Effects of Metal Micro and Nano-Particles on hASCs: An In Vitro Model
Abstract
:1. Introduction
2. Results
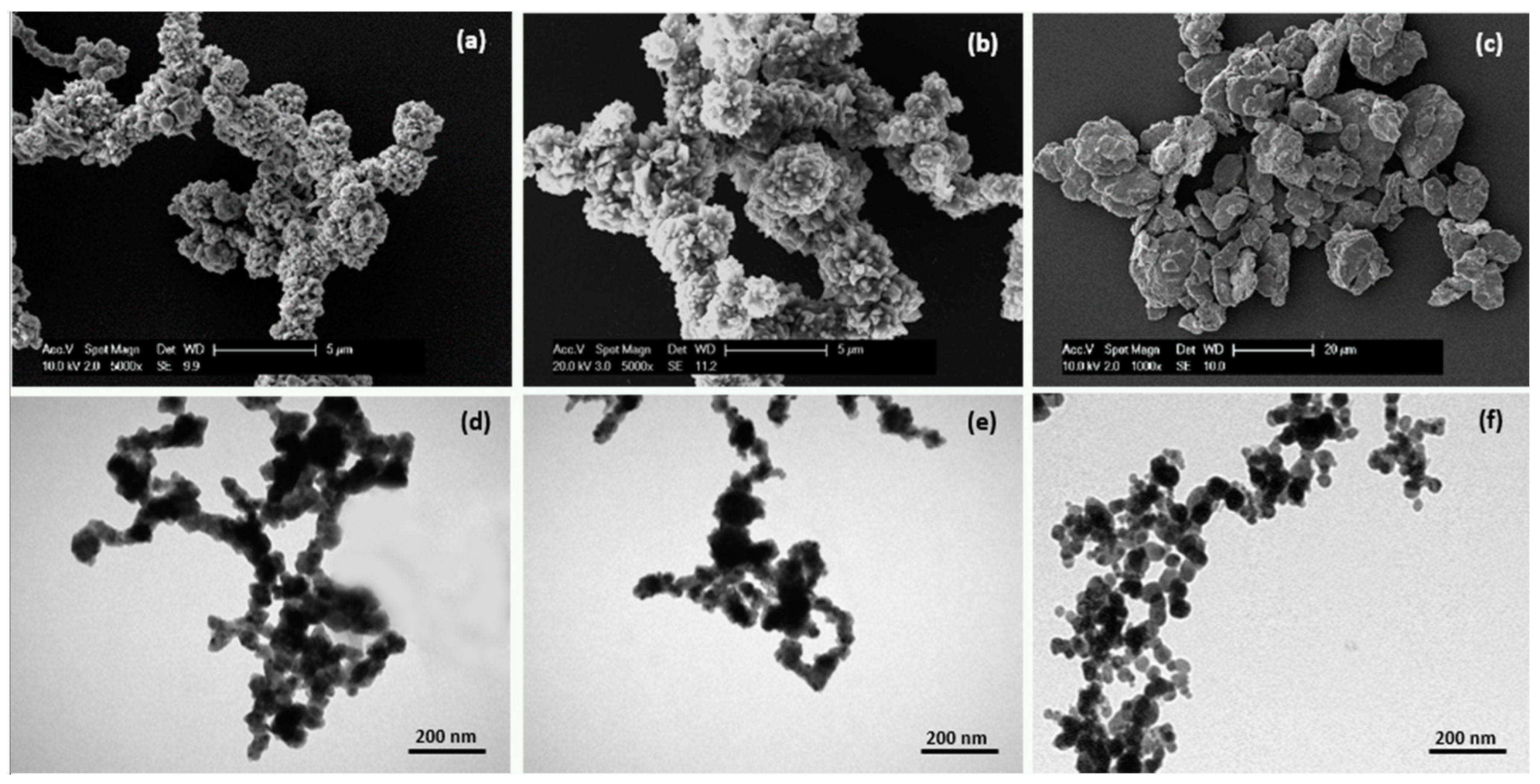
2.1. Micro- and Nanoparticle Characterization
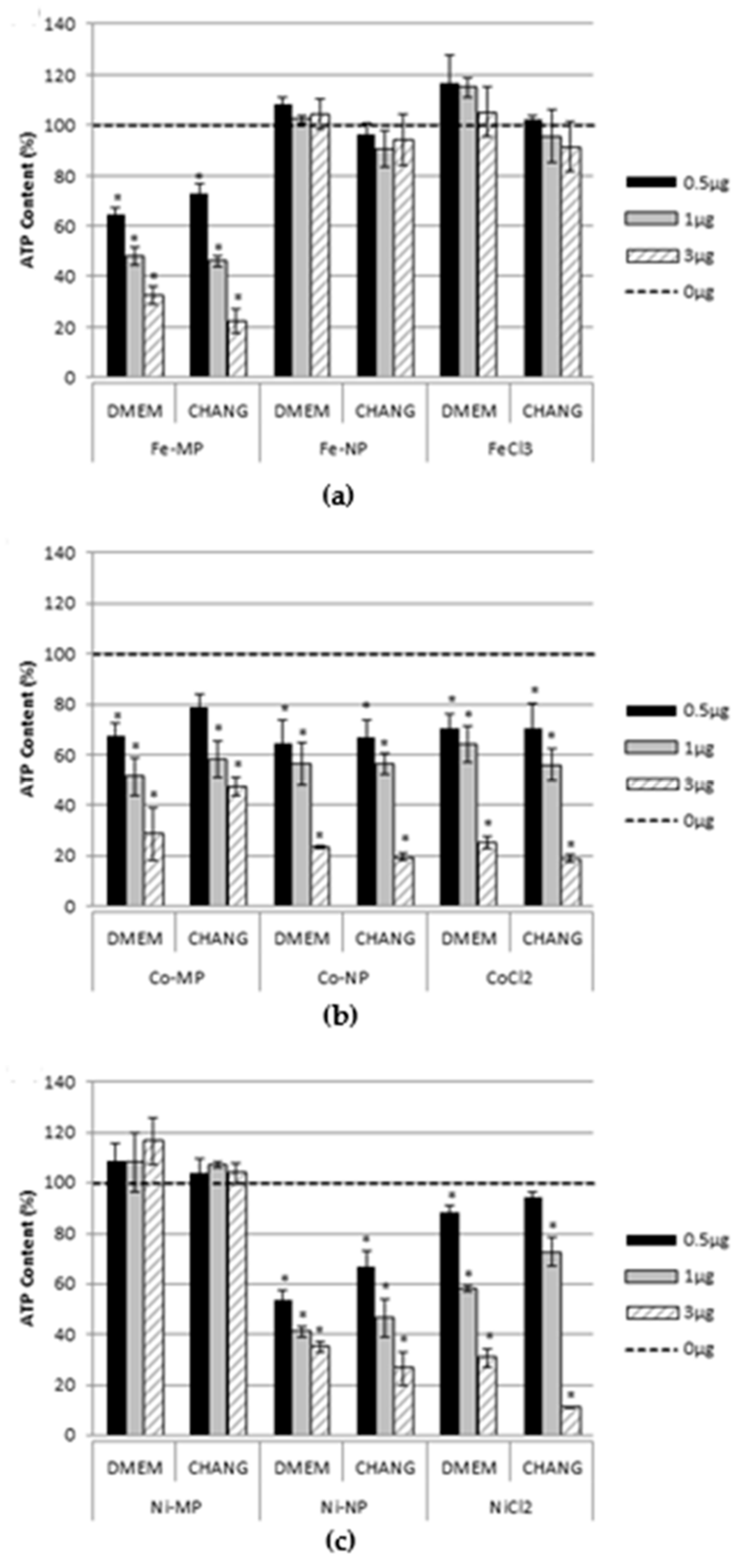
2.2. Cell Viability
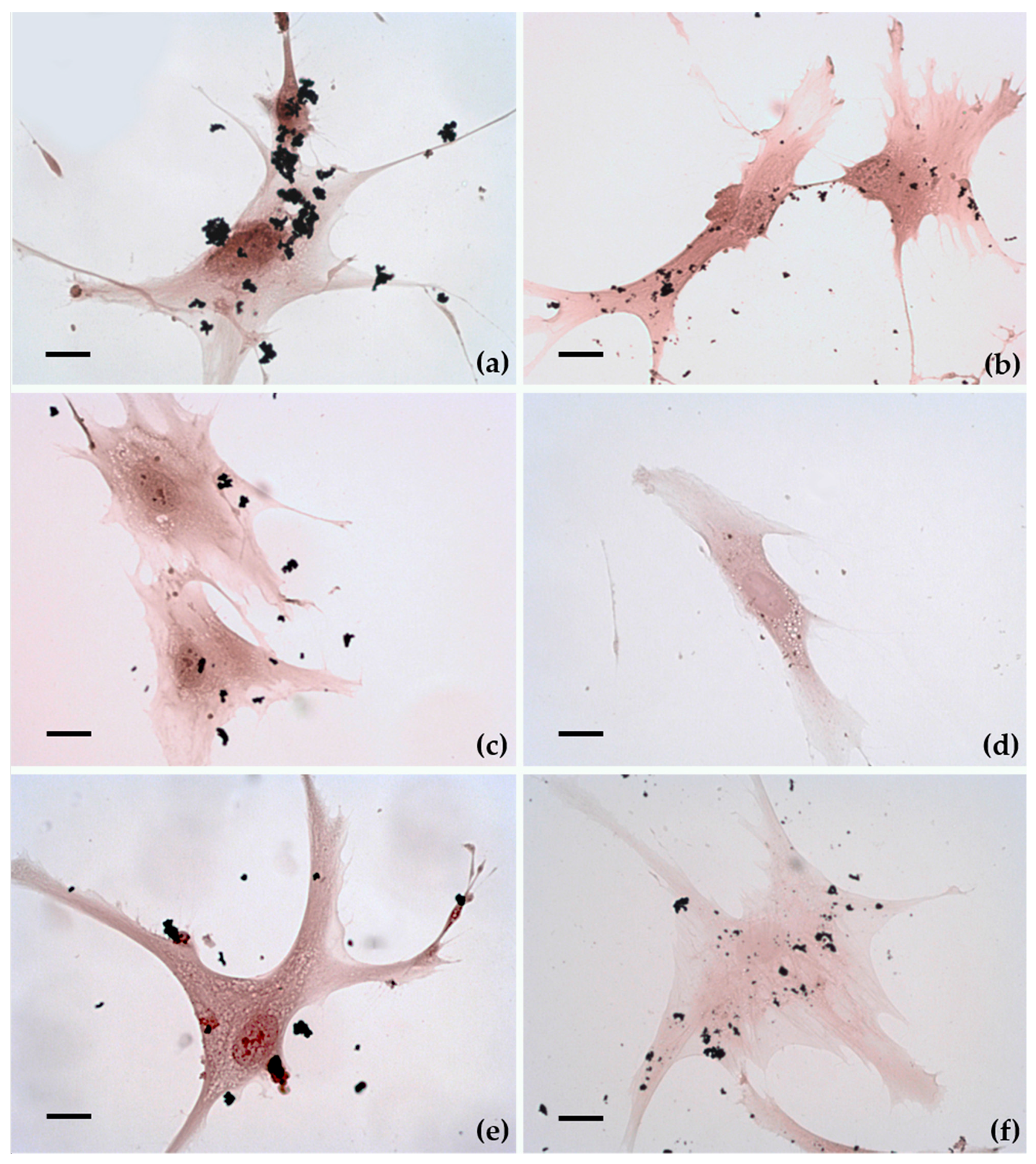
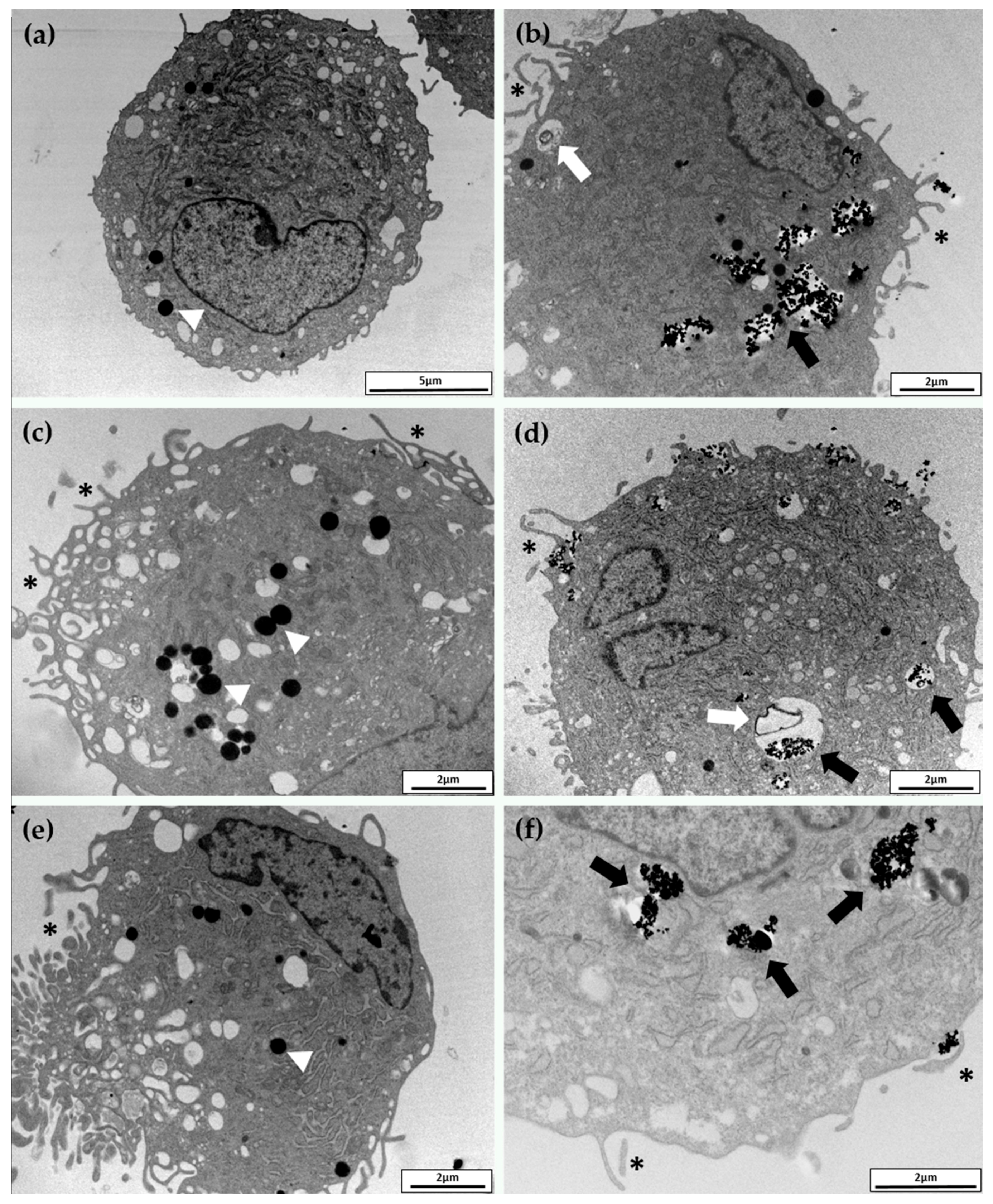
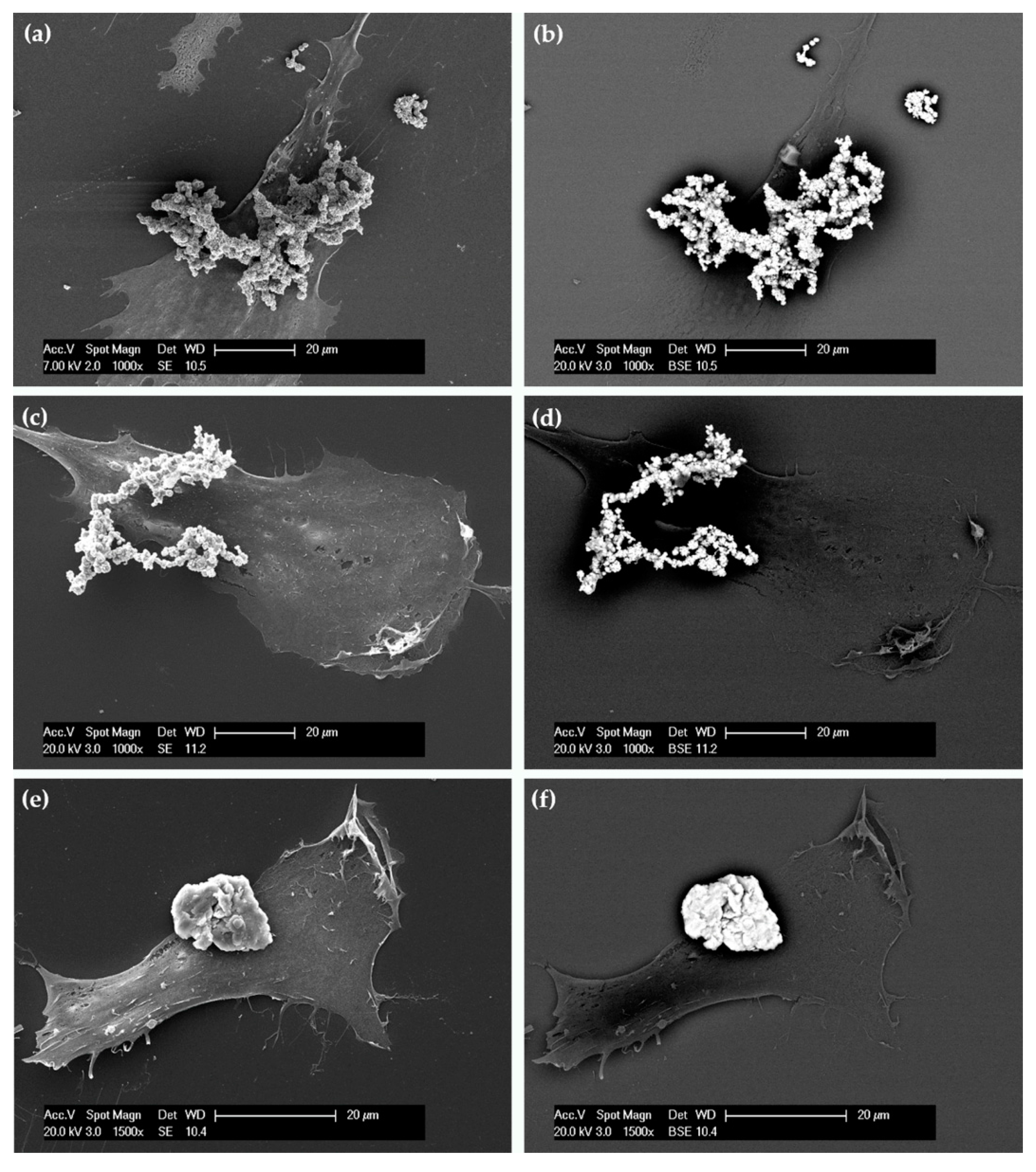
2.3. Cellular Uptake and Morphology
2.4. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Micro- and Nanoparticle Characterizations
4.2. Patient Samples
4.3. hASC Isolation and Culture
4.4. Cell Exposure and Viability
4.5. Uptake and Morphology
4.6. RNA Isolation, Reverse Transcription, and Real-Time PCR
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klimczak, A.; Kozlowska, U. Mesenchymal Stromal Cells and Tissue-Specific Progenitor. Stem Cells Int. 2016, 2016, 4285215. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Mammalian Neural Stem Cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Bruder, S.P. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol. Med. 2001, 7, 259–264. [Google Scholar] [CrossRef]
- Cherubino, M.; Valdatta, L.; Balzaretti, R.; Pellegatta, I.; Rossi, F.; Protasoni, M.; Tedeschi, A.; Accolla, R.S.; Bernardini, G.; Gornati, R. Human Adipose-Derived Stem Cells Promote Vascularization of Collagen-Based Scaffolds Transplanted into Nude Mice. Regen. Med. 2016, 11, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Huss, R. Adult Stem Cell Lines in Regenerative Medicine and Reconstructive Surgery. J. Surg. Res. 2005, 124, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Suma, R.N.; Mohanan, P.V. Stem Cells, a New Generation Model for Predictive Nano Toxicological Assessment. Curr. Drug. MeTab. 2015, 16, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Edmundson, M.; Nguyen, T.K.T.; Song, B. Nanoparticles Based Stem Cell Tracking in Regenerative Medicine. Theranostics 2013, 3, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.G.; Gornati, R.; Sabbioni, E.; Chiriva-Internati, M.; Cobos, E.; Jenkinsf, M.R.; Bernardini, G. Nanothecnology and human health: Risks and beneficts. J. Appl. Toxicol. 2010, 30, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Rancoule, C.; Magné, N.; Vallard, A.; Guy, J.B.; Rodriguez-Lafrasse, C.; Deutsch, E.; Chargari, C. Nanoparticles in radiation oncology: From bench-side to bedside. Cancer Lett. 2016, 375, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Sabbioni, E.; Fortaner, S.; Farina, M.; Del Torchio, R.; Olivato, I.; Petrarca, C.; Bernardini, G.; Mariani-Costantini, R.; Perconti, S.; Di Giampaolo, L.; et al. Cytotoxicity and morphological transforming potential of cobalt nanoparticles, microparticles and ions in Balb/3T3 mouse fibroblasts: An in vitro model. Nanotoxicology 2014, 8, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, B.; Chen, Z.; Liu, W.; Pan, J.; Hou, L.; Zhang, Z. A Multi-Functional Tumor Theranostic Nanoplatform for MRI Guided Photothermal-Chemotherapy. Pharm. Res. 2016, 33, 1472–1485. [Google Scholar] [CrossRef] [PubMed]
- Di Gioacchino, M.; Verna, N.; Gornati, R.; Sabbioni, E.; Bernardini, G. Metal nanoparticle health risk assessment. In Nanotoxicology: From In Vivo and In Vitro Models to Health Risks; Sahu, S.C., Casciano, D., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 519–542. [Google Scholar]
- Gornati, R.; Papis, E.; Di Gioacchino, M.; Sabbioni, E.; Dalle Donne, I.; Milzani, A.; Bernardini, G. In vivo and in vitro models for nanotoxicology testing. In Nanotoxicology: From In Vivo and In Vitro Models to Health Risks; Sahu, S.C., Casciano, D., Eds.; Wiley & Sons Ltd.: Chichester, UK, 2009; pp. 279–302. [Google Scholar]
- Gornati, R.; Pedretti, E.; Rossi, F.; Cappellini, F.; Zanella, M.; Olivato, I.; Sabbioni, E.; Bernardini, G. Zerovalent Fe, Co and Ni nanoparticle toxicity evaluated on SKOV-3 and U87 cell lines. J. Appl. Toxicol. 2016, 36, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Quecchia, C.; Perotta, C.; Timpini, A.; Maccarinelli, K.; Di Tommaso, L.; Di Gioacchino, M. Systemic nickel allergy syndrome: Nosologic framework and usefulness of diet regimen for diagnosis. Int. J. Immunopathol. Pharmacol. 2013, 26, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Klostergaard, J.; Seeney, C.E. Magnetic nanovectors for drug delivery. Nanomedicine 2012, 8, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, L.M.; Delpech, F.; Nayral, C.; Lachaize, S.; Chaudret, B. New generation of magnetic and luminescent nanoparticles for in vivo real-time imaging. Interface Focus 2013, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Parkes, L.M.; Hodgson, R.; Lu, L.T.; Tung, L.D.; Robinson, I.; Fernig, D.G.; Thanh, N.T.K. Cobalt nanoparticles as a novel magnetic resonance contrast agent–relaxivities at 1.5 and 3 Tesla. Contrast Media Mol. Imaging 2008, 3, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Manea, C.M.; Rusu, M.C.; Constantin, D.; Mănoiu, V.M.; Moldovan, L.; Jianu, A.M. Ultrastructural features of human adipose-derived multipotent mesenchymal stromal cells. Rom. J. Morphol. Embryol. 2014, 55, 1363–1369. [Google Scholar] [PubMed]
- Lai, J.C.K.; Lai, M.B.; Jandhyam, S.; Dukhande, V.V.; Bhushan, A.; Daniels, C.K.; Leung, S.W. Exposure to titanium dioxide and other metallic oxide nanoparticles induces cytotoxicity on human neural cells and fibroblasts. Int. J. Nanomed. 2008, 3, 533–545. [Google Scholar]
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J.; et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Genchi, G.G.; Liakos, I.; Cappello, V.; Gemmi, M.; Athanassiou, A.; Mazzola, B.; Mattoli, V. Effects of cerium oxide nanoparticles on PC12 neuronal-like cells: Proliferation, differentiation, and dopamine secretion. Pharm. Res. 2013, 30, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, A.; Ahrén, M.; Gustafsson, H.; Abrikossova, N.; Warntjes, M.; Jönsson, J.I.; Uvdal, K.; Engström, M. Gd2O3 nanoparticles in hematopoietic cells for MRI contrast enhancement. Int. J. Nanomed. 2011, 6, 3233–3240. [Google Scholar] [CrossRef]
- Samberg, M.E.; Loboa, E.G.; Oldenburg, S.J.; Monteiro-Riviere, N. Silver nanoparticles do not influence stem cell differentiation but cause minimal toxicity. Nanomedicine 2012, 7, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Tautzenberger, A.; Lorenz, S.; Kreja, L.; Zeller, A.; Musyanovych, A.; Schrezenmeier, H.; Landfester, K.; Mailänder, V.; Ignatius, A. Effects of fluorescence-labelled nanoparticles on mesenchymal stem cell differentiation. Biomaterials 2010, 31, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Song, M.S.; Ryu, P.D.; Lam, A.T.; Joo, S.W.; Lee, S.Y. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/beta-catenin signaling pathway. Int. J. Nanomed. 2015, 10, 4383–4392. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Hagen, R.; Kleinsasser, N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 2011, 201, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, L.; Cao, G. Acute toxicity test of CuO nanoparticles using human mesenchymal stem cells. Toxicol. Mech. Methods 2014, 24, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Rudge, S.R.; Kurtz, T.L.; Vessely, C.R.; Catterall, L.G.; Williamson, D.L. Preparation, characterization, and performance of magnetic iron-carbon composite microparticles for chemotherapy. Biomaterials 2000, 21, 1411–1420. [Google Scholar] [CrossRef]
- Tan, Y.K.; Best, S.L.; Donnelly, C.; Olweny, E.; Kapur, P.; Mir, S.A.; Gnade, B.; McLeroy, S.; Pearle, M.S.; Cadeddu, J.A. Novel iron oxide microparticles used to render stone fragments paramagnetic: Assessment of toxicity in a murine model. J. Urol. 2012, 188, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Indech, R. Nanotechnological Processing of Catalytic Surfaces. U.S. Patent 20060115389 A1, 1 June 2006. [Google Scholar]
- Toghill, K.E.; Xiao, L.; Phillips, M.A.; Compton, R.G. The non-enzymatic determination of glucose using an electrolytically fabricated nickel microparticle modified boron-doped diamond electrode or nickel foil electrode. Sens. Actuators B 2010, 147, 642–652. [Google Scholar] [CrossRef]
- Ahamed, M. Toxic response of nickel nanoparticles in human lung epithelial A549 cells. Toxicol 2011, 25, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Greulich, C.; Diendorf, J.; Simon, T.; Eggeler, G.; Epple, M.; Köller, M. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater. 2011, 7, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Papis, E.; Rossi, F.; Raspanti, M.; Dalle-Donne, I.; Colombo, G.; Milzani, A.; Bernardini, G.; Gornati, R. Engineered cobalt oxide nanoparticles readily enter cells. Toxicol. Lett. 2009, 189, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Bossi, E.; Zanella, D.; Gornati, R.; Bernardini, G. Cobalt oxide nanoparticles can enter inside the cells by crossing plasma membranes. Sci. Rep. 2016, 6, 22254. [Google Scholar] [CrossRef] [PubMed]
- Petrarca, C.; Clemente, E.; Amato, V.; Pedata, P.; Sabbioni, E.; Bernardini, G.; Iavicoli, I.; Cortese, S.; Niu, Q.; Otsuki, T.; et al. Engineered metal based nanoparticles and innate immunity. Clin. Mol. Allergy 2015, 13, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, D.H.; Jang, J.; Kim, S.; Kang, T.; Lee, K.; Choi, I.H. The effects of sub-lethal concentrations of silver nanoparticles on inflammatory and stress genes in human macrophages using cDNA microarray analysis. Biomaterials 2012, 33, 4690–4699. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Kim, S.; Kim, J.S.; Choi, I.H. Inflammasome formation and IL-1β release by human blood monocytes in response to silver nanoparticles. Biomaterials 2012, 33, 6858–6867. [Google Scholar] [CrossRef] [PubMed]
- Macro, L.; Jaiswal, J.K.; Simon, S.M. Dynamics of clathrin-mediated endocytosis and its requirement for organelle biogenesis in Dictyostelium. J. Cell Sci. 2012, 125, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Hammacher, A.; Smith, D.K.; Matthews, J.M.; Ward, L.D. Interleukin-6: Structure-function relationships. Protein Sci. 1997, 6, 929–955. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signalling and therapy. Circ. Res. 2015, 103, 1204–1219. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Zannettino, A.C. Methods for the purification and characterization of human adipose-derived stem cells. Methods Mol. Biol. 2011, 702, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Bava, A.; Cappellini, F.; Pedretti, E.; Rossi, F.; Caruso, E.; Vismara, E.; Chiriva-Internati, M.; Bernardini, G.; Gornati, R. Heparin and carboxymethylchitosan metal nanoparticles: An evaluation of their cytotoxicity. BioMed Res. Int. 2013, 2013, 314091. [Google Scholar] [CrossRef] [PubMed]
- Bava, A.; Gornati, R.; Cappellini, F.; Caldinelli, L.; Pollegioni, L.; Bernardini, G. D-amino acid oxidase-nanoparticle system: A potential novel approach for cancer enzymatic therapy. Nanomedicine 2013, 8, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Ali, D.; Alhadlaq, H.A.; Akhtar, M.J. Nickel oxide nanoparticles exert cytotoxicity via oxidative stress and induce apoptotic response in human liver cells (HepG2). Chemosphere 2013, 93, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Bardack, S.; Dalgard, C.L.; Kalinich, J.F.; Kasper, C.E. Genotoxic changes to rodent cells exposed in Vitro to Tungsten. Nickel, Cobalt and Iron. Int. J. Environ. Res. Public Health 2014, 11, 2922–2940. [Google Scholar] [CrossRef] [PubMed]
- Defo, M.A.; Bernatchez, L.; Campbell, P.G.C.; Couture, P. Waterborne cadmium and nickel impact oxidative stress responses and retinoid metabolism in yellow perch. Aquat. Toxicol. 2014, 154, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, A.Y.; Campbell, J.L.; Dodd, D.E.; Oller, A.R.; Clewell, H.J. Time- and concentration-dependent genomic responses of the rat airway to inhaled nickel subsulfide. Toxicol. Appl. Pharmacol. 2014, 279, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Hussainzada, N.; Lewis, J.A.; Baer, C.E.; Ippolito, D.L.; Jackson, D.A.; Stallings, J.D. Whole adult organism transcriptional profiling of acute metal exposures in male zebrafish. BMC Pharmacol. Toxicol. 2014, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Palomäki, S.; Pietilä, M.; Laitinen, S.; Pesälä, J.; Sormunen, R.; Lehenkari, P.; Koivunen, P. HIF-1α is upregulated in human mesenchymal stem cells. Stem Cells 2013, 31, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.A.; Mooney, D.J. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials 2010, 31, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Palombella, S.; Pirrone, C.; Cherubino, M.; Valdatta, L.; Bernardini, G.; Gornati, R. Identification of reference genes for qPCR analysis during hASC long culture maintenance. PLoS ONE 2017, 12, e0170918. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Sequence 5′–3′ | Amplicon Leght (bp) | NCBI Accession Number | |
|---|---|---|---|---|
| ACTβ | Fw | ATGGGTCAGAAGGATTCC | 78 | NM_001101.3 |
| Rv | CTCGATGGGGTACTTCAG | |||
| B2M | Fw | CTATCCAGCGTACTCCAA | 93 | NM_004048.2 |
| Rv | GAAACCCAGACACATAGC | |||
| GAPDH | Fw | TTTGGCTACAGCAACAGG | 107 | NM_001289746.1 |
| Rv | GGTCTCTCTCTTCCTCTTG | |||
| RPL13A | Fw | TATGAGTGAAAGGGAGCC | 82 | NM_001270491.1 |
| Rv | ATGACCAGGTGGAAAGTC | |||
| RPS18 | Fw | GAGGTGGAACGTGTGATC | 109 | NM_022551.2 |
| Rv | GGACCTGGCTGTATTTTC | |||
| PPIA | Fw | AACCACCAGATCATTCCTT | 86 | NM_001300981.1 |
| Rv | GCGAGAGCACAAAGATTC | |||
| MT1A | Fw | CTCCTGCAAGAAGAGCTG | 87 | NM_005946.2 |
| Rv | TTCTCTGATGCCCCTTTG | |||
| Hsp70 | Fw | AGGCGGAGAAGTACAAAG | 85 | NM_005345.5 |
| Rv | ATGTTGAAGGCGTAGGAC | |||
| CAT | Fw | TACCCTCTCATCCCAGTT | 85 | NM_001752.3 |
| Rv | GGTCGAAGGCTATCTGTT | |||
| SOD | Fw | CAGATGACTTGGGCAAAG | 82 | NM_000454.4 |
| Rv | CCAATTACACCACAAGCC | |||
| P53 | Fw | CCACCATCCACTACAACT | 92 | NM_000546.5 |
| Rv | GGAGTCTTCCAGTGTGAT | |||
| CASP3 | Fw | GAGGCCGACTTCTTGTAT | 92 | NM_004346.3 |
| Rv | CAAAGCGACTGGATGAAC | |||
| BCL2 | Fw | CCTTCTTTGAGTTCGGTG | 98 | NM_000633.2 |
| Rv | CAGGTACTCAGTCATCCA | |||
| EGR1 | Fw | GCAGAAGGACAAGAAAGC | 94 | NM_001964.2 |
| Rv | CGGGTAAGAGGTAGCAAC | |||
| HIF1α | Fw | CAAGTCCTCAAAGCACAG | 75 | NM_001530.3 |
| Rv | TGGTAGTGGTGGCATTAG | |||
| VEGF | Fw | GGAGTCCAACATCACCAT | 80 | NM_001171623.1 |
| Rv | GCTGTAGGAAGCTCATCT | |||
| IL6 | Fw | ACTCACCTCTTCAGAACG | 113 | NM_000600.3 |
| Rv | CCTCTTTGCTGCTTTCAC | |||
| IL8 | Fw | GCCAAGGAGTGCTAAAGA | 103 | NM_000584.3 |
| Rv | TGGTCCACTCTCAATCAC | |||
| IL1b | Fw | CTACGAATCTCCGACCAC | 90 | NM_000576.2 |
| Rv | AACCAGCATCTTCCTCAG | |||
| AP2A1 | Fw | CTGGTGGAATGTCTGGAG | 117 | NM_014203.2 |
| Rv | GATGATGAGGCTGATGGT | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palombella, S.; Pirrone, C.; Rossi, F.; Armenia, I.; Cherubino, M.; Valdatta, L.; Raspanti, M.; Bernardini, G.; Gornati, R. Effects of Metal Micro and Nano-Particles on hASCs: An In Vitro Model. Nanomaterials 2017, 7, 212. https://doi.org/10.3390/nano7080212
Palombella S, Pirrone C, Rossi F, Armenia I, Cherubino M, Valdatta L, Raspanti M, Bernardini G, Gornati R. Effects of Metal Micro and Nano-Particles on hASCs: An In Vitro Model. Nanomaterials. 2017; 7(8):212. https://doi.org/10.3390/nano7080212
Chicago/Turabian StylePalombella, Silvia, Cristina Pirrone, Federica Rossi, Ilaria Armenia, Mario Cherubino, Luigi Valdatta, Mario Raspanti, Giovanni Bernardini, and Rosalba Gornati. 2017. "Effects of Metal Micro and Nano-Particles on hASCs: An In Vitro Model" Nanomaterials 7, no. 8: 212. https://doi.org/10.3390/nano7080212






