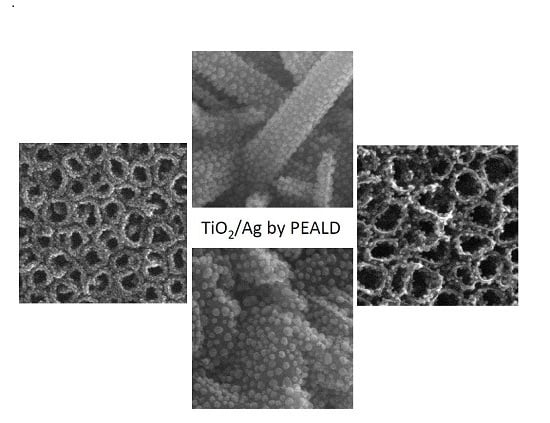
Optimization of the Silver Nanoparticles PEALD Process on the Surface of 1-D Titania Coatings
Abstract
:1. Introduction
2. Results
2.1. The Production of Titania 1-D Coatings (TNT and TNN) and Their Characterization
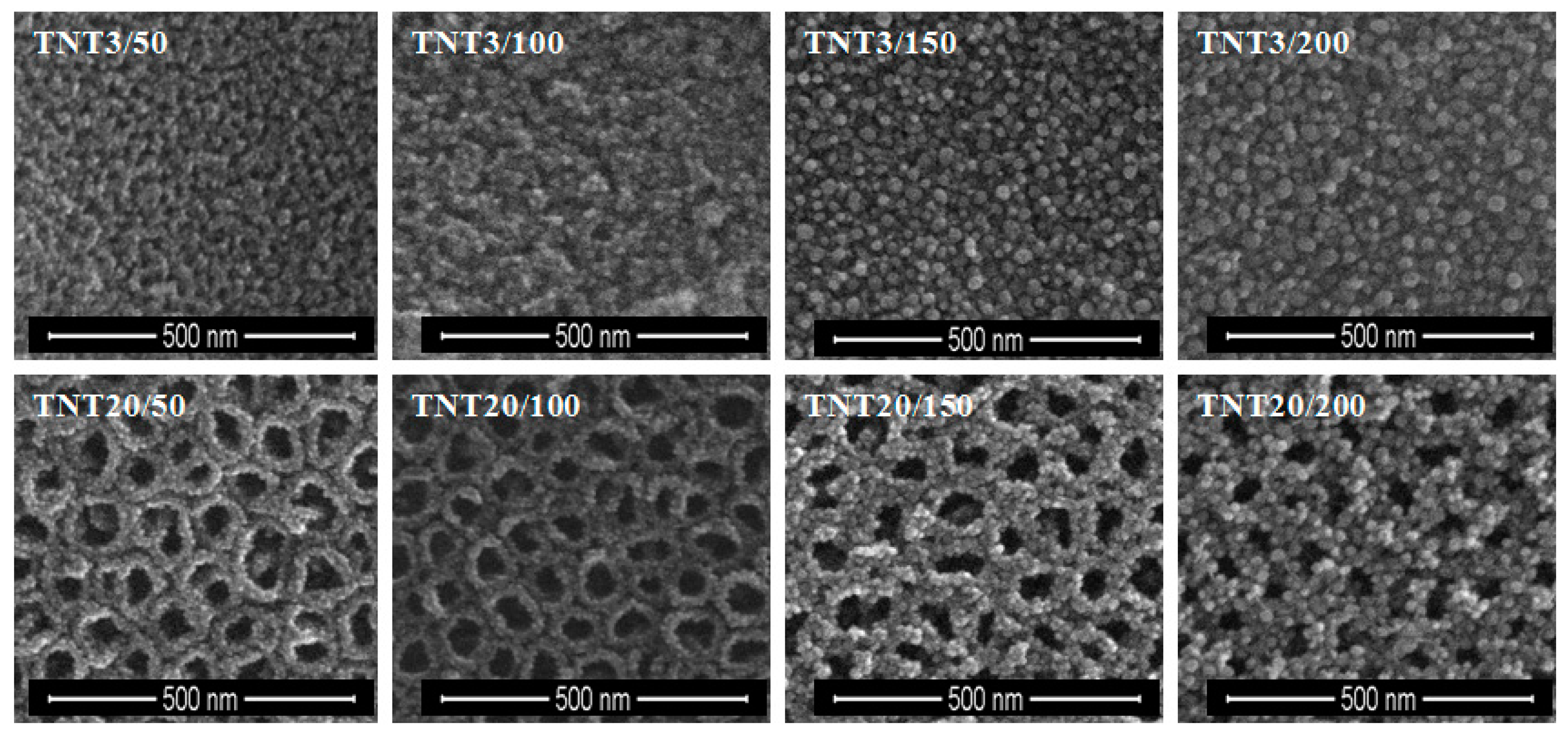
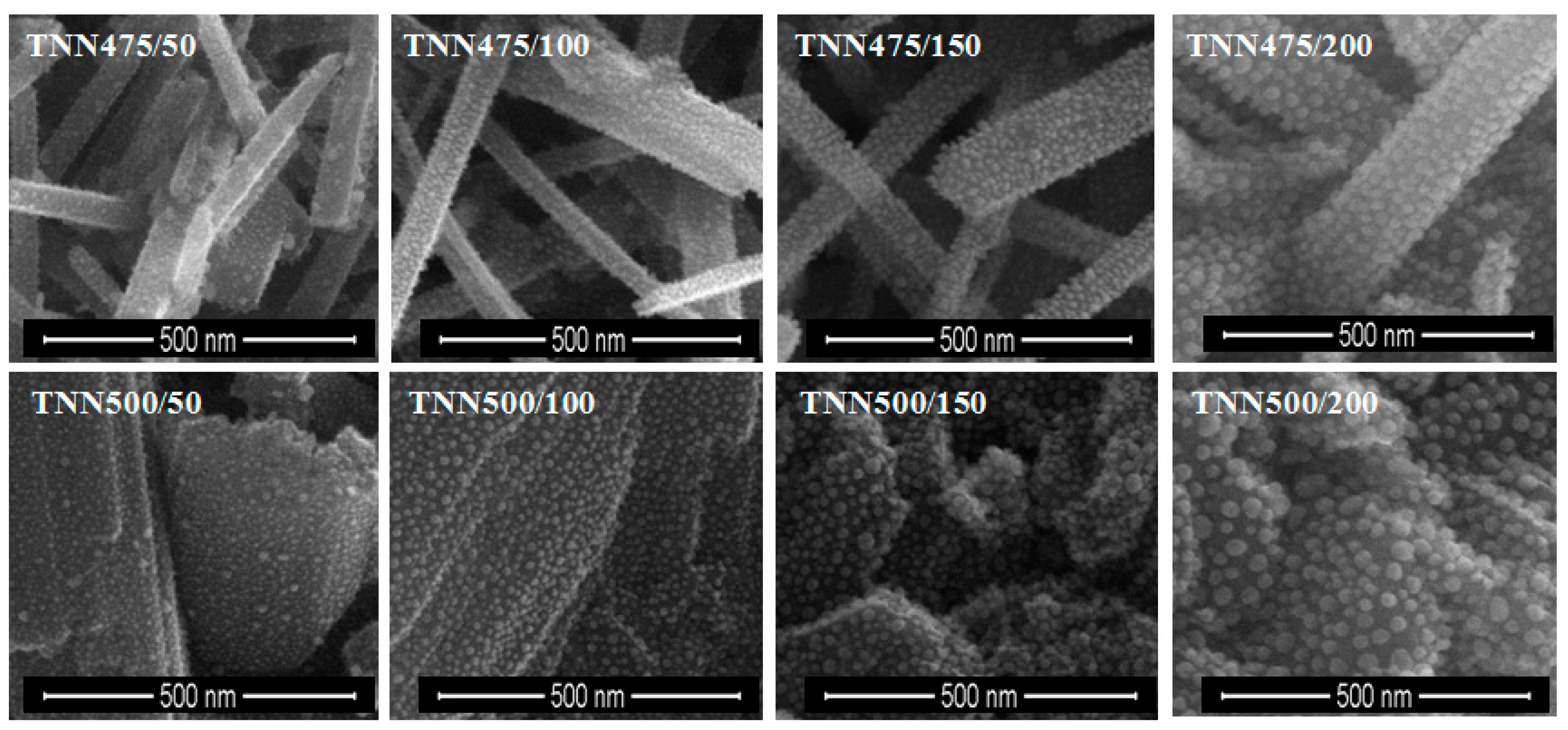
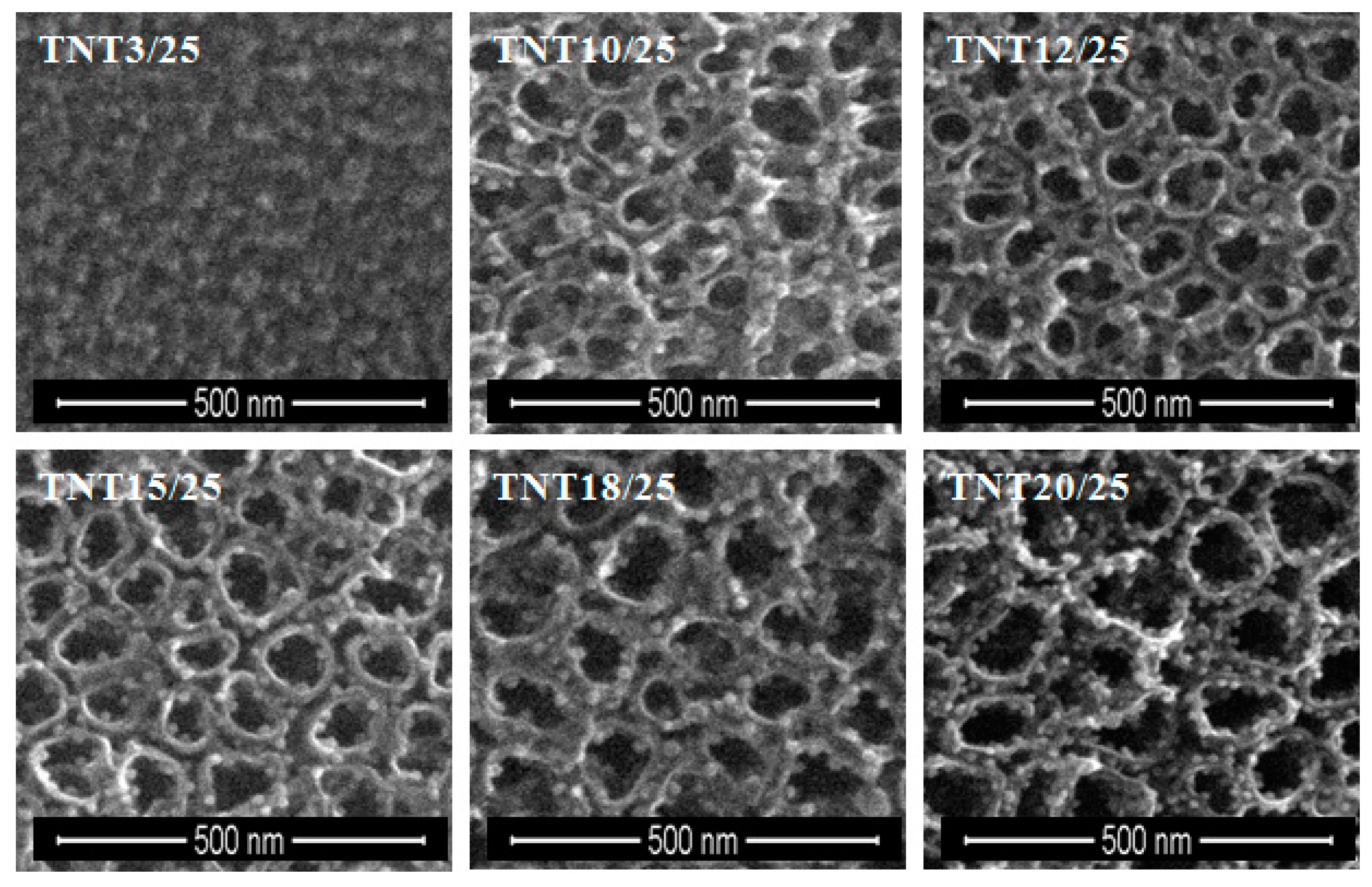
2.2. The Deposition of Silver Particles on the Surface of Titania 1-D Coatings (TNT and TNN) by PEALD and the Characterization of Systems: TNT/Ag and TNN/Ag
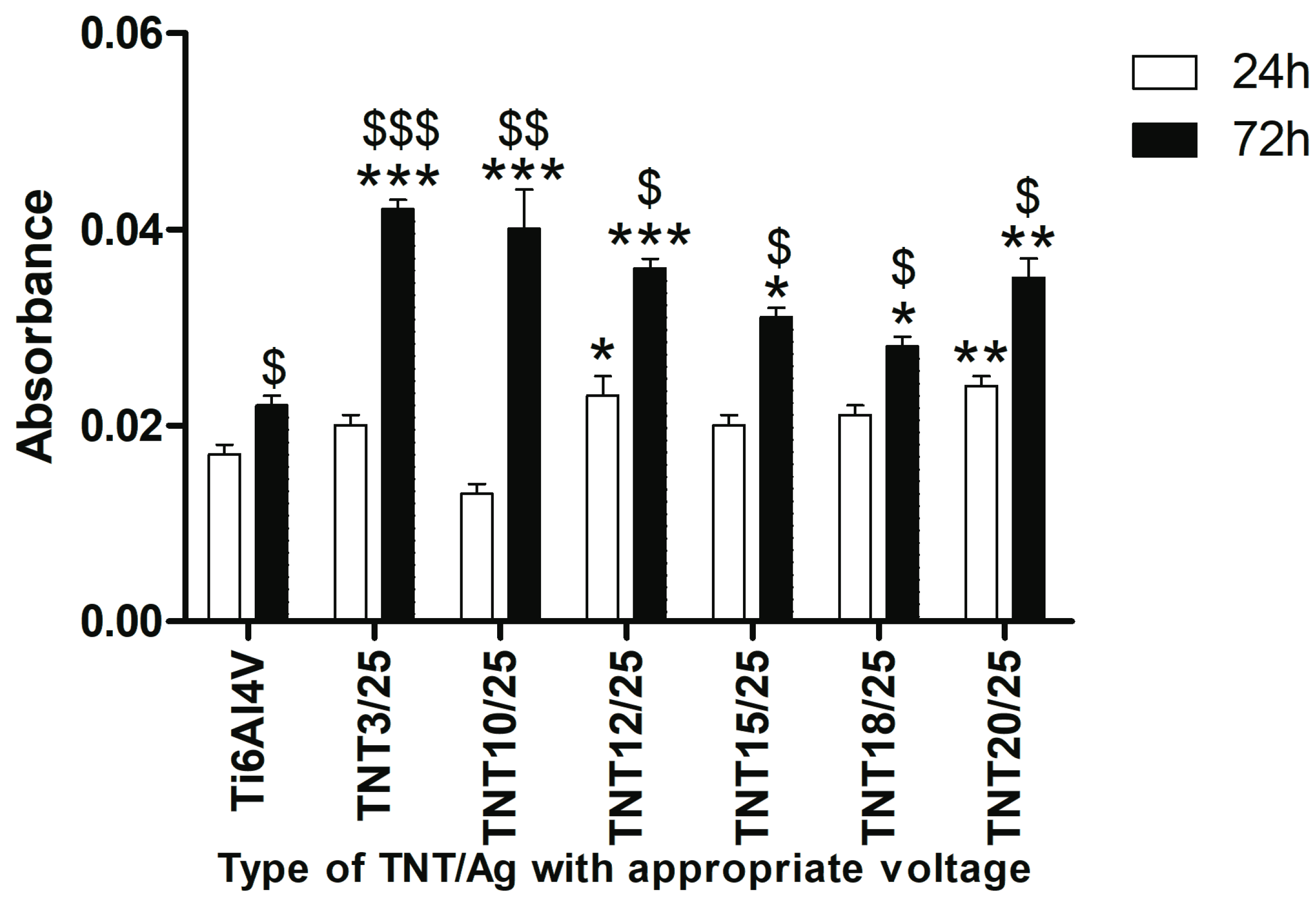
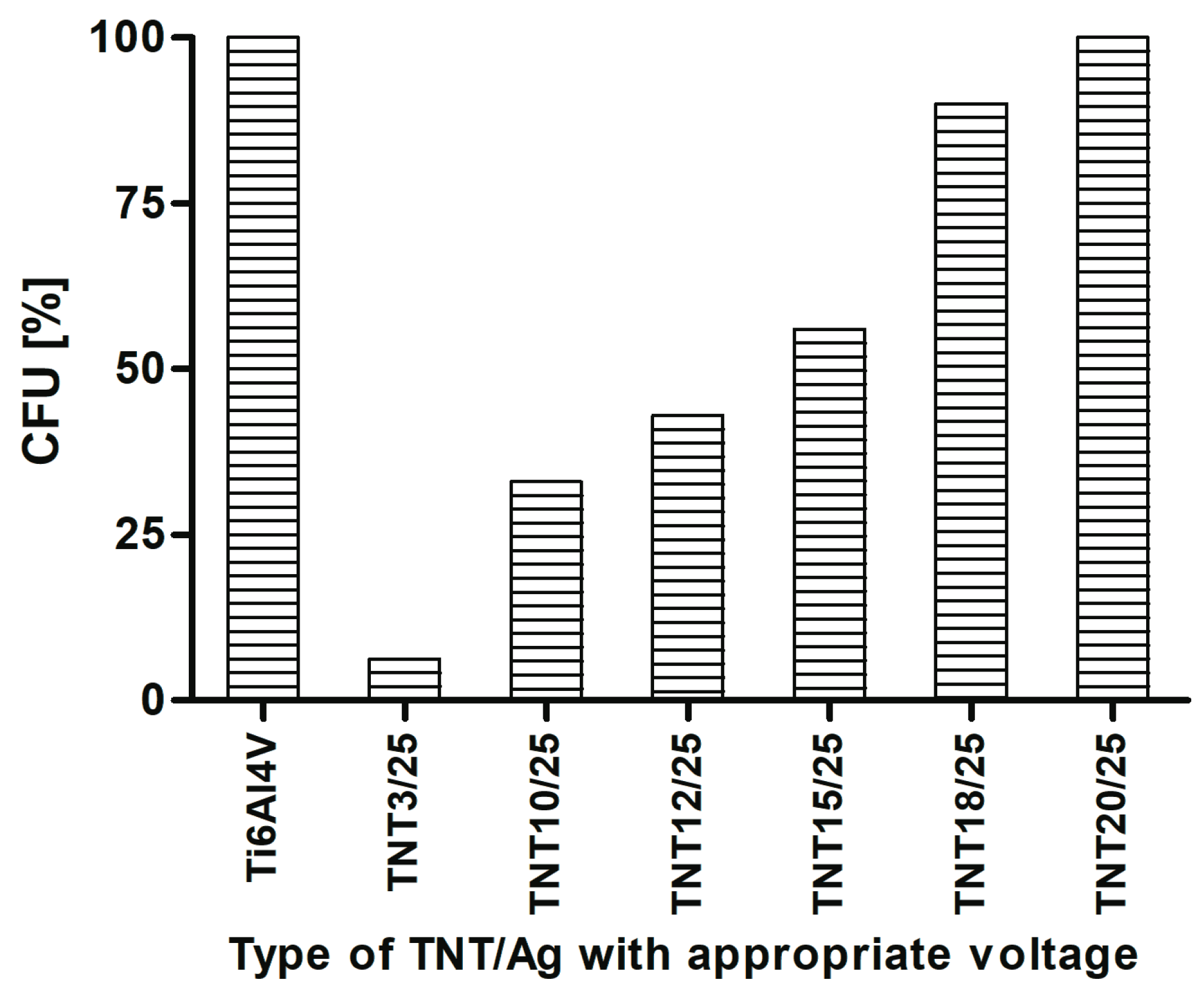
2.3. Fibroblast Adhesion and Proliferation on TNT/Ag Detected by MTT ((3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide) Assay
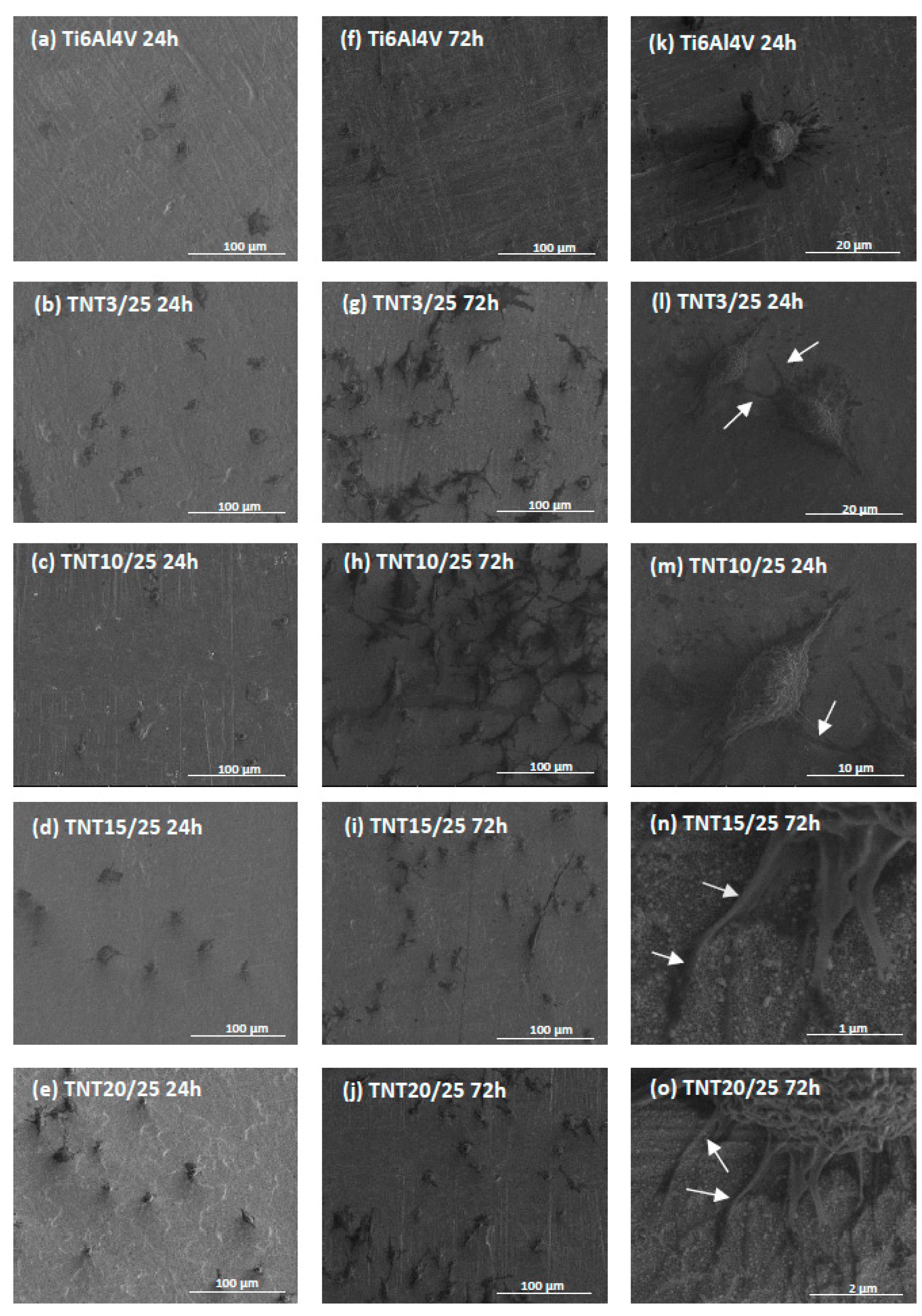
2.4. Cell Morphology, Adhesion and Proliferation Observed by Scanning Electron Microscopy
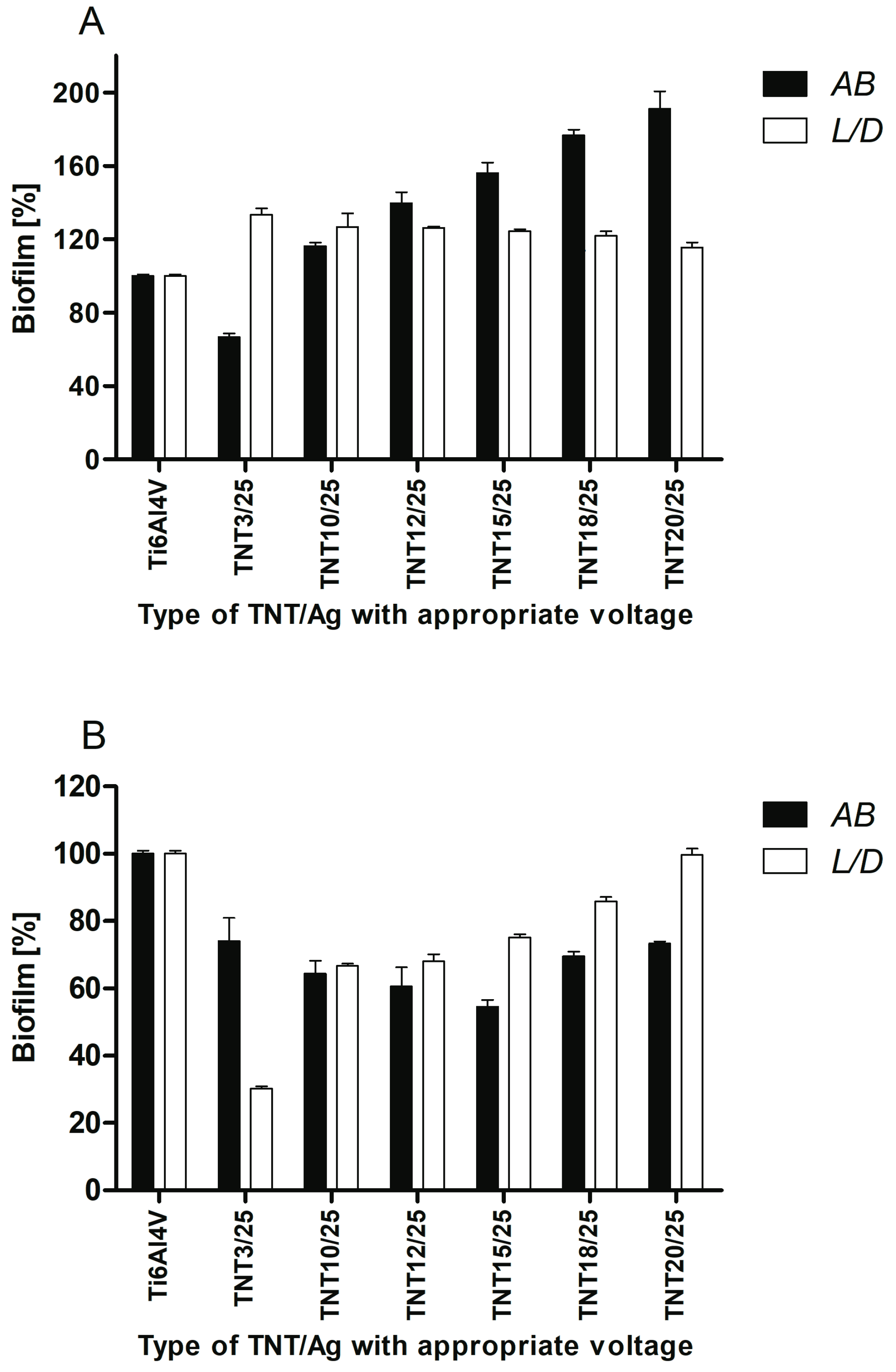
2.5. Antimicrobial Properties Studies on the Base of S. aureus Biofilm Assessment on Titanium Foil
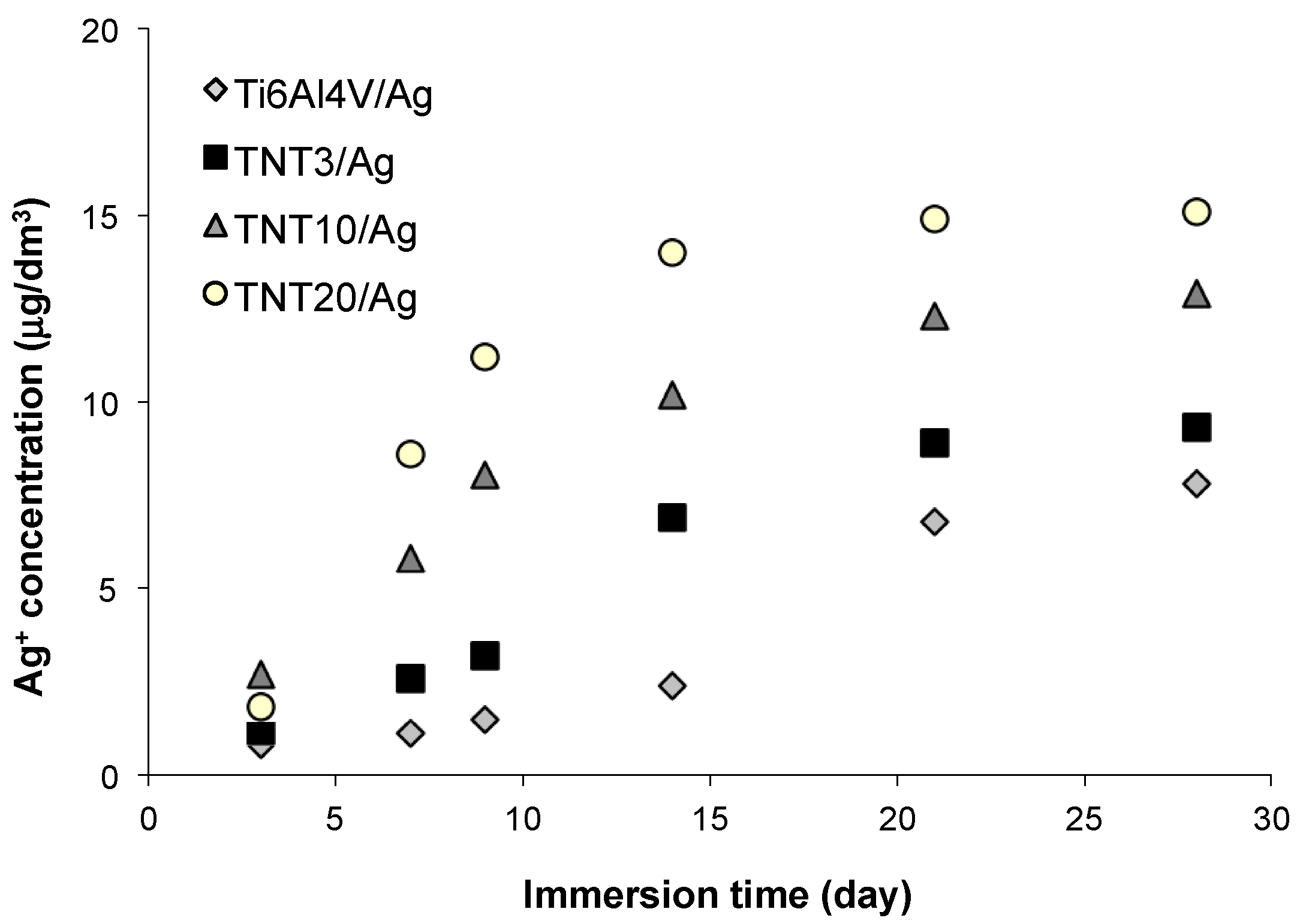
2.6. Silver Ions Releasing from TNT/Ag Nanocomposites
3. Discussion
4. Materials and Methods
4.1. Ti6Al4V Substrates
4.2. Electrochemical Oxidation of Ti6Al4V
4.3. Thermal Oxidation of Ti6Al4V
4.4. Characterization of Titania Coatings
4.5. PEALD of Silver Nanoparticles
4.6. Characterization of TiO2/Ag Coatings
4.7. Cell Adhesion and Proliferation Assay on TNT/Ag
4.8. Statistical Analysis
4.9. Bacterial Strains and Biofilm Formation on Titanium Biomaterials
4.10. The Assessment of S. aureus Biofilm on Titanium Foil
4.11. Statistical Analysis
4.12. Silver Ions Releasing
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Mehta, R.; Kumar, A.; Kumar, V.; Bhayana, G.; Wadhwa, S. Implant Surface Modification and Osseointegration-Past, Present and Future. J. Oral Health Community Dent. 2014, 8, 113–118. [Google Scholar]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface Treatments and Functional Coatings for Biocompatibility Improvement and Bacterial Adhesion Reduction in Dental Implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Sobieszczyk, S. Surface Modifications of Ti and its Alloys. Adv. Mater. Sci. 2010, 10, 29–42. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Tan, A.W.; Pingguan-Murphy, B.; Ahmad, R.; Akbar, S.A. Review of titania nanotubes: Fabrication and cellular response. Ceram. Int. 2012, 38, 4421–4435. [Google Scholar] [CrossRef]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; van der Heyde, H.; Jin, S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y. Fabrication of Titania Nanofibers by Electrospinning. Nano Lett. 2003, 3, 555–560. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, J.; Yin, L.; Liu, S.; Zhang, X.; Ao, Y.; Chen, H. Evaluation of the morphology and osteogenic potential of titania-based electrospun nanofibers. J. Nanomater. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Kiran, A.S.K.; Balu, R.; Kumar, T.S.S.J. Vero cell viability and human osteoblast cell response to electrospun phase controlled titania nanofibers. J. Biomater. Tissue Eng. 2012, 2, 292–298. [Google Scholar] [CrossRef]
- Venugopal, J.; Vadgama, P.; Kumar, T.S.S.; Ramakrishna, S. Biocomposite nanofibres and osteoblasts for bone tissue engineering. Nanotechnology 2007, 18, 55101–55109. [Google Scholar] [CrossRef]
- Park, Y.-J.; Song, H.-J.; Kim, I.; Yang, H.-S. Surface characteristics and bioactivity of oxide film on titanium metal formed by thermal oxidation. J. Mater. Sci. Mater. Med. 2007, 18, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dregia, S.; Akbar, S.; Alhoshan, M. Growth of 1-D TiO2 Nanowires on Ti and Ti alloys by Oxidation. J. Nanomater. 2010, 2010, 7. [Google Scholar] [CrossRef]
- Tavangar, A.; Tan, B.; Venkatakrishnan, K. Synthesis of Bio-Functionalized Three-Dimensional Titania Nanofibrous Structures Using Femtosecond Laser Ablation. Acta Biomater. 2011, 7, 2726–2732. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Pingguan-Murphy, B.; Osman, N.A.A. Progress of key strategies in development of electrospun scaffolds: Bone tissue. Sci. Technol. Adv. Mater. 2012, 13, 043002. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-Functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Christenson, E.M.; Anseth, K.S.; van den Beucken, L. Nanobiomaterial applications in orthopedics. J. Orthop. Res. 2007, 25, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ding, B. Applications of electrospun fibers. Recent Pat. Nanotechnol. 2008, 2, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 034002. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Suzuki, Y.; Yoshikawa, S. Syntheses of TiO2 (B) nanowires and TiO2 anatase nanowires by hydrothermal and post-heat treatments. J. Solid State Chem. 2008, 178, 2179–2185. [Google Scholar] [CrossRef]
- Azad, A.M.; Hershey, R.; Ali, S.; Goel, V. Bactericidal efficacy of electrospun pure and Fe-doped titania nanofibers. J. Mater. Res. 2010, 25, 1761–1770. [Google Scholar] [CrossRef]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T. Diameter of titanium nanotubes influences anti-bacterial efficacy. J. Nanotechnol. 2011, 22, 295102. [Google Scholar] [CrossRef] [PubMed]
- Ercan, B.; Kummer, K.M.; Tarquinio, K.M.; Webster, T.J. Decreased Staphylococcus aureus biofilm growth on anodized nanotubular titanium and the effect of electrical stimulation. Acta Biomater. 2011, 7, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Popat, K.C.; Eltgroth, M.; Latempa, T.J.; Grimes, C.A.; Desai, T.A. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials 2007, 28, 4880–4888. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, A.F.; Miller, C.; Liu, H. Anodic growth and biomedical applications of TiO2 nanotubes. J. Biomed. Nanotechnol. 2014, 10, 2977–3003. [Google Scholar] [CrossRef] [PubMed]
- Puckett, D.S.; Tayler, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Piszczek, P.; Topolski, A.; Lewandowska, Ż.; Talik, E.; Andersen, I.H.; Nielsen, L.P.; Heikkilä, M.; Leskelä, M. The structure and the photocatalytic activity of titania based nanotube and nanofiber coatings. Appl. Surf. Sci. 2016, 368, 165–172. [Google Scholar] [CrossRef]
- Davies, R.L.; Etris, S.F. The development and functions of silver in water purification and disease control. Catal. Today 1997, 36, 107–114. [Google Scholar] [CrossRef]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiminezhad, A.; Raee, M.J.; Manafi, Z.; Jahromi, A.S.; Ghasemi, Y. Ancient and Novel Forms of Silver in Medicine and Biomedicine. J. Adv. Med. Sci. Appl. Technol. 2016, 2, 122–128. [Google Scholar] [CrossRef]
- Martinez-Castanon, G.A.; Nino-Martinez, N.; Martinez-Gutierrez, F.; Martinez-Mendoza, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 83, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Rehan, M.; Rahier, H.; Bechelany, M.; Assche, G.V. Seed-mediated hot-injection synthesis of tiny Ag nanocrystals on nanoscale solid supports and reaction mechanism. ACS Appl. Mater. Interfaces 2016, 8, 10551–10561. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhu, H.; Peng, B.; Chen, H.; Sun, Y.; Gang, X.; Jin, P.; Wang, J. Synthesis of PS/Ag nanocomposite spheres with catalytic and antibacterial activities. ACS Appl. Mater. Interfaces 2012, 4, 5625–5632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic Potential of Silver Nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, C.; Gao, Q.; Li, Y.; Zhang, L. Fabrication of silver-incorporated TiO2 nanotubes and evaluation on its antibacterial activity. Mater. Lett. 2014, 137, 464–467. [Google Scholar] [CrossRef]
- Nagarajan, S.; Soussan, L.; Bechelany, M.; Teyssier, C.; Cavailles, V.; Pochat-Bohatier, C.; Miele, P.; Kalkura, N.; Janot, J.M.; Balme, S. Novel biocompatible electrospun gelatin fiber mats with antibiotic drug delivery properties. J. Mater. Chem. B 2016, 4, 1134–1141. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Films 2002, 409, 138–146. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Zaera, F. The Surface Chemistry of Atomic Layer Depositions of Solid Thin Films. J. Phys. Chem. Lett. 2012, 3, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Elam, J.W.; Zinovev, A.; Pellin, M.J.; Comstock, D.J.; Hersam, M.C. Nucleation and growth of noble metals on oxide surfaces using atomic layer deposition. ECS Trans. 2007, 3, 271–278. [Google Scholar]
- Kariniemi, M.; Niinisto, J.; Hatanpää, T.; Kemell, M.; Sajavaara, T.; Ritala, M.; Leskelä, M. Plasma-enhanced atomic layer deposition of silver thin films. Chem. Mater. 2011, 23, 2901–2907. [Google Scholar] [CrossRef]
- Lewandowska, Ż.; Piszczek, P.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B. The evaluation of the impact of titania nanotube covers morphology and crystal phase on their biological properties. J. Mater. Sci. Mater. Med. 2015, 26, 163. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Topolski, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Piszczek, P. Bioactivity studies on titania coatings and the estimation of their usefulness in the modification of implant surfaces. Nanomaterials 2017, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A. 1D-titania nanoarchitecture as bioactive and photoactive coatings for modern implants—A review. TiO2 2017. accepted. [Google Scholar]
- Lai, Y.; Sun, L.; Chen, Y.; Zhuang, H.; Lin, C.; Chin, J.W. Effects of the structure of TiO2 nanotube array on Ti substrate on its photocatalytic activity. J. Electrochem. Soc. 2006, 153, D123–D127. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Capping antibacterial Ag nanorods aligned on Ti interlayer by mesoporous TiO2 layer. Surf. Coat. Technol. 2009, 203, 3123–3128. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Self-accumulated Ag nanoparticles on mesoporous TiO2 thin film with high bactericidal activities. Surf. Coat. Technol. 2010, 204, 3676–3683. [Google Scholar] [CrossRef]
- Ly, X.; Gao, F.; Yang, Y.; Wang, T. A Facile Electrochemical Approach to Form TiO2/Ag Heterostructure Films with Enhanced Photocatalytic Activity. Ind. Eng. Chem. Res. 2016, 55, 107–115. [Google Scholar]
- Plodinec, M.; Gajović, A.; Jakša, G.; Žagar, K.; Čeh, M. High-temperature hydrogenation of pure and silver-decorated titanate nanotubes to increase their solar absorbance for photocatalytic applications. J. Alloys Compd. 2014, 591, 147–155. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Tan, X.; Fan, Q.; Wang, X.; Grambow, B. Eu(III) sorption to TiO2 (anatase and rutile): Batch, XPS, and EXAFS studies. Environ. Sci. Technol. 2009, 43, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Praveen Kumar, D.; Lakshmana Reddy, N.; Karthic, M.; Neppolian, B.; Madhavan, J.; Shankar, M.V. Solar light sensitized p-Ag2O/n-TiO2 nanotubes heterojunction photocatalysts for enhanced hydrogen production in aqueous-glycerol solution. Sol. Energy Mater. Sol. Cells 2016, 154, 78–87. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, V.; Alfara, S.O.; Torres-Martinez, L.M.; Lee, S.-W. Silver-TiO2 nanocomposites: Synthesis and harmful algae bloom UV-photoelimination. Appl. Catal. B Environ. 2010, 98, 229–234. [Google Scholar] [CrossRef]
- Mäkelä, M.; Hatanpää, T.; Mizohata, K.; Meinander, K.; Niinistö, J.; Räisänen, J.; Ritala, M.; Leskelä, M. Studies on thermal atomic layer deposition of silver thin films. Chem. Mater. 2017, 29, 2040–2045. [Google Scholar] [CrossRef]
- Mackus, A.J.M.; Verheijen, M.A.; Leick, N.; Bol, A.A.; Kessel, W.M.M. Influence of Oxygen Exposure on the Nucleation of Platinum Atomic Layer Deposition: Consequences for Film Growth, Nanopatterning, and Nanoparticle Synthesis. Chem. Mater. 2013, 25, 1905–1911. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, K.B.; Jeon, H.S.; Park, H.K. Effects of surface nano-topography on human osteoblast filopodia. Anal. Sci. 2011, 27, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Riehle, M.O.; Johnstone, H.; Affrossman, S.; Curtis, A.S. Investigating the limits of filopodial sensing: A brief report using SEM to image the interaction between 10 nm high nano-topography and fibroblast filopodia. Cell Biol. Int. 2004, 28, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhu, Z.; Liu, J.; Hu, W.; Li, C.M. ZnO nanorod-templated well-aligned ZrO2 nanotube arrays for fibroblast adhesion and proliferation. Nanotechnology 2014, 25, 215102. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrata with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.-Y.; Liu, C.-P.; Huang, H.-H.; Lee, S.-W. Both Enhanced Biocompatibility and Antibacterial Activity in Ag-Decorated TiO2 Nanotubes. PLoS ONE 2013, 8, e75364. [Google Scholar] [CrossRef] [PubMed]
- Takagi, J.; Petre, B.M.; Walz, T.; Springer, T.A. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 2002, 110, 599–611. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewczyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Sadekuzzaman, M.; Yang, S.; Mizan, M.F.R.; Ha, S.D. Current and recent advanced strategies for combating biofilms. Comp. Rev. Food Sci. Food Saf. 2015, 14, 491–509. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Raman, S.; He, F.; Huang, Y. Surface modification of medical metals by ion implantation of silver and copper. Vaccum 2007, 81, 1114–1118. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
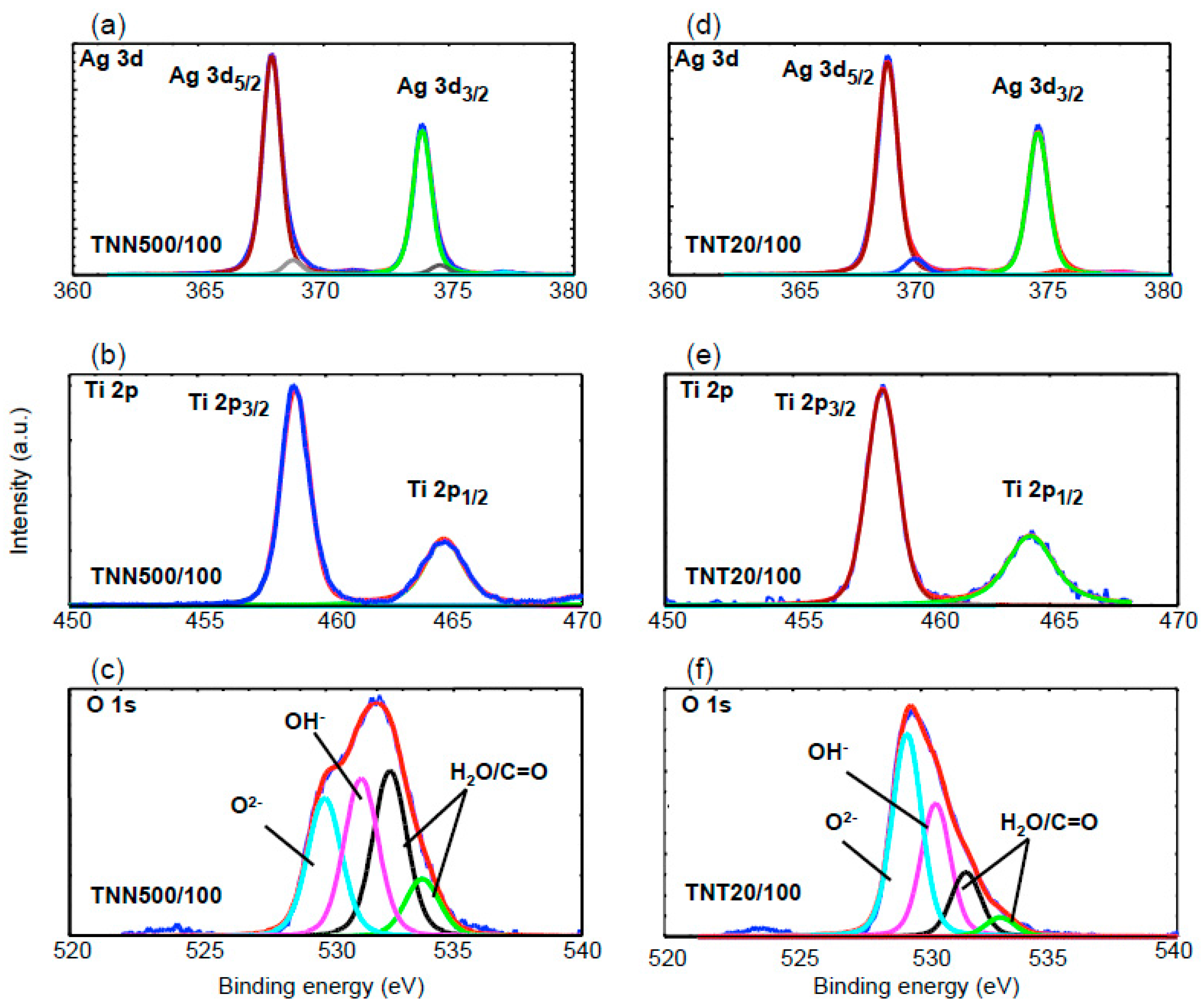

| Ti4+ | O2− | OH− | H2O | C=O | ||
| TiO2 Samples | Ti(2p3/2) (eV) | Ti(2p1/2) (eV) | O(1s) (eV) | O(1s) (eV) | O(1s) (eV) | O(1s) (eV) |
| TNT3/50 | 458.7 | 464.4 | 529.9 (47%) | 531.0 (32%) | 532.2 (17%) | 533.5 (4%) |
| TNT3/100 | 458.5 | 464.2 | 529.9 (32%) | 531.0 (36%) | 532.0 (24%) | 533.2 (8%) |
| TNT6/50 | 458.7 | 464.4 | 529.9 (43%) | 531.1 (21%) | 532.2 (29%) | 533.7 (7%) |
| TNT6/100 | 458.5 | 464.2 | 530.0 (45%) | 531.1 (31%) | 532.3 (16%) | 533.4 (7%) |
| TNT15/50 | 458.2 | 463.9 | 529.9 (66%) | 531.2 (20%) | 532.2 (10%) | 533.3 (4%) |
| TNT15/100 | 458.5 | 464.2 | 529.9 (51%) | 531.0 (22%) | 532.1 (21%) | 533.4 (6%) |
| TNN500/100 | 458.4 | 464.2 | 529.9 (27%) | 531.4 (30%) | 532.5 (32%) | 533.8 (11%) |
| TNN475/100 | 458.0 | 464.8 | 529.5 (48%) | 530.5 (32%) | 532.5 (15%) | 533.2 (3%) |
| Ag | Ag+ | Ag | ||||
| TiO2 Samples | Ag(3d5/2) (eV) | Ag(3d3/2) (eV) | Ag(3d5/2) (eV) | Ag(3d3/2) (eV) | Ag(3d5/2) (eV) | Ag(3d3/2) (eV) |
| TNT3/50 | 368.7 (96%) | 374.6 (96%) | - | - | 369.8 (4%) | 375.4 (4%) |
| TNT3/100 | 368.4 (96%) | 374.4 (96%) | - | - | 369.4 (4%) | 375.2 (4%) |
| TNT6/50 | 368.8 (100%) | 374.8 (100%) | - | - | - | - |
| TNT6/100 | 368.5 (82%) | 374.5 (82%) | 367.8 (14%) | 373.9 (14%) | 369.4 (4%) | 375.3 (4%) |
| TNT15/50 | 368.2 (97%) | 374.2 (97%) | - | - | 369.2 (3%) | 375.1 (3%) |
| TNT15/100 | 368.4 (94%) | 374.4 (94%) | - | - | 369.3 (6%) | 375.1 (6%) |
| TNN500/100 | 368.9 (6%) | 374.6 (6%) | 367.9 (94%) | 373.9 (94%) | - | - |
| TNN475/100 | 368.8 (97%) | 374.8 (97%) | - | - | 369.8 (3%) | 375.6 (3%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Talik, E.; Mäkelä, M.; Leskelä, M.; Piszczek, P. Optimization of the Silver Nanoparticles PEALD Process on the Surface of 1-D Titania Coatings. Nanomaterials 2017, 7, 193. https://doi.org/10.3390/nano7070193
Radtke A, Jędrzejewski T, Kozak W, Sadowska B, Więckowska-Szakiel M, Talik E, Mäkelä M, Leskelä M, Piszczek P. Optimization of the Silver Nanoparticles PEALD Process on the Surface of 1-D Titania Coatings. Nanomaterials. 2017; 7(7):193. https://doi.org/10.3390/nano7070193
Chicago/Turabian StyleRadtke, Aleksandra, Tomasz Jędrzejewski, Wiesław Kozak, Beata Sadowska, Marzena Więckowska-Szakiel, Ewa Talik, Maarit Mäkelä, Markku Leskelä, and Piotr Piszczek. 2017. "Optimization of the Silver Nanoparticles PEALD Process on the Surface of 1-D Titania Coatings" Nanomaterials 7, no. 7: 193. https://doi.org/10.3390/nano7070193