Solvent Engineering for High-Performance PbS Quantum Dots Solar Cells
Abstract
:1. Introduction
2. Experiment Procedure
2.1. Materials
2.2. Ti-Sols, Zn2+ Precursor and PbS CQDs Fabrication
2.3. Devices Fabrication
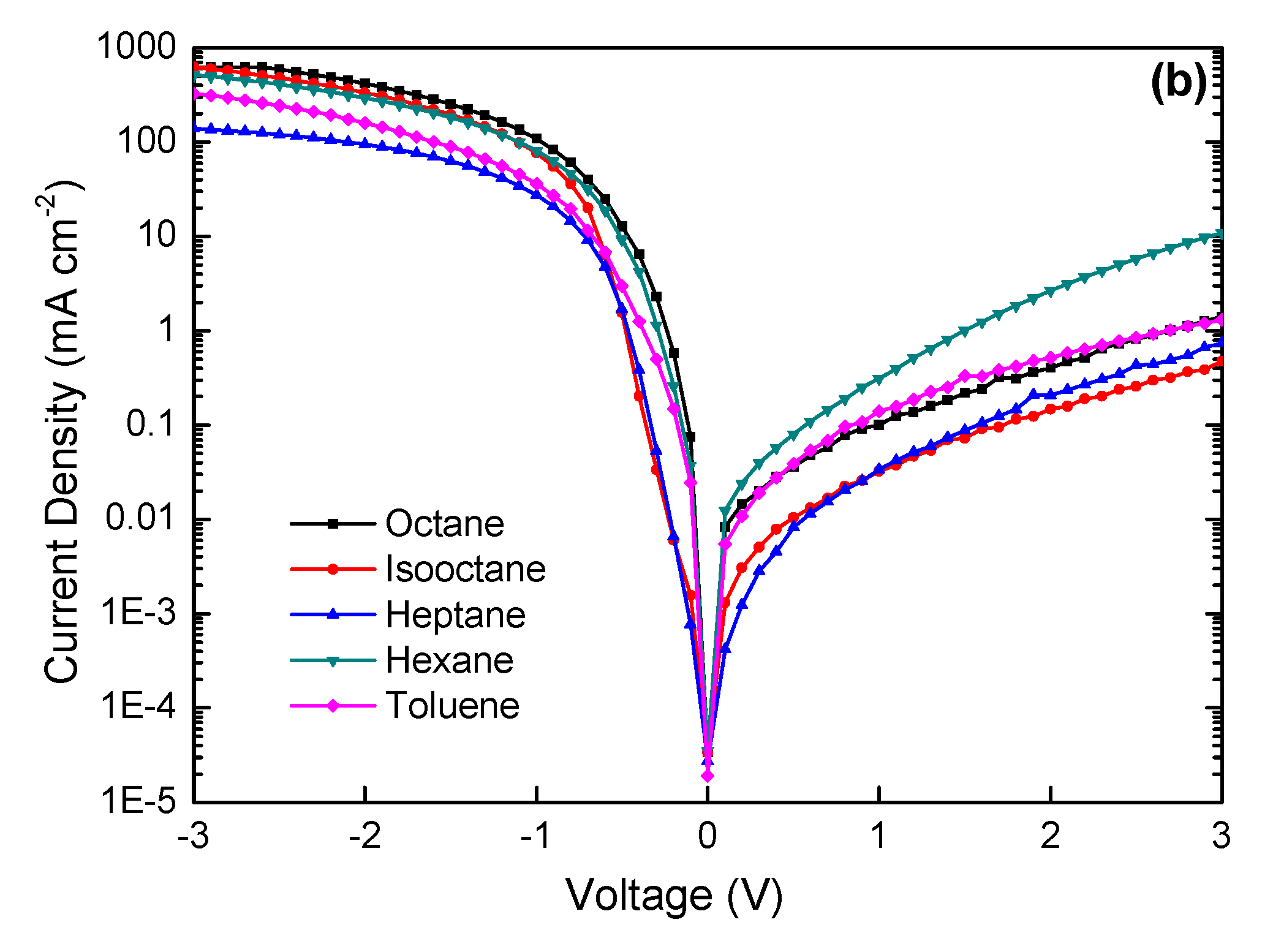
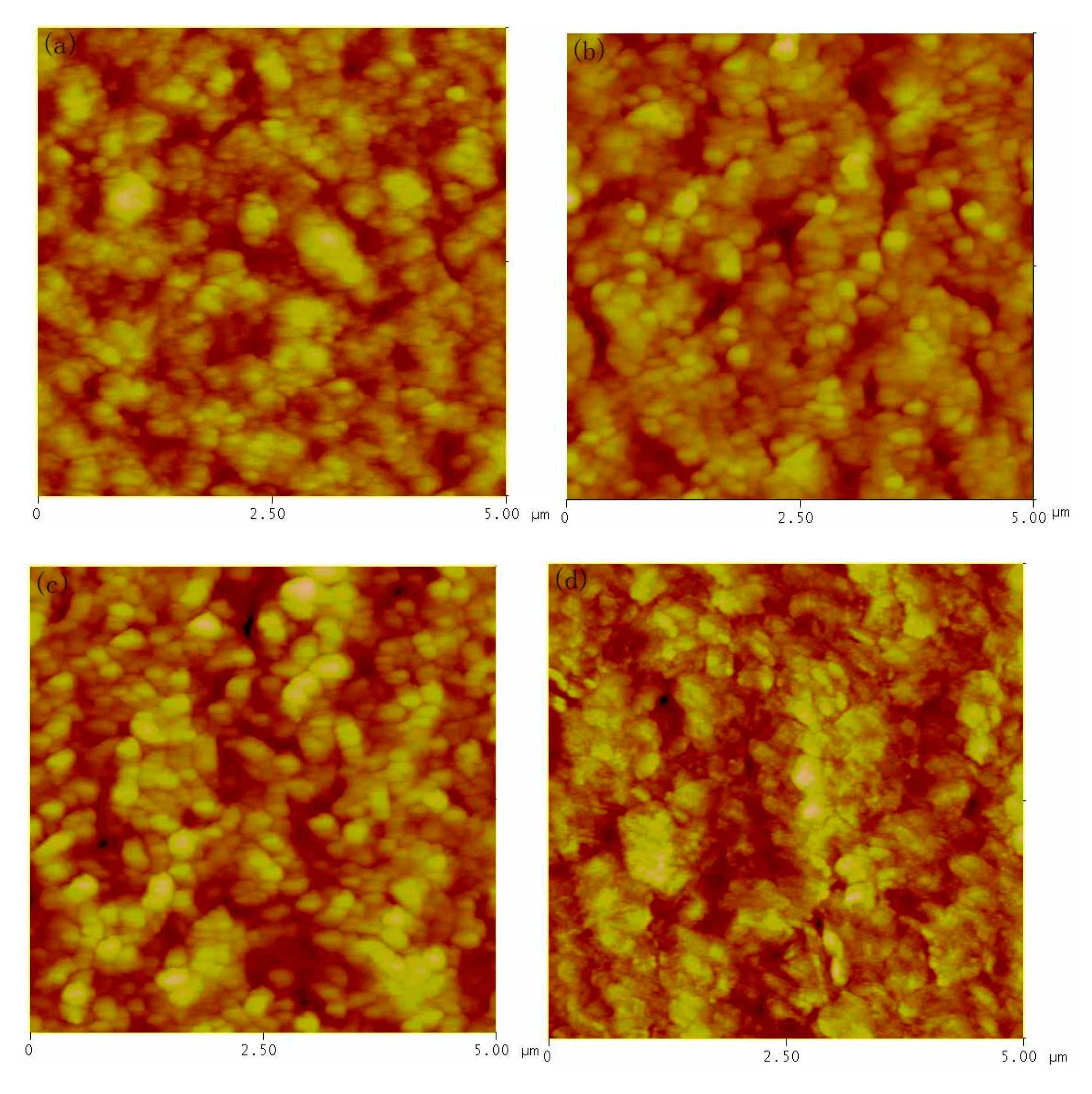
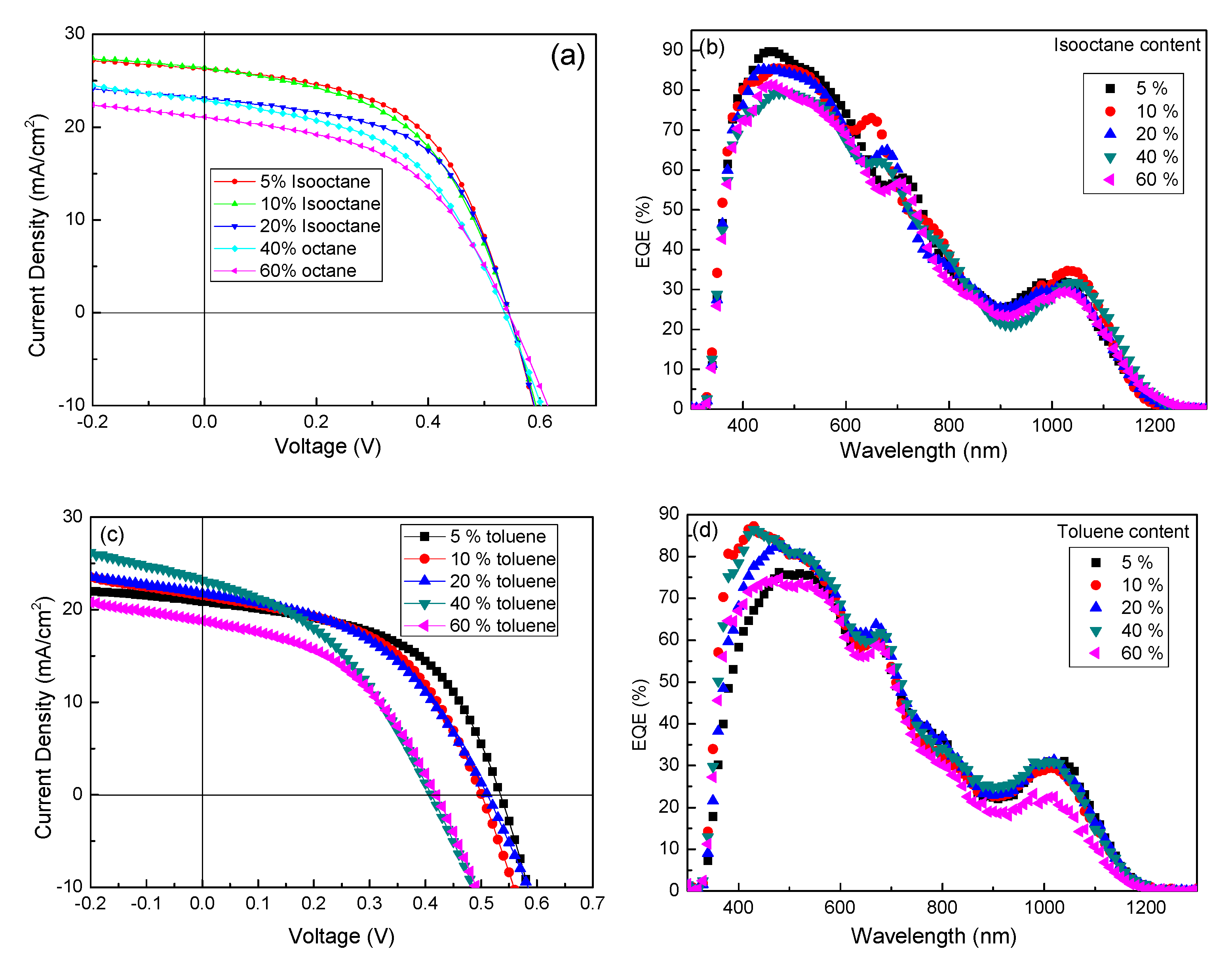
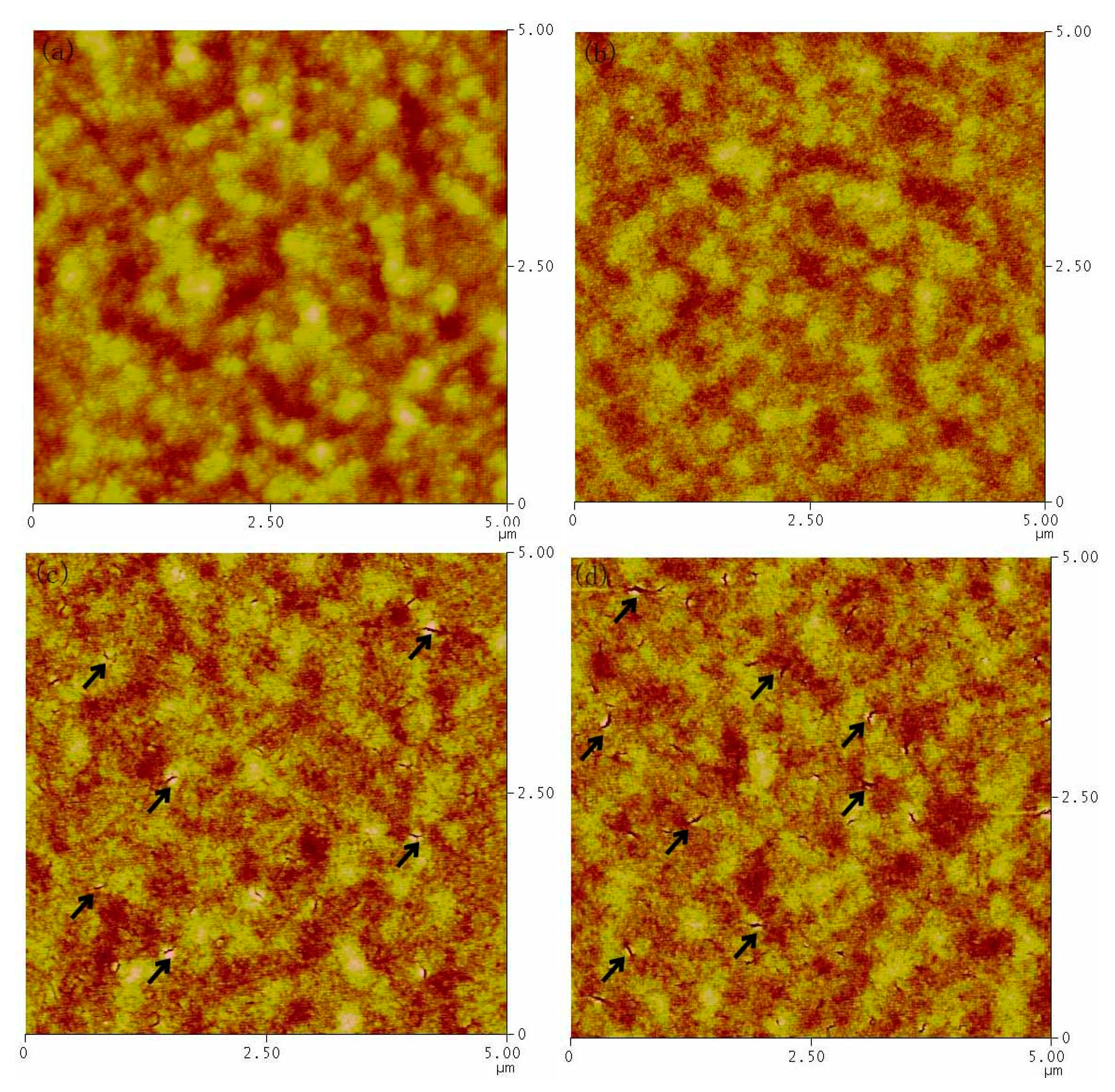
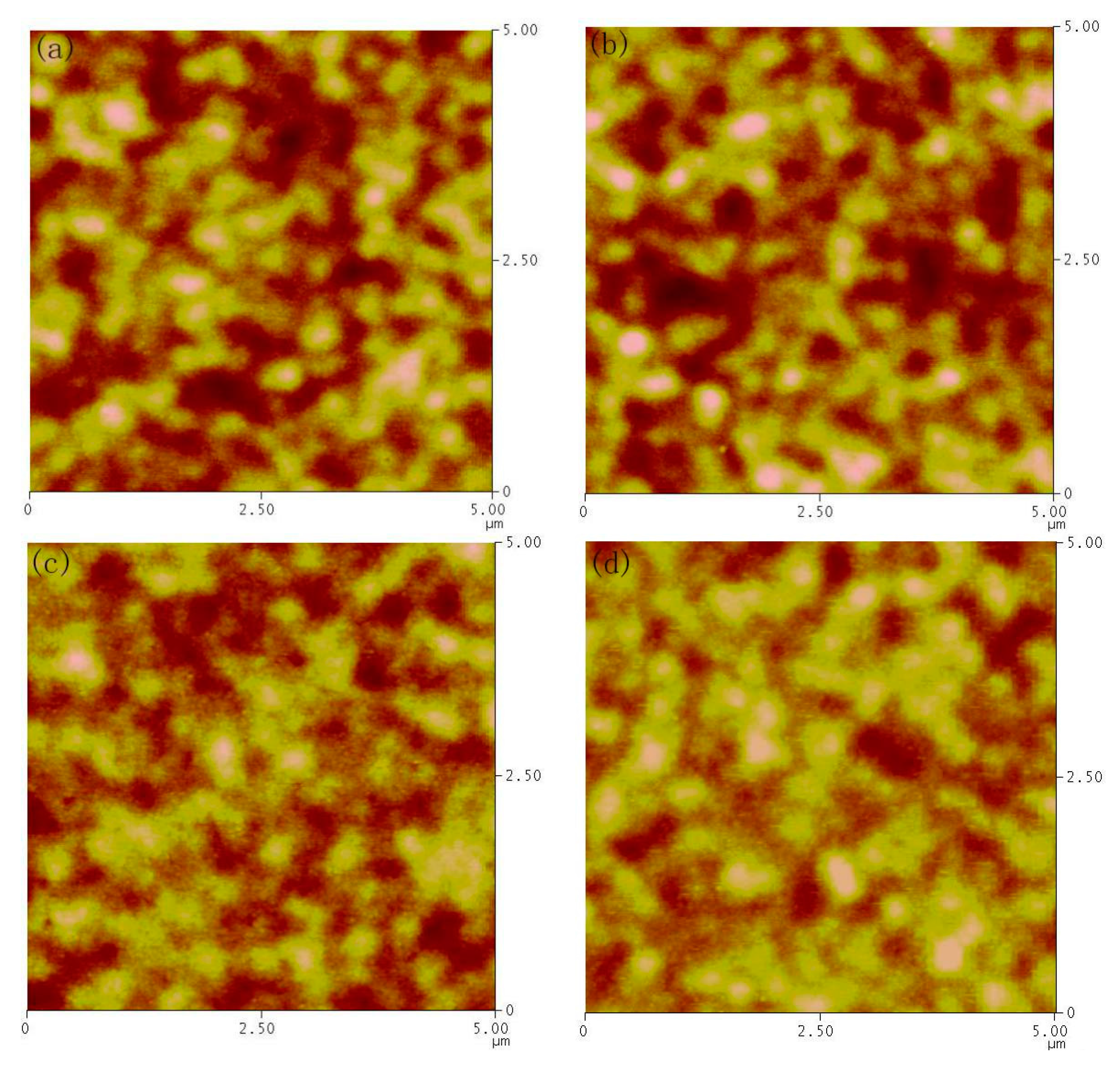
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Neo, D.C.J.; Cheng, C.; Stranks, S.D.; Fairclough, S.M.; Kim, J.S.; Kirkland, A.I.; Smith, J.M.; Snaith, H.J.; Assender, H.E.; Watt, A.A.R. Influence of Shell Thickness and Surface Passivation on PbS/CdS Core/Shell Colloidal Quantum Dot Solar Cells. Chem. Mater. 2014, 26, 4004–4013. [Google Scholar] [CrossRef]
- Klem, E.J.D.; Gregory, C.W.; Cunningham, G.B.; Hall, S.; Temple, D.S.; Lewis, J.S. Planar PbS quantum dot/C60 heterojunction photovoltaic devices with 5.2% power conversion efficiency. Appl. Phys. Lett. 2012, 100, 173109. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Wen, S.; Liu, S.; Heng, J.; Qin, D.; Hou, L.; Wu, H.; Xu, W.; Huang, W.; et al. CdTe nanocrystal hetero-junction solar cells with high open circuit voltage based on Sb-doped TiO2 electron acceptor materials. Nanomaterials 2017, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, L.; Huang, J.; Ning, Z.; Sun, L.; Ågren, H. Improving the Photocurrent in Quantum-Dot-Sensitized Solar Cells by Employing Alloy PbxCd1xS Quantum Dots as Photosensitizers. Nanomaterials 2016, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Panthani, M.G.; Kurley, J.M.; Crisp, R.W.; Dietz, T.C.; Ezzyat, T.; Luther, J.M.; Talapin, D.V. High Efficiency Solution Processed Sintered CdTe Nanocrystal Solar cells: The Role of interfaces. Nano Lett. 2014, 14, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, Y.; Gao, Y.; Qin, D.; Wu, H.; Huang, W. Enhancement of open-circuit voltage and the fill factor in CdTe nanocrystal solar cells by using interface materials. Nanotechnology 2014, 25, 365203. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, Y.; Lin, Y.; Gao, K.; Zhang, Y.; Liu, K.; Yang, Q.; Zhou, X.; Qin, D.; Wu, H.; et al. Solution-processed efficient CdTe nanocrystal/CBD-CdS heterojunction solar cells with ZnO interlayer. J. Nanopart. Res. 2013, 15, 2053. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Du, X.; Cheng, X.; Chen, X.; Jiang, Y.; Yang, B. From planar-heterojunction to n-i structure: An efficient strategy to improve short-circuit current and power conversion efficiency of aqueous-solution-processed hybrid solar cells. Energy Environ. Sci. 2013, 6, 1597–1603. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Li, Z.; Hao, H.; Zeng, Q.; Wang, Y.; Du, X.; Wang, L.; Yang, B. In situ construction of nanoscale CdTe-CdS bulk heterojunctions for inorganic nanocrystal solar cells. Adv. Energy Mater. 2014, 4, 1400235. [Google Scholar] [CrossRef]
- Nir, Y.-G.; Michal, S.-H.; Marina, Z.; Shifi, K.; Asher, S.; Nir, T. Molecular control of quantum-dot internal electric field and its application to CdSe-based solar cells. Nat. Mater. 2011, 10, 974–979. [Google Scholar]
- Semonin, O.E.; Luther, J.M.; Choi, S.; Chen, H.Y.; Gao, J.; Nozik, A.J.; Beard, M.C. Peak External Photocurrent Quantum Efficiency Exceeding 100% via MEG in a Quantum Dot Solar Cell. Science 2011, 334, 1530–1533. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.J.; Levina, L.; Debnath, R.; Zhitomirsky, D.; Sargent, E.H. Solar Cells Using Quantum Funnels. Nano Lett. 2011, 11, 3701–3706. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Walker, B.; Kim, H.B.; Kim, J.Y.; Sargent, E.H.; Park, J.; Kim, J.Y. Inverted Colloidal Quantum Dot Solar Cells. Adv. Mater. 2014, 26, 3321–3327. [Google Scholar] [CrossRef] [PubMed]
- Gonfa, B.A.; Zhao, H.; Li, J.; Qiu, J.; Saidani, M.; Zhang, S.; Izquierdo, R.; Wu, N.; El Khakani, M.A.; Ma, D.; et al. Air-processed depleted bulk heterojunction solar cells based on PbS/CdS core-shell quantum dots and TiO2 nanorod arrays. Sol. Energy Mater. Sol. Cells 2014, 124, 67–74. [Google Scholar] [CrossRef]
- Yuan, M.; Kemp, K.W.; Thon, S.M.; Kim, J.Y.; Chou, K.W.; Amassian, A.; Sargent, E.H. High-Performance Quantum-Dot Solids via Elemental Sulfur Synthesis. Adv. Mater. 2014, 26, 3513–3519. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.A.; Konstantatos, G.; Zhang, S.; Paul, W.; Cyr, E.J.; Klem, D.; Levina, L.; Sargent, E.H. Solution-processed PbS quantum dot infrared photodetectors and photovoltaics. Nat. Mater. 2005, 4, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Voznyy, O.; Arquer, F.P.G.; Liu, M.; Xu, J.; Proppe, A.H.; Walters, G.; Fan, F.; Tan, H.; Liu, M.; et al. 10.6% Certified Colloidal Quantum Dot Solar Cells via Solvent-Polarity-Engineered Halide Passivation. Nano Lett. 2016, 16, 4630–4634. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Ren, Y.; Hoogland, S.; Voznyy, O.; Levina, L.; Stadler, P.; Lan, X.; Zhitomirsky, D.; Sargent, E.H. All-inorganic colloidal quantum dot photovoltaics employing solution-phase-halide passivation. Adv. Mater. 2012, 24, 6295–6299. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Wang, L.; Gao, R.; Li, W.; Guo, X.; Dong, H.; Qiu, Y. Inorganic halogen ligands in quantum dots: I-, Br-, Cl- and film fabrication through electrophoretic deposition. Phys. Chem. Chem. Phys. 2013, 15, 19595–19600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jang, J.; Liu, W.; Talapin, D.V. Colloidal nanocrystals with inorganic halide, pseudohalide, and halometallate ligands. ACS Nano 2014, 8, 7359–7369. [Google Scholar] [CrossRef] [PubMed]
- Dirin, D.N.; Dreyfuss, S.; Bodnarchuk, M.I.; Nedelcu, G.; Papagiorgis, P.; Itskos, G.; Kovalenko, M.V. Lead halide perovskites and other metal halide complexes as inorganic capping ligands for colloidal nanocrystals. J. Am. Chem. Soc. 2014, 136, 6550–6553. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tang, J.; Kramer, I.J.; Debnath, R.; Koleilat, G.I.; Wang, X.; Fisher, A.; Li, R.; Brzozowski, L.; Levina, L.; et al. Electron acceptor materials engineering in colloidal quantum dot solar cells. Adv. Mater. 2011, 23, 3832–3837. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, D.; Furukawa, M.; Tang, J.; Stadler, P.; Hoogland, S.; Voznyy, O.; Liu, H.; Sargent, E.H. N-Type Colloidal-Quantum-Dot Solids for Photovoltaics. Adv. Mater. 2012, 24, 6181–6185. [Google Scholar] [CrossRef] [PubMed]
- Ip, A.H.; Thon, S.M.; Hoogland, S.; Voznyy, O.; Zhitomirsky, D.; Debnath, R.; Levina, L.; Amassian, A.; Sargent, E.H. Hybrid passivated colloidal quantum dot solids. Nat. Nanotechnol. 2012, 7, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.M.; Brown, P.R.; Bulovic, V.; Bawendi, M.G. Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat. Mater. 2014, 13, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Voznyy, O.; Pan, J.; Hoogland, S.; Adinolfi, V.; Xu, J.; Li, M.; Kirmani, A.R.; Sun, J.P.; Minor, J.; et al. Air-stable n-type colloidal quantum dot solids. Nat. Mater. 2014, 13, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.J.; Sargent, E.H. The architecture of colloidal quantum dot solar cells: Materials to devices. Chem. Rev. 2014, 114, 863–882. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.; Boercker, J.E.; Lumb, M.P.; Placencia, D.; Foos, E.E.; Tischler, J.G. Enhanced Open-Circuit Voltage of PbS Nanocrystal Quantum Dot Solar Cells. Sci. Rep. 2013, 3, 2225. [Google Scholar] [CrossRef] [PubMed]
- Malgras, V.; Nattestad, A.; Yamauchi, Y.; Dou, S.X.; Kim, J.H. The effect of surface passivation on the structure of sulphur-rich PbS colloidal quantum dots for photovoltaic application. Nanoscale 2015, 7, 5706–5711. [Google Scholar] [CrossRef] [PubMed]
- Ciach, R.; Dotsenko, Y.P.; Naumov, V.V.; Shmyryeva, A.N. Injection technique for the study of solar cell test structures. Sol. Energy Mater. Sol. Cells 2003, 76, 613–624. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, B.; Gao, Y.; Liu, H.; Tian, Y.; Qin, D.; Wu, H.; Hou, L.; Huang, W. Novel hybrid ligands for passivating PbS colloidal quantum dots to enhance the performance of solar cells. Nano-Micro Lett. 2015, 7, 325–331. [Google Scholar] [CrossRef]
- Tu, Y.; Wu, J.; He, X.; Guo, P.; Wu, T.; Luo, H.; Liu, Q.; Wang, K.; Lin, J.; Huang, M.; et al. Solvent engineering for forming stonehenge-like PbI2 nano-structures towards efficient perovskite solar cells. J. Mater. Chem. A 2017, 5, 4376–4383. [Google Scholar] [CrossRef]
- Luo, P.; Xia, W.; Zhou, S.; Sun, L.; Cheng, J.; Xu, C.; Lu, Y. Solvent Engineering for Ambient-Air-Processed, Phase-Stable CsPbI3 in Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 3603–3608. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Jang, W.; Ahn, S.; Park, C.E.; Wang, D.H. Dramatically enhanced performances and ideally controlled nano-morphology via co-solvent processing in low bandgap polymer solar cells. Organ. Electron. 2016, 34, 42–49. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Z.; He, H.; Zheng, X.; Wong, K.S.; Yang, S. Solvent Engineering Boosts the Efficiency of Paintable Carbon-Based Perovskite Solar Cells to Beyond 14%. Adv. Energy Mater. 2016, 6, 1502087. [Google Scholar] [CrossRef]
- Perumallapelli, G.R.; Vasa, S.R.; Jang, J. Improved morphology and enhanced stability via solvent engineering for planar heterojunction perovskite solar cells. Organ. Electron. 2016, 31, 142–148. [Google Scholar] [CrossRef]
- Wang, R.; Shang, Y.; Kanjanaboos, P.; Zhou, W.; Ning, Z.; Sargent, E.H. Colloidal quantum dot ligand engineering for high performance solar cells. Energy Environ. Sci. 2016, 9, 1130. [Google Scholar] [CrossRef]
- Kirmani, A.R.; Carey, G.H.; Abdelsamie, M.; Yan, B.; Cha, D.; Rollny, L.R.; Cui, X.; Sargent, E.H.; Amassian, A. Effect of Solvent Environment on Colloidal-Quantum-Dot Solar-Cell Manufacturability and Performance. Adv. Mater. 2014, 26, 4717–4723. [Google Scholar] [CrossRef] [PubMed]
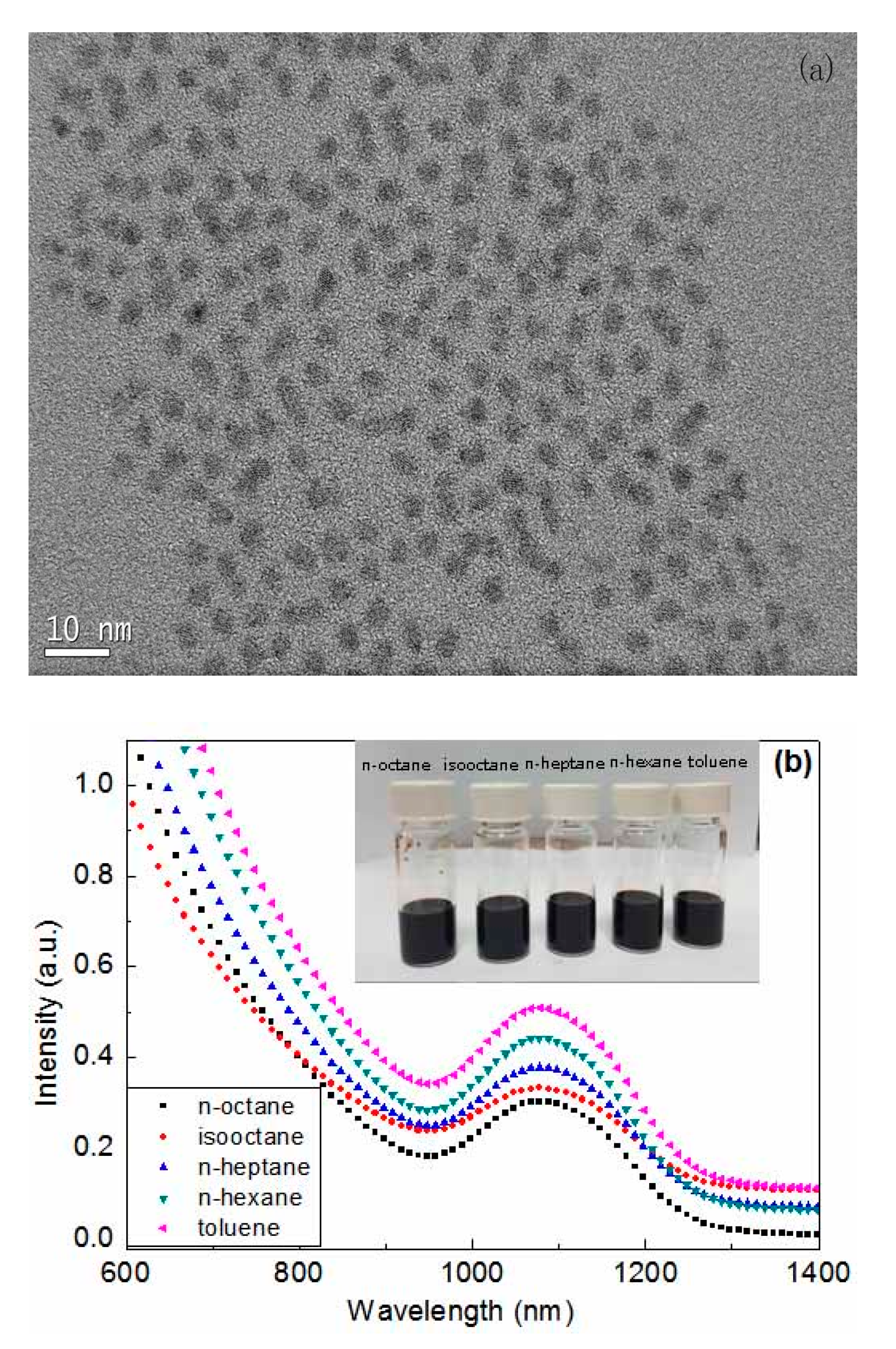
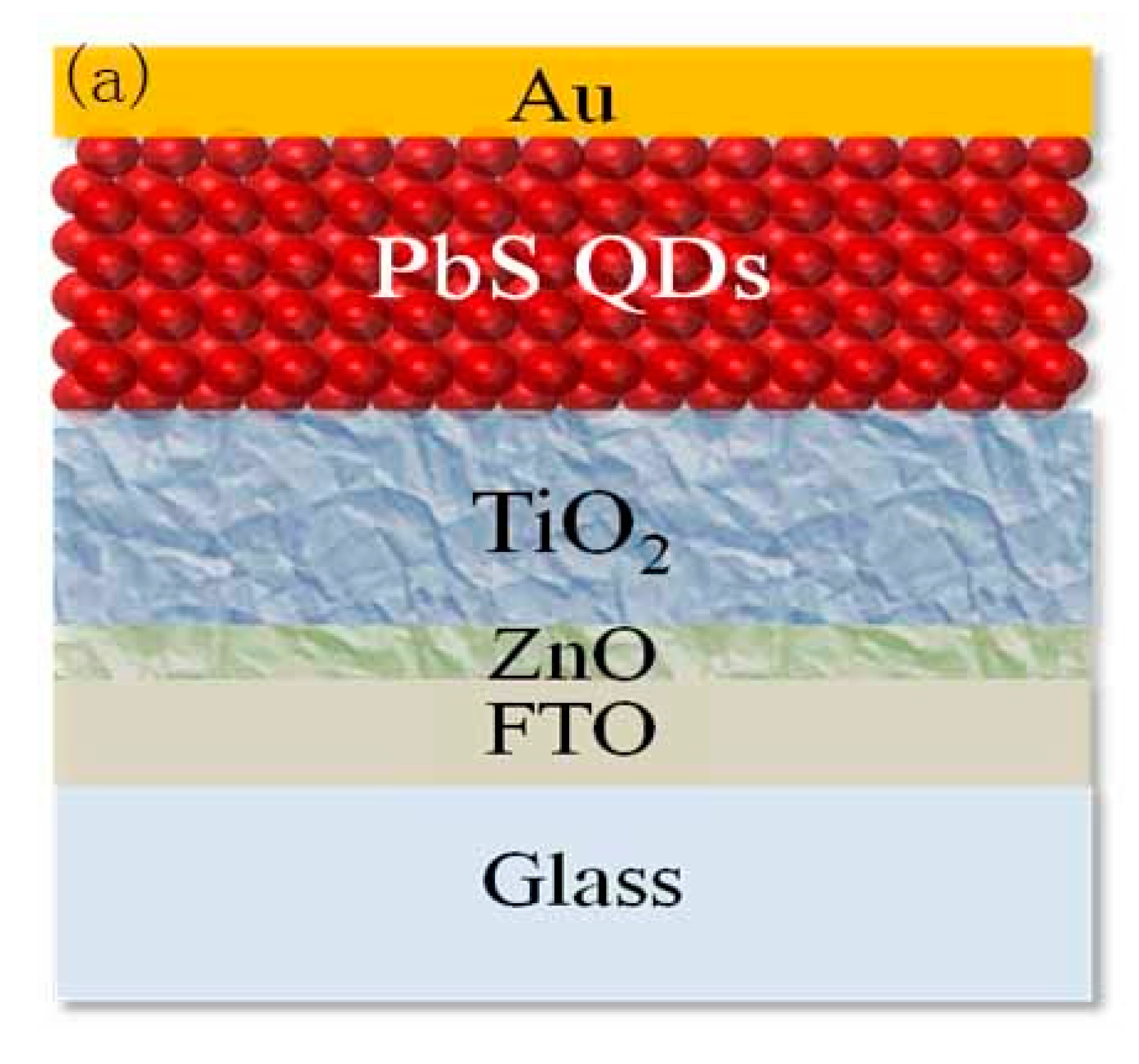
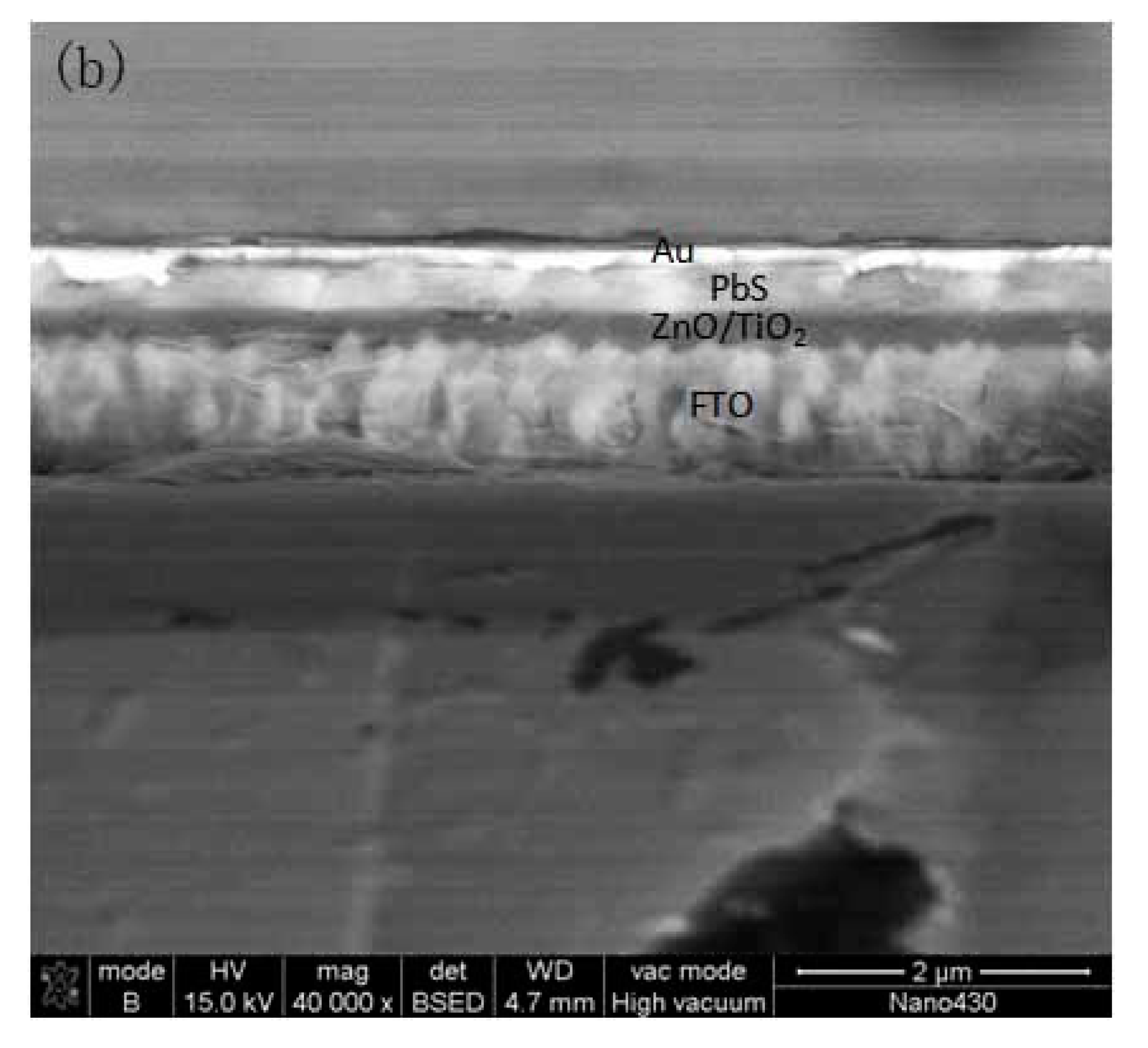
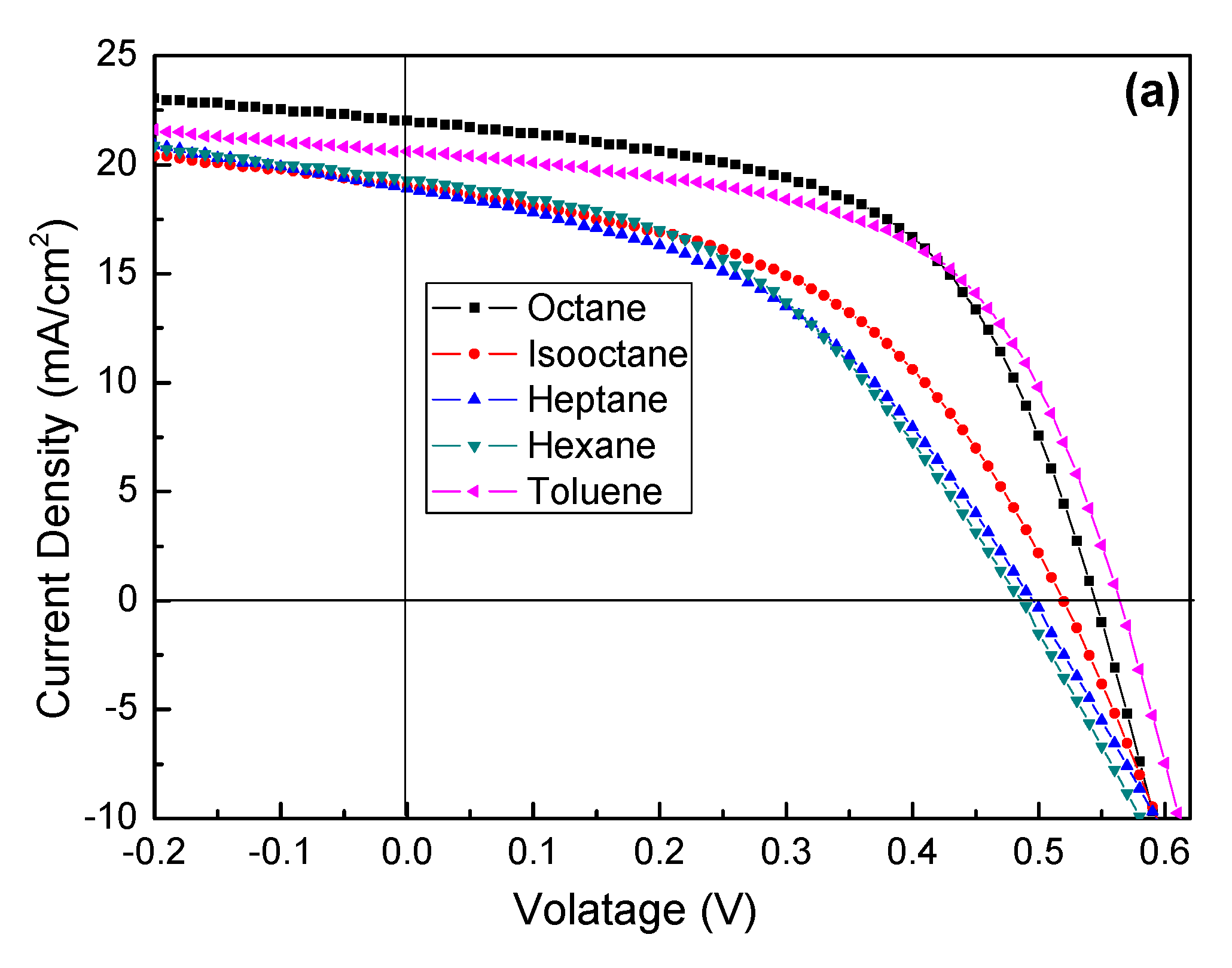
| Solvent Name | Polarity | Viscosity (Pa·s) | Boiling Point (°C) | Absorption Wavelength (nm) |
|---|---|---|---|---|
| n-octane | 0.06 | 0.53 | 125 | 200 |
| isooctane | 0.10 | 0.53 | 99 | 210 |
| heptane | 0.20 | 0.41 | 98 | 200 |
| n-hexane | 0.06 | 0.33 | 69 | 210 |
| toluene | 2.40 | 0.59 | 111 | 285 |
| Solvent | Power Conversion Efficiency (PCE) (%) | Jsc (mA cm−2) | Voc (V) | Fill Factor (FF) (%) |
|---|---|---|---|---|
| n-octane | 6.67 | 22.04 | 0.54 | 56.04 |
| isooctane | 4.62 | 19.00 | 0.52 | 46.76 |
| heptane | 4.69 | 21.63 | 0.52 | 41.70 |
| n-hexane | 4.12 | 19.30 | 0.49 | 43.57 |
| toluene | 6.59 | 20.60 | 0.56 | 57.13 |
| Isooctane/n-octane (v/v) | PCE (%) | Jsc (mA cm−2) | Voc (V) | FF (%) | Rs (Ω × cm−2) | Rsh (Ω × cm−2) |
| 5% | 7.64 | 26.29 | 0.54 | 53.82 | 4.97 | 205.55 |
| 10% | 7.26 | 26.38 | 0.54 | 50.93 | 5.47 | 109.21 |
| 20% | 6.99 | 23.10 | 0.54 | 56.04 | 4.83 | 173.90 |
| 40% | 6.08 | 22.90 | 0.54 | 49.17 | 7.02 | 104.33 |
| 60% | 5.64 | 21.10 | 0.53 | 50.43 | 7.94 | 132.37 |
| Toluene/n-octane (v/v) | PCE (%) | Jsc (mA cm−2) | Voc (V) | FF (%) | Rs (Ω × cm−2) | Rsh (Ω × cm−2) |
| 5% | 5.84 | 20.87 | 0.53 | 52.80 | 9.29 | 131.23 |
| 10% | 5.31 | 21.57 | 0.50 | 49.24 | 11.37 | 109.94 |
| 20% | 5.09 | 21.74 | 0.51 | 45.91 | 12.19 | 104.65 |
| 40% | 3.85 | 23.20 | 0.41 | 40.48 | 8.20 | 55.56 |
| 60% | 3.51 | 18.80 | 0.42 | 44.45 | 8.52 | 100.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Yang, Y.; Li, M.; Qin, D.; Zhang, Y.; Hou, L. Solvent Engineering for High-Performance PbS Quantum Dots Solar Cells. Nanomaterials 2017, 7, 201. https://doi.org/10.3390/nano7080201
Wu R, Yang Y, Li M, Qin D, Zhang Y, Hou L. Solvent Engineering for High-Performance PbS Quantum Dots Solar Cells. Nanomaterials. 2017; 7(8):201. https://doi.org/10.3390/nano7080201
Chicago/Turabian StyleWu, Rongfang, Yuehua Yang, Miaozi Li, Donghuan Qin, Yangdong Zhang, and Lintao Hou. 2017. "Solvent Engineering for High-Performance PbS Quantum Dots Solar Cells" Nanomaterials 7, no. 8: 201. https://doi.org/10.3390/nano7080201





