Development of Poly(lactic acid)/Chitosan Fibers Loaded with Essential Oil for Antimicrobial Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of CEO-Loaded CS Particles
2.2. Morphology of PLA/CS-CEO Fiber Mats
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Surface Hydrophilicity of PLA/CS-CEO Fibers
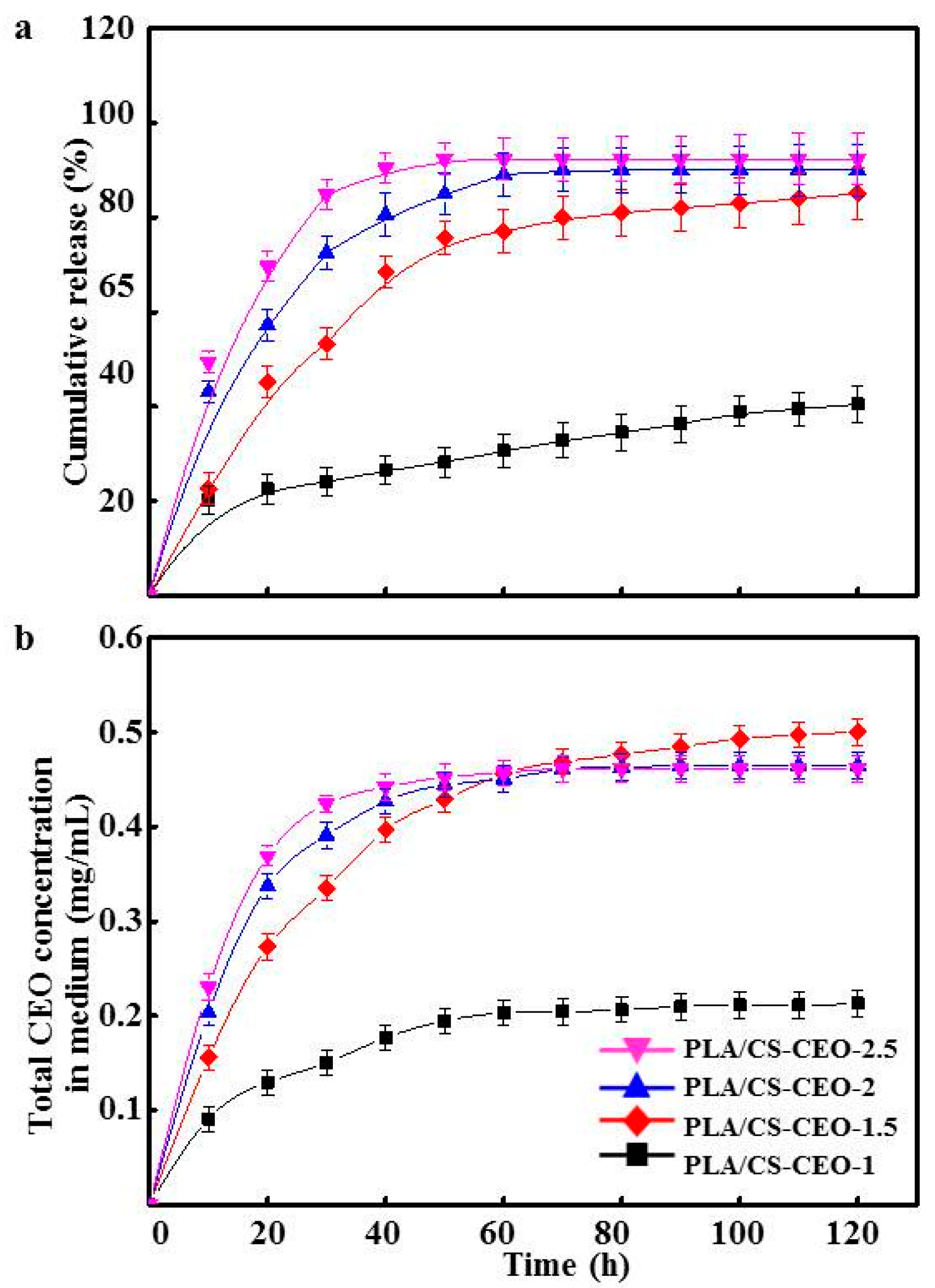
2.5. Release Characteristics of PLA/CS-CEO Fiber Mats
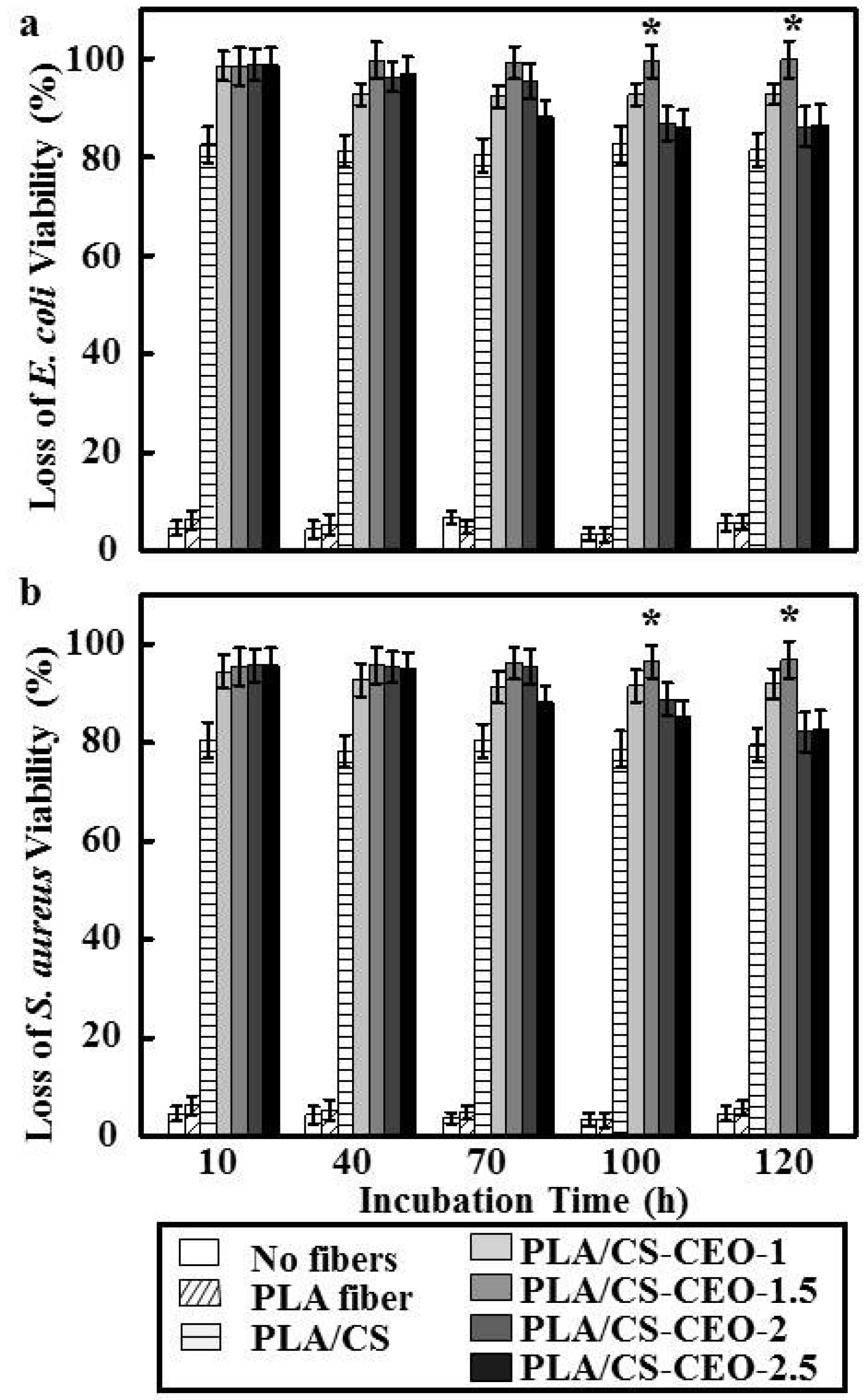
2.6. Antibacterial Activity of PLA/CS-CEO Fibers
3. Materials and Methods
3.1. Materials
3.2. Preparation of CS-CEO Nanoparticles
3.3. Characterization of CS-CEO Nanoparticles
3.4. Preparation of PLA/CS-CEO Fibers
3.5. Characterization of PLA/CS-CEO Fibers
3.6. Antibacterial Properties of PLA/CS-CEO Fibers
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, H.; Jung, J.; Zhao, Y. Preparation and characterization of cellulose nanocrystals films incorporated with essential oil loaded β-chitosan beads. Food Hydrocoll. 2017, 69, 164–172. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cran, M.J.; Bigger, S.W.; Hernández-Muñoz, P.; Gavara, R. Antioxidant and antimicrobial properties of ethylene vinyl alcohol copolymer films based on the release of oregano essential oil and green tea extract components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols a review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Ziani, K.; Fang, Y.; Mclements, D.J. Encapsulation of functional lipophilic components in surfactant-based colloidal delivery systems: Vitamin E, vitamin D, and lemon oil. Food Chem. 2012, 134, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, X.; Xiao, Z.; Bi, W. Effect of chitosan nanoparticles loaded with cinnamon essential oil on the quality of chilled pork. LWT Food Sci. Technol. 2015, 63, 519–526. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Gavlighi, H.A.; Gardini, F. Chitosan-cinnamon essential oil nano-formulation: Application as a novel additive for controlled release and shelf life extension of beef patties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT Food Sci. Technol. 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.E.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Corona-Rangel, M.L. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT Food Sci. Technol. 2017, 77, 15–20. [Google Scholar]
- Kashiri, M.; Maghsoudlo, Y.; Khomeiri, M. Incorporating Zataria multiflora Boiss. essential oil and sodium bentonite nano-clay open a new perspective to use zein films as bioactive packaging materials. Food Sci. Technol. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Schiffman, J.D. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly (ethylene oxide) nanofibers. Carbohydr. Polym. 2014, 113, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Zhu, D.H.; Feng, K.; Liu, F.J.; Lou, W.Y.; Li, N.; Zong, M.H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, H.; Soto-Valdez, H.; Auras, R. Poly(lactic acid) film incorporated with marigold flower extract (Tagetes erecta) intended for fatty-food application. Food Control 2014, 46, 55–66. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.H.; Wu, H.; Zong, M.H.; Jing, Y.R.; Han, S.Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Luque-Alcaraz, A.G.; Cortez-Rocha, M.O.; Zquez-Contreras, C.A.; Acosta-Silva, A.L.; Burgos-Hern, A.; Argüelles-Monal, W.M.; Plascencia-Jatomea, M. Enhanced antifungal effect of chitosan/pepper tree (Schinus molle) essential oil bionanocomposites on the viability of Aspergillus parasiticus spores. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- González, J.O.W.; Gutiérrez, M.M.; Ferrero, A.A.; Band, B.F. Essential oils nanoformulations for stored-product pest control-characterization and biological properties. Chemosphere 2014, 100, 130–138. [Google Scholar]
- Lozano, M.V.; Torrecilla, D.; Torres, D.; Vidal, A.; Domínguez, F.; Alonso, M.J. Highly efficient system to deliver taxanes into tumor cells: Docetaxel-loaded chitosan oligomer colloidal carriers. Biomacromolecules 2008, 9, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.M.D.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface application to cyclosporine. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Rojsitthisak, P.; Nimmannit, U. Preparation of turmeric oil-loaded chitosan-alginate biopolymeric nanocapsules. Mater. Sci. Eng. C Mater. 2009, 29, 856–860. [Google Scholar] [CrossRef]
- Sadeghi, A.M.; Dorkoosh, F.A.; Avadi, M.R.; Saadat, P.; Rafieetehrani, M.; Junginger, H.E. Preparation, characterization and antibacterial activities of chitosan, N-trimethyl chitosan (TMC) and N-diethylmethyl chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation methods. Int. J. Pharm. 2008, 355, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Anacristina, V.L.; Loongtak, L. Controlled release of allyl isothiocyanate using soy protein and poly(lactic acid) electrospun fibers. Food Res. Int. 2009, 42, 933–940. [Google Scholar]
- Wang, B.; Zheng, H.; Chang, M.W.; Ahmad, Z.; Li, J.S. Hollow polycaprolactone composite fibers for controlled magnetic responsive antifungal drug release. Colloids Surf. B 2016, 145, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.P.; George, T.S.; Guru, K.S.S. Extraction, purification and characterization of chitosan from endophytic fungi isolated from medicinal plants. World J. Sci. Technol. 2011, 1, 43–48. [Google Scholar]
- Lagaron, J.M.; Fernandez-Saiz, P.; Ocio, M.J. Using atr-ftir spectroscopy to design active antimicrobial food packaging structures based on high molecular weight chitosan polysaccharide. J. Agric. Food Chem. 2007, 55, 2554–2562. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Maderuelo, C.; Zarzuelo, A.; Lanao, J.M. Critical factor in the release of drugs from sustained release hydrophilic matrices. J. Control. Release 2011, 154, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Lv, J.; Ding, M.; Li, Y.; Wang, H.; Jiang, S. Release behavior of tetracycline hydrochloride loaded chitosan/poly (lactic acid) antimicrobial nanofibrous membranes. Mater. Sci. Eng. C Mater. 2016, 59, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Pătraşcu, L.; Cantaragiu, A.; Alexe, P.; Dima, S. The kinetics of the swelling process and the release mechanisms of Coriandrum sativum L. essential oil from chitosan/alginate/inulin microcapsules. Food Chem. 2015, 195, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic antimicrobial activities of natural essential oils with chitosan films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Valdramidis, V.P.; Donnell, C.P.O.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Mulla, M.Z.; Arfat, T.A. Thermo-mechanical, structural characterization and antibacterial performance of solvent casted polylactide/cinnamon oil composite films. Food Control 2016, 69, 196–204. [Google Scholar] [CrossRef]
- Deng, X.; Zhou, S.; Li, X.; Zhao, J.; Yuan, M. In vitro degradation and release profiles for poly-dl-lactide-poly(ethylene glycol) microspheres containing human serum albumin. J. Control. Release 2001, 71, 165–173. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B 2011, 84, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Parris, N.; Cooke, P.H.; Hicks, K.B. Encapsulation of essential oils in zein nanospherical particles. J. Agric. Food Chem. 2005, 53, 4788–4792. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of essential oil-loaded chitosan—Alginate nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef]

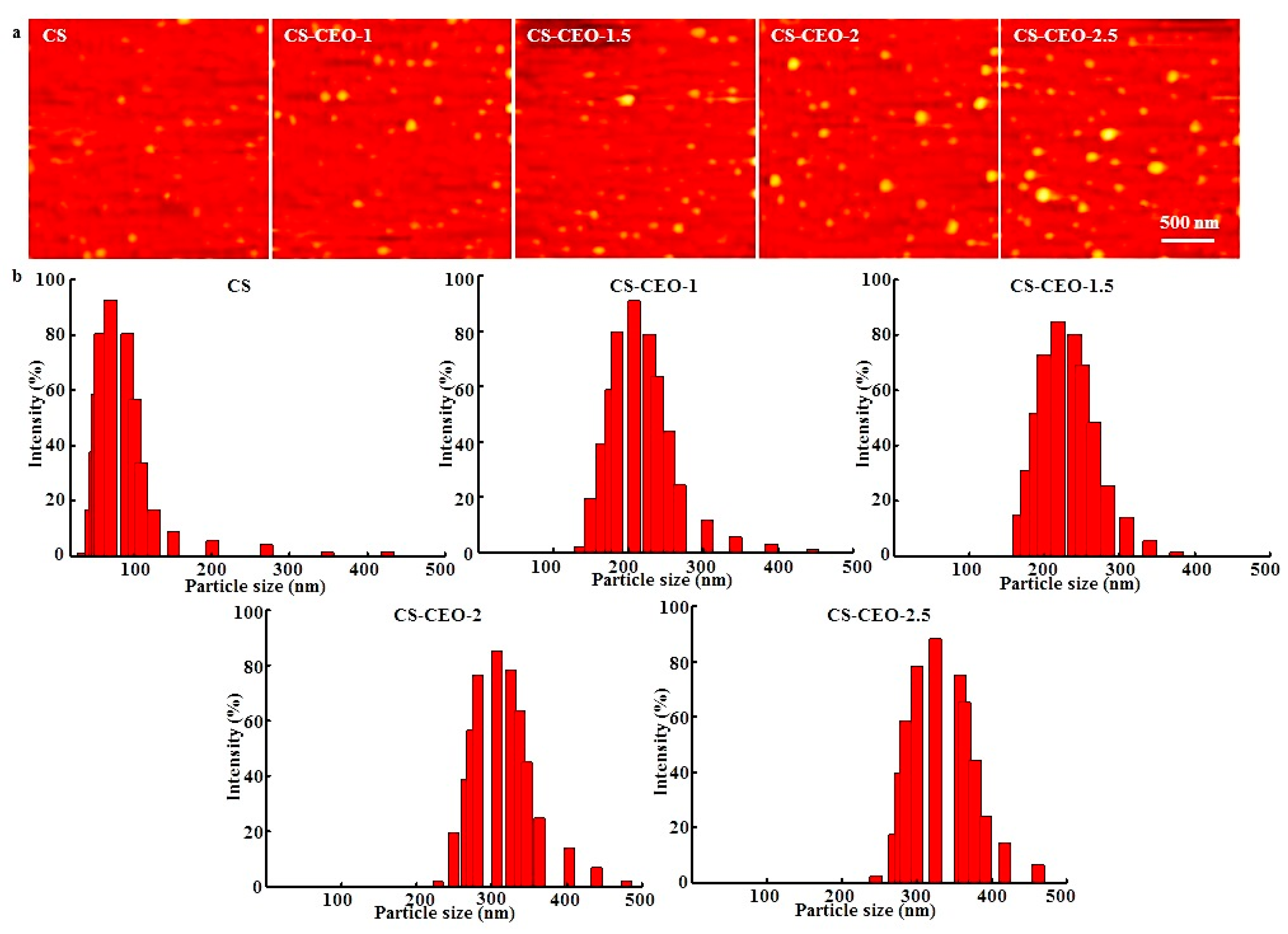
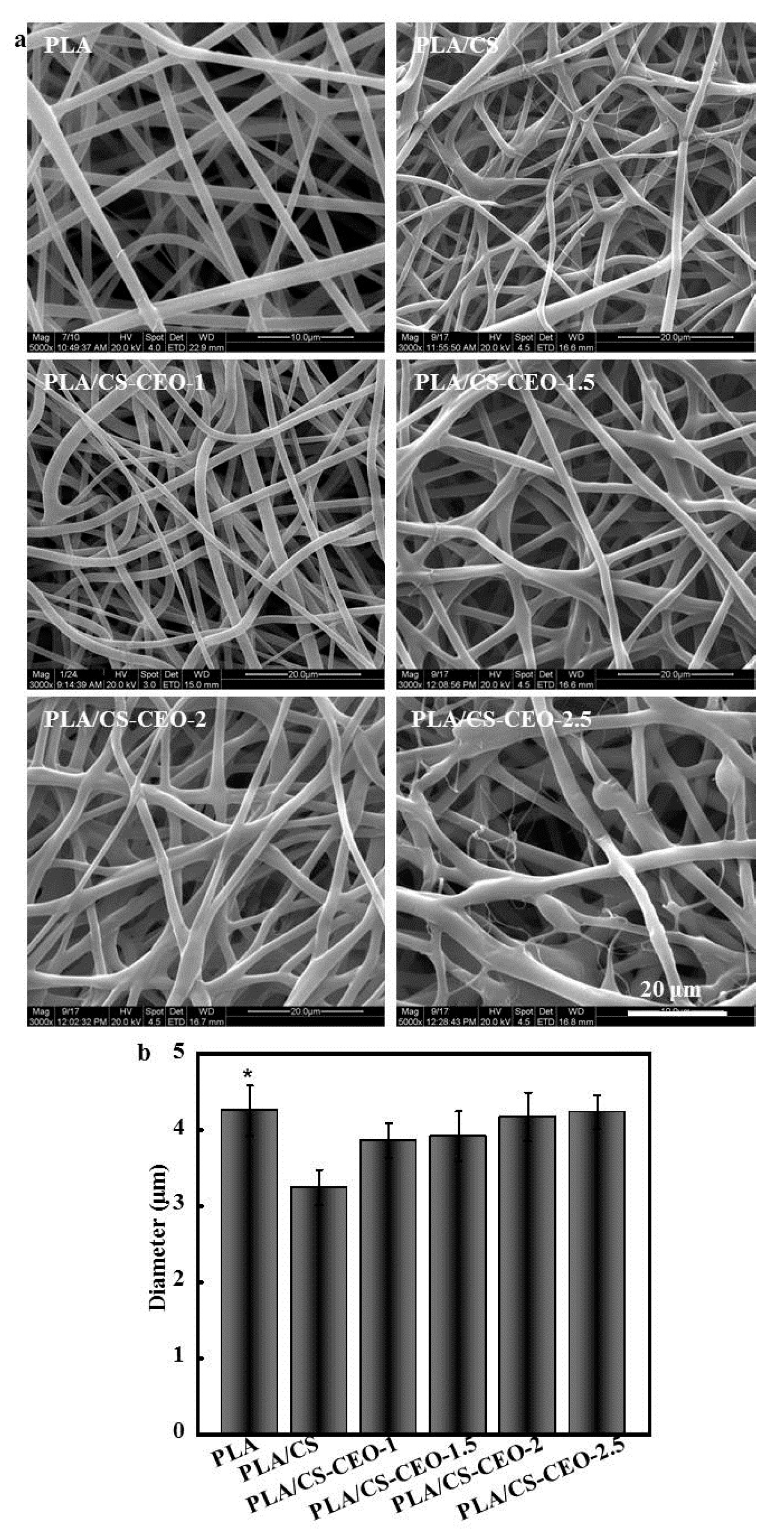
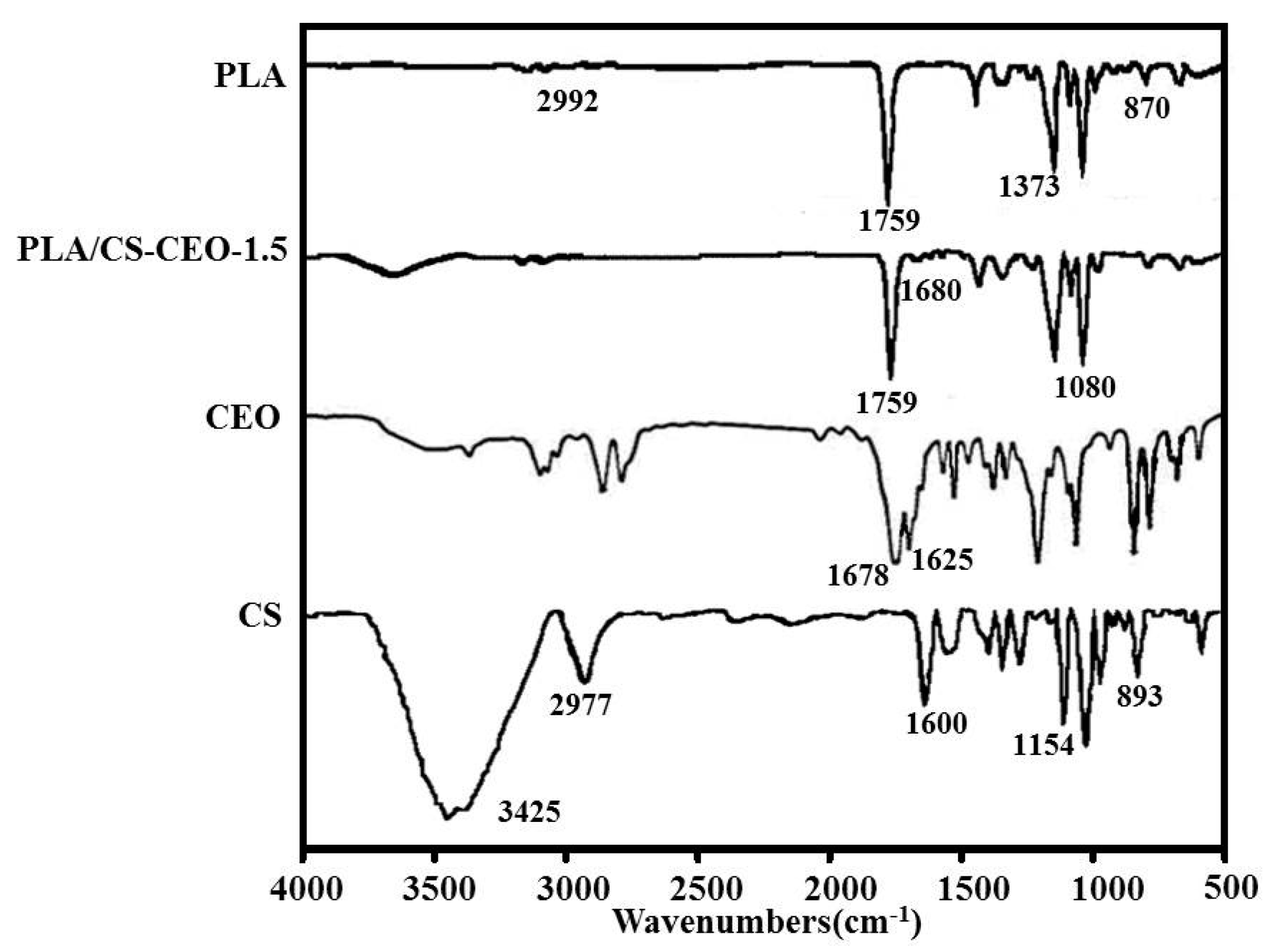
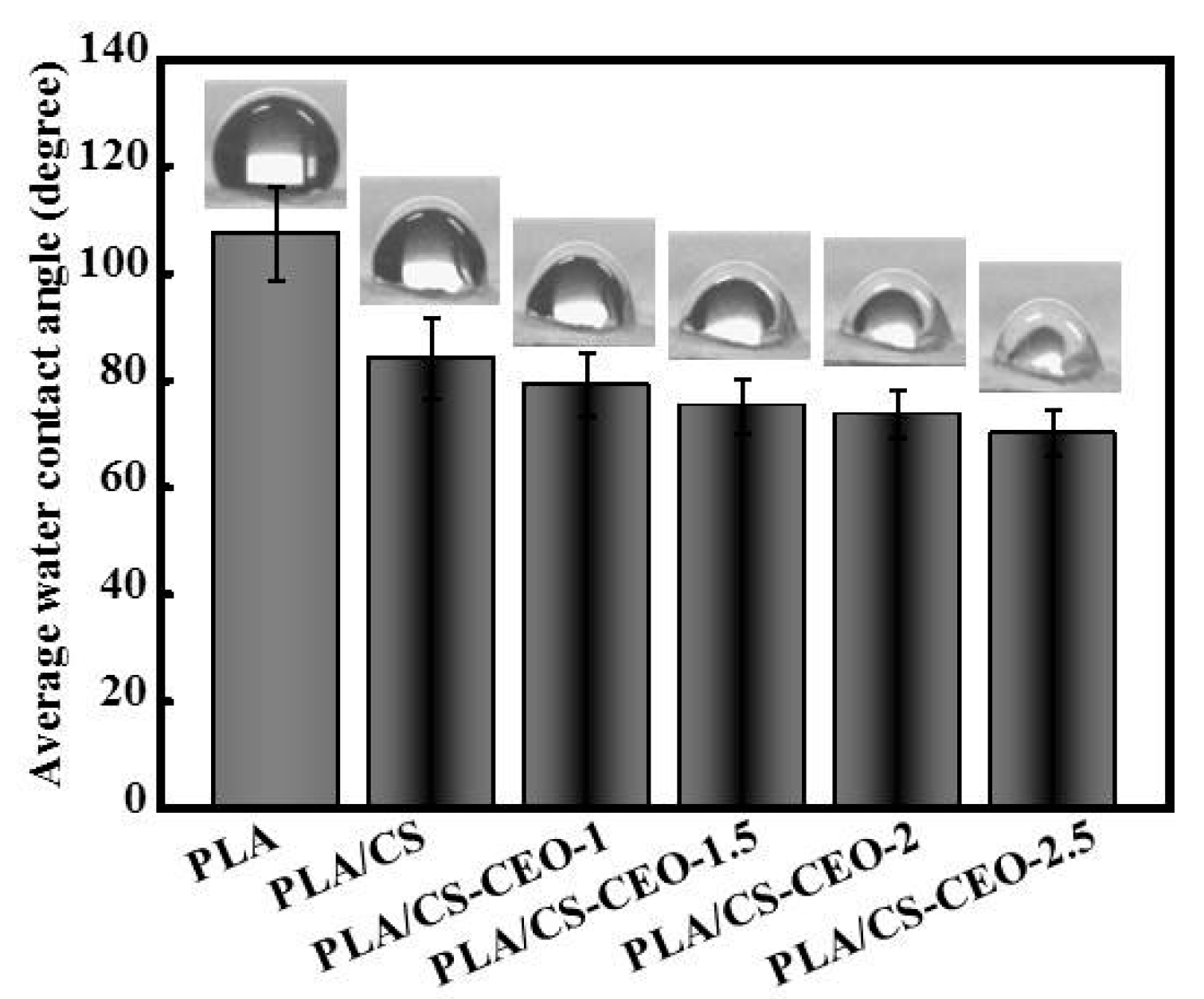
| Nanoparticles | Zeta Potential (mV) | Amount of Encapsulated EO (g) | Encapsulation Efficlency (%) | Nanoparticle Formation Efficiency (%) |
|---|---|---|---|---|
| CS | −8.6 ± 0.5 | - | - | 76.4 ± 12.2 |
| CS/CEO-1 | −11.2 ± 0.8 | 0.32 ± 0.06 | 55.64 ± 9.57 | 74.7 ± 11.8 |
| CS/CEO-1.5 | −13.6 ± 1.1 | 0.46 ± 0.07 | 53.64 ± 8.44 | 73.6 ± 11.3 |
| CS/CEO-2 | −16.9 ± 1.3 | 0.58 ± 0.08 | 50.21 ± 7.58 | 71.1 ± 10.5 |
| CS/CEO-2.5 | −22.4 ± 1.8 | 0.63 ± 0.09 | 47.67 ± 6.94 | 70.3 ± 10.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, S.; Zhang, R.; Lan, W.; Qin, W. Development of Poly(lactic acid)/Chitosan Fibers Loaded with Essential Oil for Antimicrobial Applications. Nanomaterials 2017, 7, 194. https://doi.org/10.3390/nano7070194
Liu Y, Wang S, Zhang R, Lan W, Qin W. Development of Poly(lactic acid)/Chitosan Fibers Loaded with Essential Oil for Antimicrobial Applications. Nanomaterials. 2017; 7(7):194. https://doi.org/10.3390/nano7070194
Chicago/Turabian StyleLiu, Yaowen, Shuyao Wang, Rong Zhang, Wenting Lan, and Wen Qin. 2017. "Development of Poly(lactic acid)/Chitosan Fibers Loaded with Essential Oil for Antimicrobial Applications" Nanomaterials 7, no. 7: 194. https://doi.org/10.3390/nano7070194




