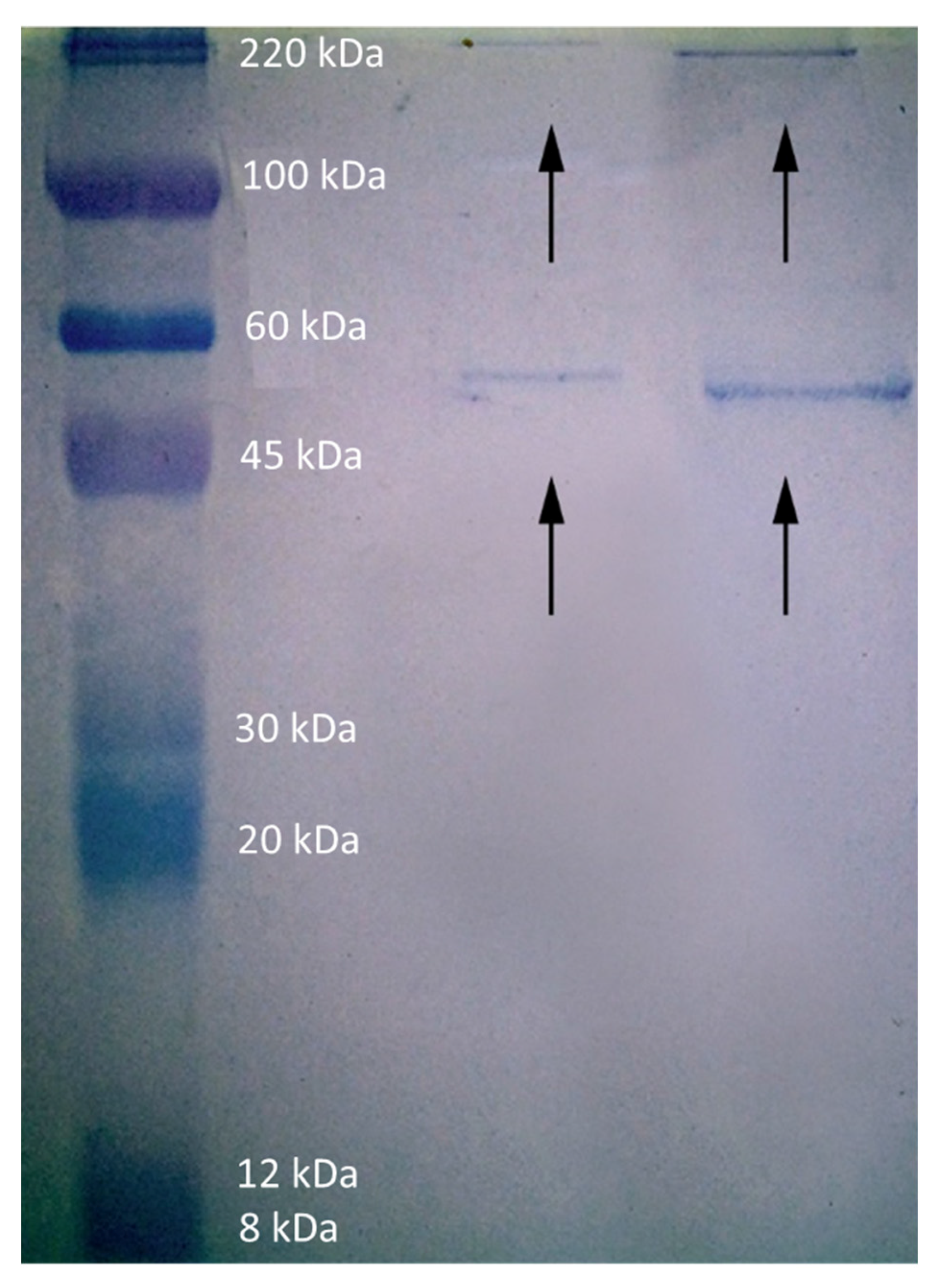
2.5. Interaction with Proteins
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Nanoemulsions
4.2.1. High Energy (HE) Method
4.2.2. Low Energy (LE) Method
4.2.3. Particle Size Measurements
4.2.4. Magnetic Measurements
4.2.5. Thermogravimetric Measurements
4.2.6. Lipid Determination
4.2.7. Iron Determination
4.2.8. IND Determination
4.2.9. In Vitro Release Assay
4.2.10. Modelling of Release Kinetics
4.2.11. Long-Term Stability Test
4.2.12. Interaction with Proteins
4.2.13. SDS-PAGE Electrophoresis
Author Contributions
Conflicts of Interest
References
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; García-Celma, M. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; González, C.; Maestro, A.; Solé, I.; Pey, C.M.; Nolla, J. Nano-emulsions: New applications and optimization of their preparation. Curr. Opin. Colloid Interface Sci. 2008, 13, 245–251. [Google Scholar] [CrossRef]
- Kumar, M.; Misra, A.; Babbar, A.K.; Mishra, A.K.; Mishra, P.; Pathak, K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int. J. Pharm. 2008, 358, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.K. Engineering of nanoemulsions for drug delivery. Curr. Drug Deliv. 2005, 2, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Lovelyn, C. Current state of nanoemulsions in drug delivery. J. Biomater. Nanobiotechnol. 2011, 2, 626–639. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Shafiq, S. Celecoxib nanoemulsion: Skin permeation mechanism and bioavailability assessment. J. Drug Target. 2008, 16, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Kwasigroch, B.; Escribano, E.; Moran, M.D.; Queralt, J.; Busquets, M.A.; Estelrich, J. Oil-in-water nanoemulsions are suitable for carrying hydrophobic compounds: Indomethacin as a model of anti-inflammatory drug. Int J. Pharm. 2016, 515, 749–756. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef]
- Rao, J.J.; McClements, D.J. Lemon oil solubilization in mixed surfactant solutions: Rationalizing microemulsion and nanoemulsion formation. Food Hydrocoll. 2012, 26, 268–276. [Google Scholar] [CrossRef]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physicochemical properties and antimicrobial efficacy of carvacrol nanoemulsions formed by spontaneous emulsification. J. Agric. Food Chem. 2013, 61, 8906–8913. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Fabrication of vitamin e-enriched nanoemulsions: Factors affecting particle size using spontaneous emulsification. J. Colloid Interface Sci. 2013, 391, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Sonneville-Aubrun, O.; Simonnet, J.T.; L’Alloret, F. Nanoemulsions: A new vehicle for skincare products. Adv. Colloid Interface Sci. 2004, 108–109, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Snehalatha, K. Esterolytic reactivities of (dialkylamino)pyridine amphiphiles solubilized in different pseudo-three-component cationic microemulsions. Langmuir 1997, 13, 378–384. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, S.; Reimers, G. Preparation of dilution-stable aqueous magnetic fluids. IEEE Trans. Magn. 1980, 16, 178–183. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2015, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Sabate, R.; Barnadas-Rodriguez, R.; Callejas-Fernandez, J.; Hidalgo-Alvarez, R.; Estelrich, J. Preparation and characterization of extruded magnetoliposomes. Int. J. Pharm. 2008, 347, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Veyret, R.; Delair, T.; Pichot, C.; Elaissari, A. Amino-containing magnetic nanoemulsions: Elaboration and nucleic acid extraction. J. Magn. Magn. Mater. 2005, 295, 155–163. [Google Scholar] [CrossRef]
- Mahendran, V.; Philip, J. Sensing of biologically important cations such as Na+, K+, Ca2+, Cu2+, and Fe3+ using magnetic nanoemulsions. Langmuir 2013, 29, 4252–4258. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, V.; Philip, J. Non-enzymatic glucose detection usinhg magnetic nanoemulsions. Appl. Phys. Lett. 2014, 105, 123110. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Amemiya, F.; Fuchigami, T.; Machida, K.; Takeda, S.; Tamamitsu, K.; Atobe, M. Highly clear and transparent nanoemulsion preparation under surfactant-free conditions using tandem acoustic emulsification. Chem. Commun. 2011, 47, 5765–5767. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.; Meleson, K.; Wilking, J.; Lin, M.Y.; Mason, T.G. Structure of concentrated nanoemulsions. J. Chem. Phys. 2005, 122, 134703. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. 2006, 18, R635–R666. [Google Scholar] [CrossRef]
- Yu, L.; Li, C.; Xu, J.; Hao, J.; Sun, D. Highly stable concentrated nanoemulsions by the phase inversion composition method at elevated temperature. Langmuir 2012, 28, 14547–14552. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McClements, D.J. Optimization of orange oil nanoemulsion formation by isothermal low-energy methods: Influence of the oil phase, surfactant, and temperature. J. Agric. Food Chem. 2014, 62, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.M. Thermoresponsive magnetic colloids. Colloid Polym. Sci. 2007, 285, 953–966. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Bombelli, F.B.; Dawson, K.A. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Anton, N.; Vandamme, T.F. The universality of low-energy nano-emulsification. Int. J. Pharm. 2009, 377, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Steward-Marshall, J.C. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar]
- Kiwada, H.; Sato, J.; Yamada, S.; Kato, Y. Feasibility of magnetic liposomes as a targeting device for drugs. Chem. Pharm. Bull. 1986, 34, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
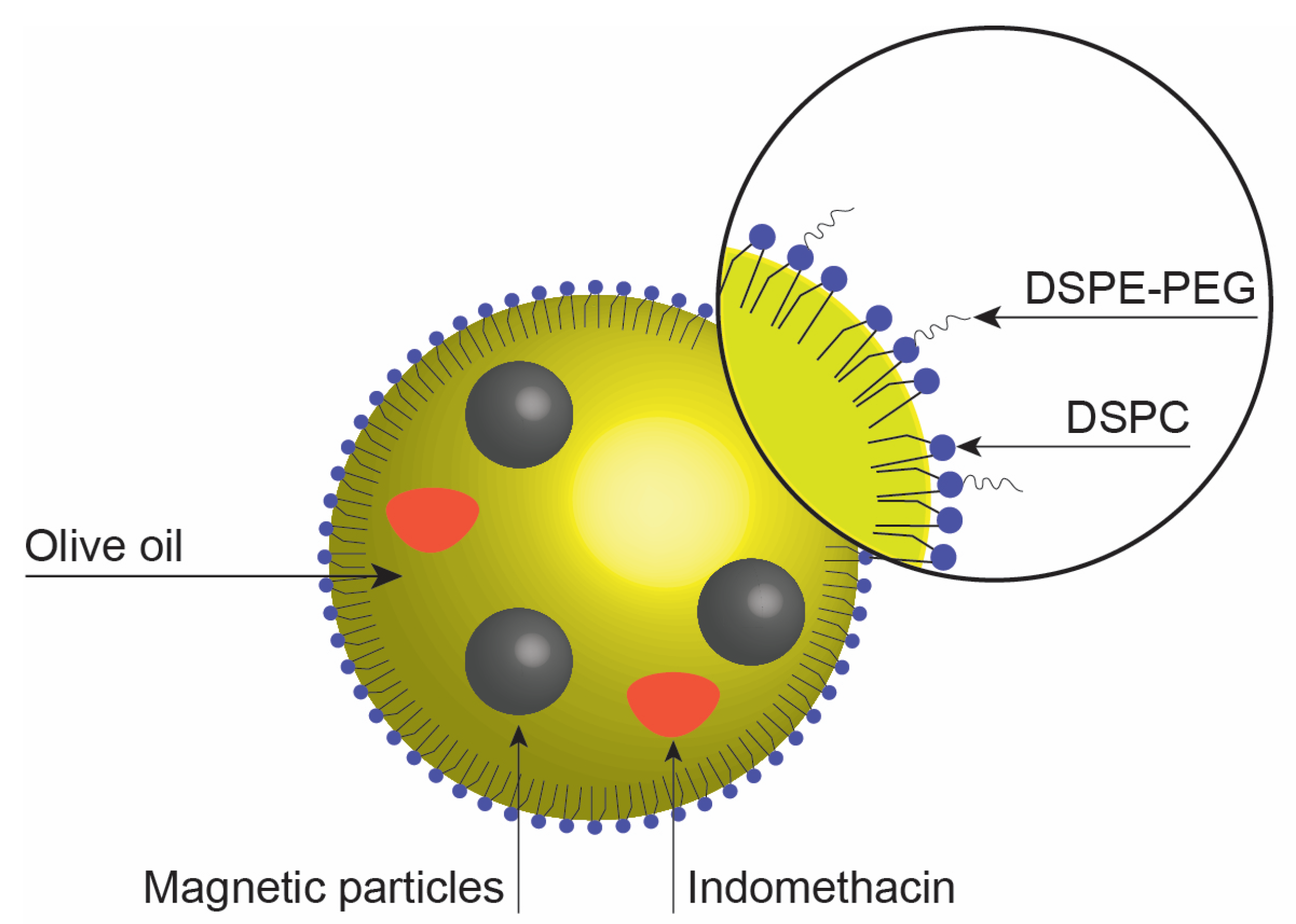
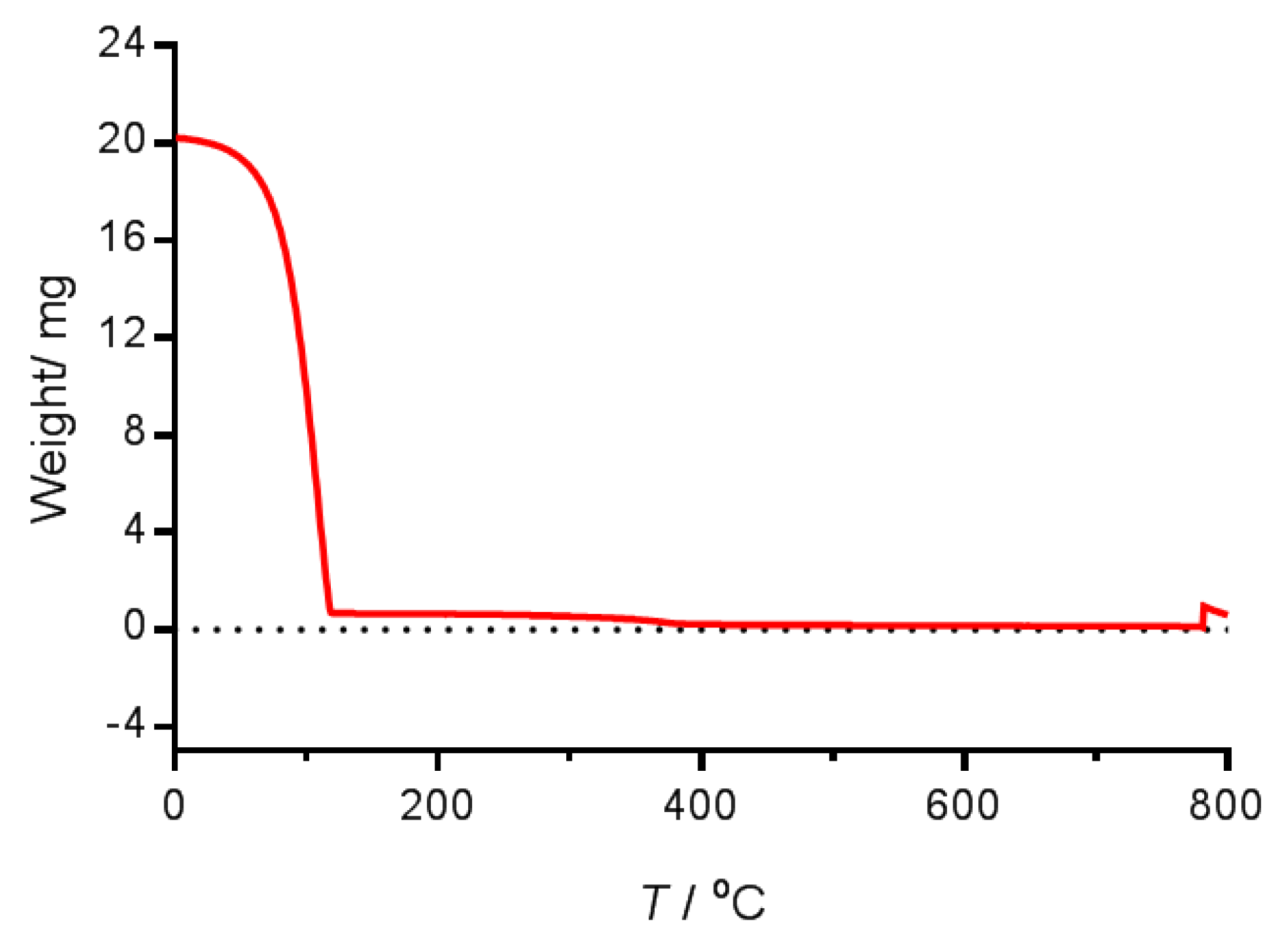
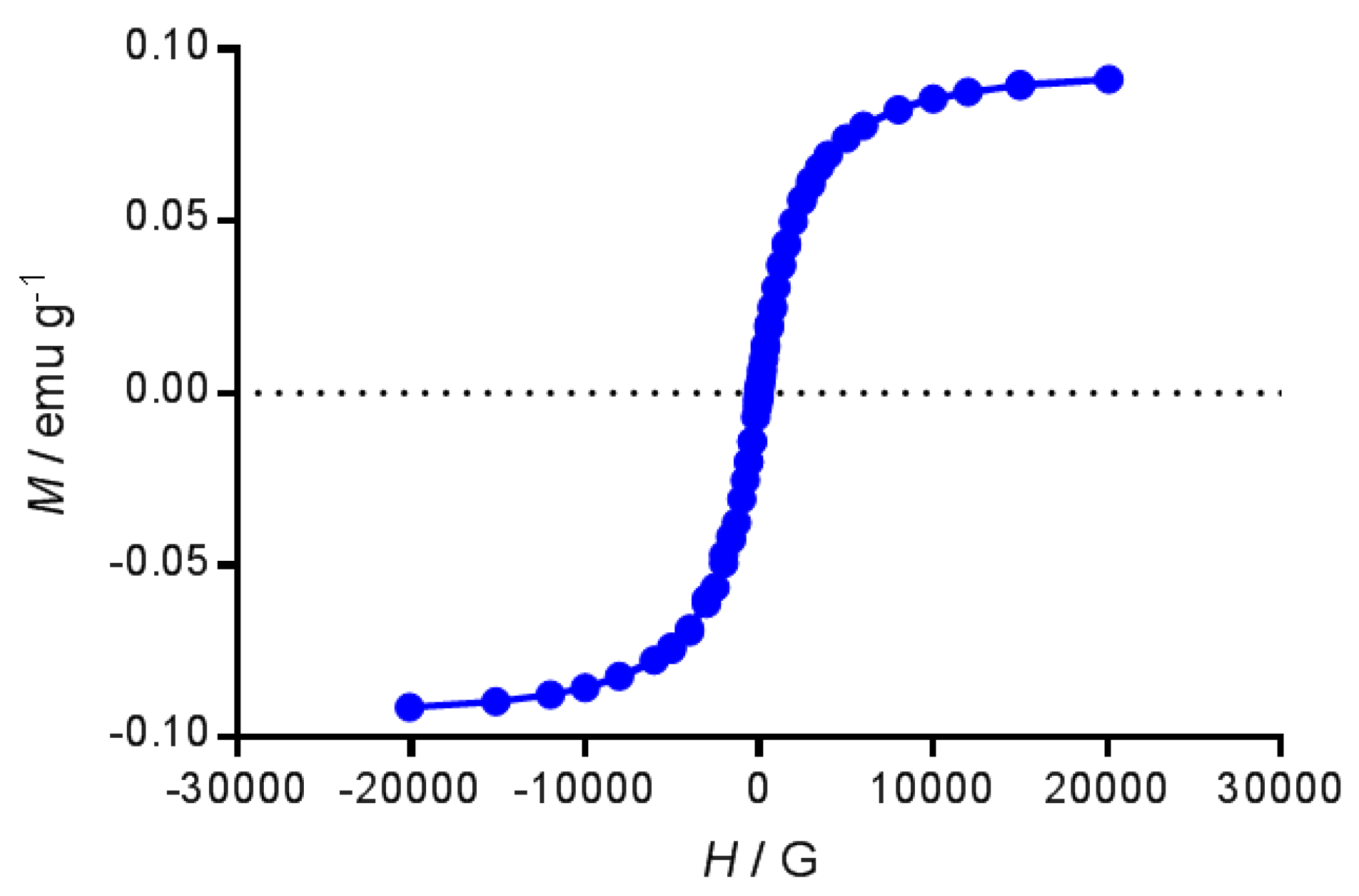
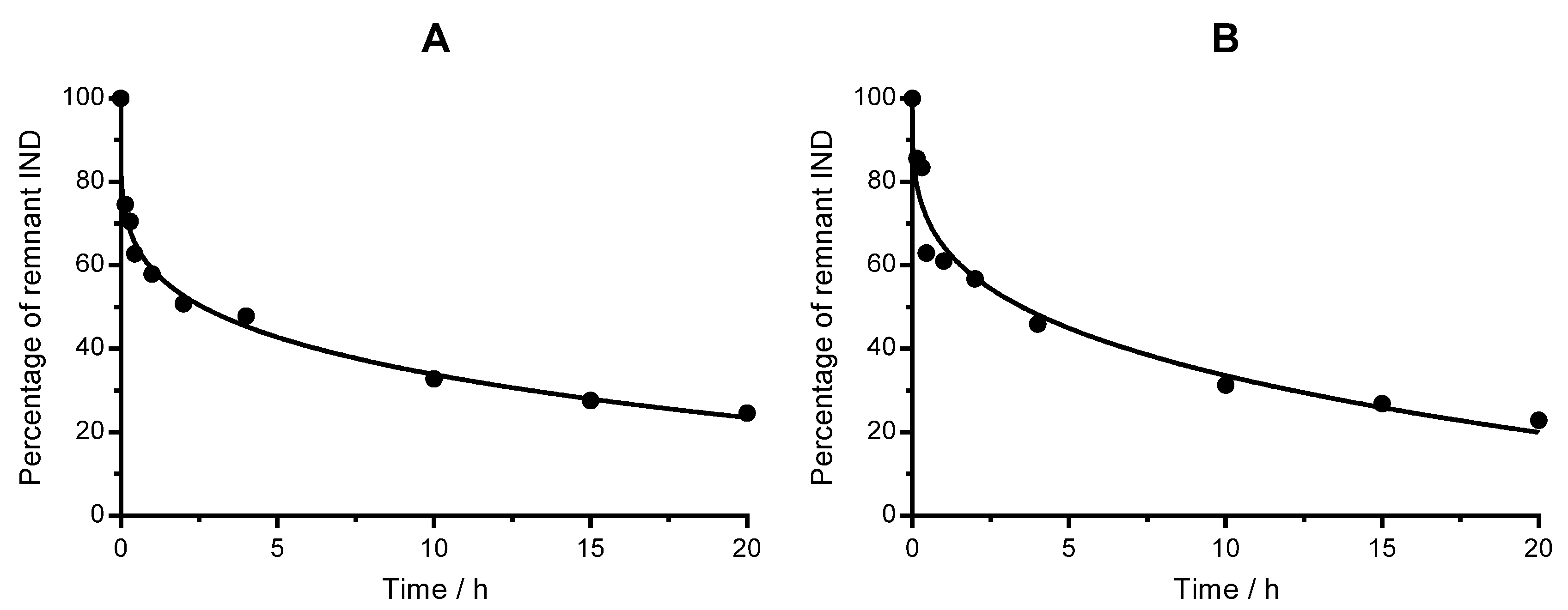


| Nanoemulsion | Lipid/mg mL−1 | Iron/mg mL−1 | IND/mg mL−1 |
|---|---|---|---|
| HE | 0.824 ± 0.054 | 0.250 ± 0.030 | 0.46 ± 0.07 |
| LE | 1.058 ± 0.040 | 0.210 ± 0.010 | 0.52 ± 0.08 |
| Nanoemulsion | Mathematical Model | R2 | Equation |
|---|---|---|---|
| HE | Zero order First order Higuchi Hixson-Crowell Korsmeyer-Peppas | 0.5827 0.8078 0.7996 0.7384 0.9918 | Zero order First order Higuchi Hixson-Crowell Korsmeyer-Peppas |
| LE | Zero order First order Higuchi Hixson-Crowell Korsmeyer-Peppas | 0.6219 0.8271 0.8340 0.7630 0.9458 |
| Nanoemulsion | Parameters | Values |
|---|---|---|
| HE | kKP/min−n | 41 ± 3 |
| n | 0.20 ± 0.02 | |
| LE | kKP/min−n | 37 ± 7.1 |
| n | 0.25 ± 0.05 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Burneo, N.; Busquets, M.A.; Estelrich, J. Magnetic Nanoemulsions: Comparison between Nanoemulsions Formed by Ultrasonication and by Spontaneous Emulsification. Nanomaterials 2017, 7, 190. https://doi.org/10.3390/nano7070190
Rodríguez-Burneo N, Busquets MA, Estelrich J. Magnetic Nanoemulsions: Comparison between Nanoemulsions Formed by Ultrasonication and by Spontaneous Emulsification. Nanomaterials. 2017; 7(7):190. https://doi.org/10.3390/nano7070190
Chicago/Turabian StyleRodríguez-Burneo, Nathalia, Maria Antònia Busquets, and Joan Estelrich. 2017. "Magnetic Nanoemulsions: Comparison between Nanoemulsions Formed by Ultrasonication and by Spontaneous Emulsification" Nanomaterials 7, no. 7: 190. https://doi.org/10.3390/nano7070190





