Facile synthesis of Mesoporouscobalt Hexacyanoferrate Nanocubes for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Discussion and Results
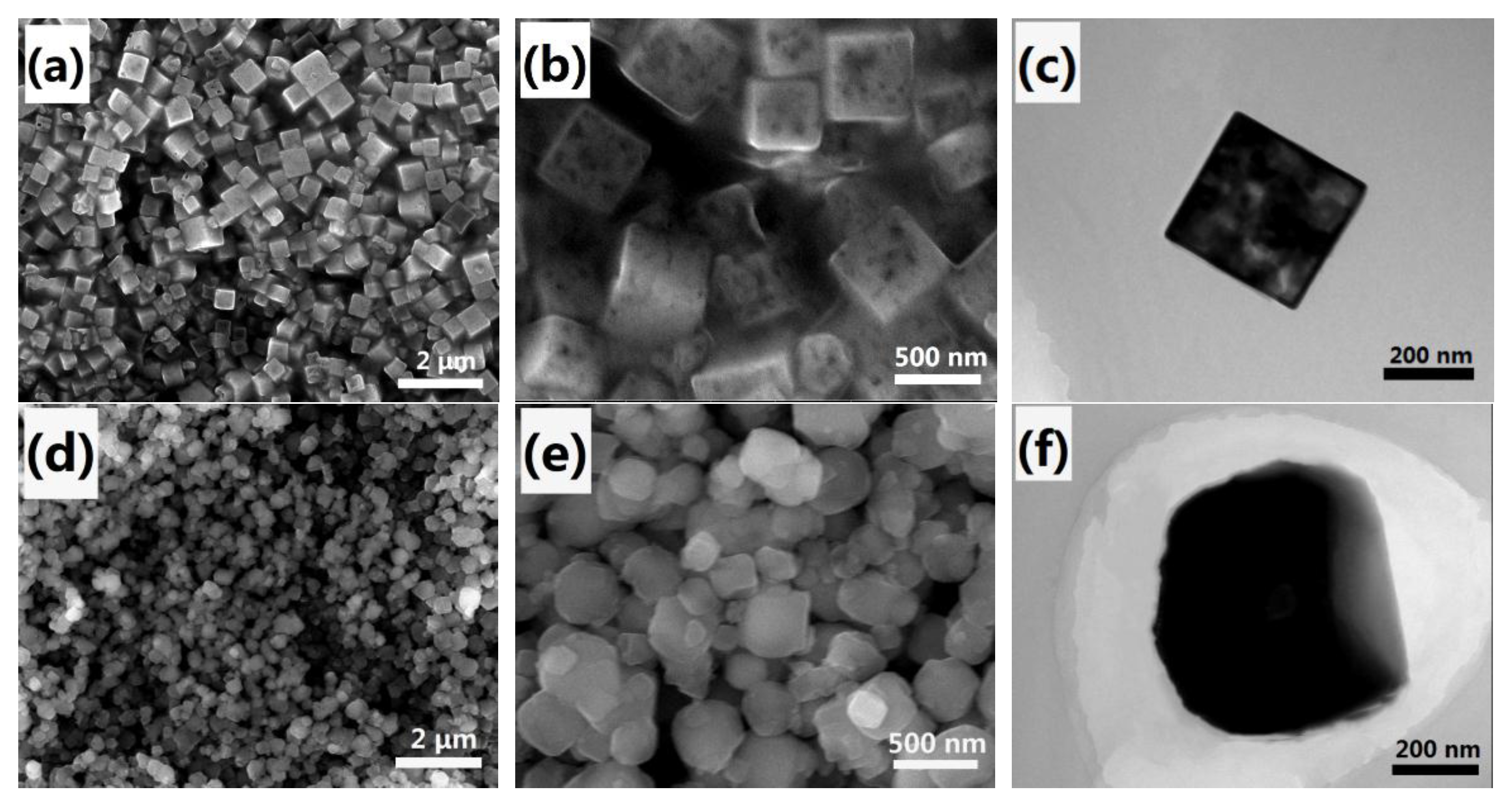
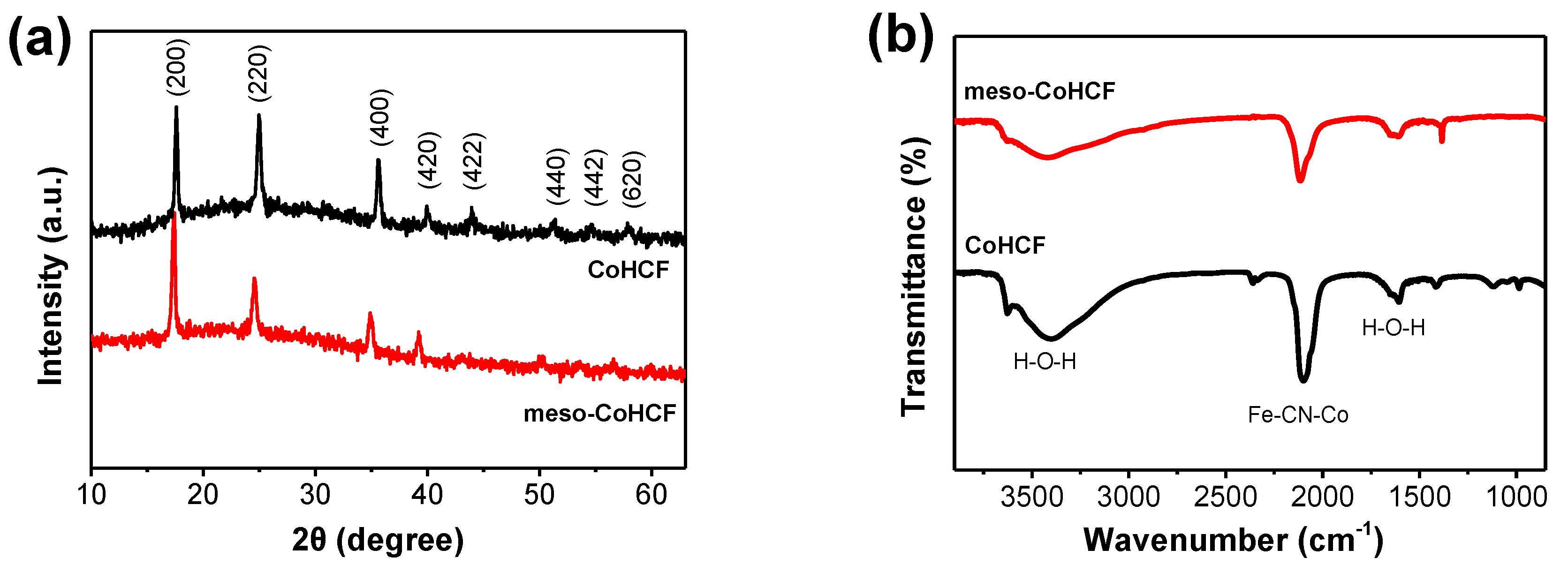
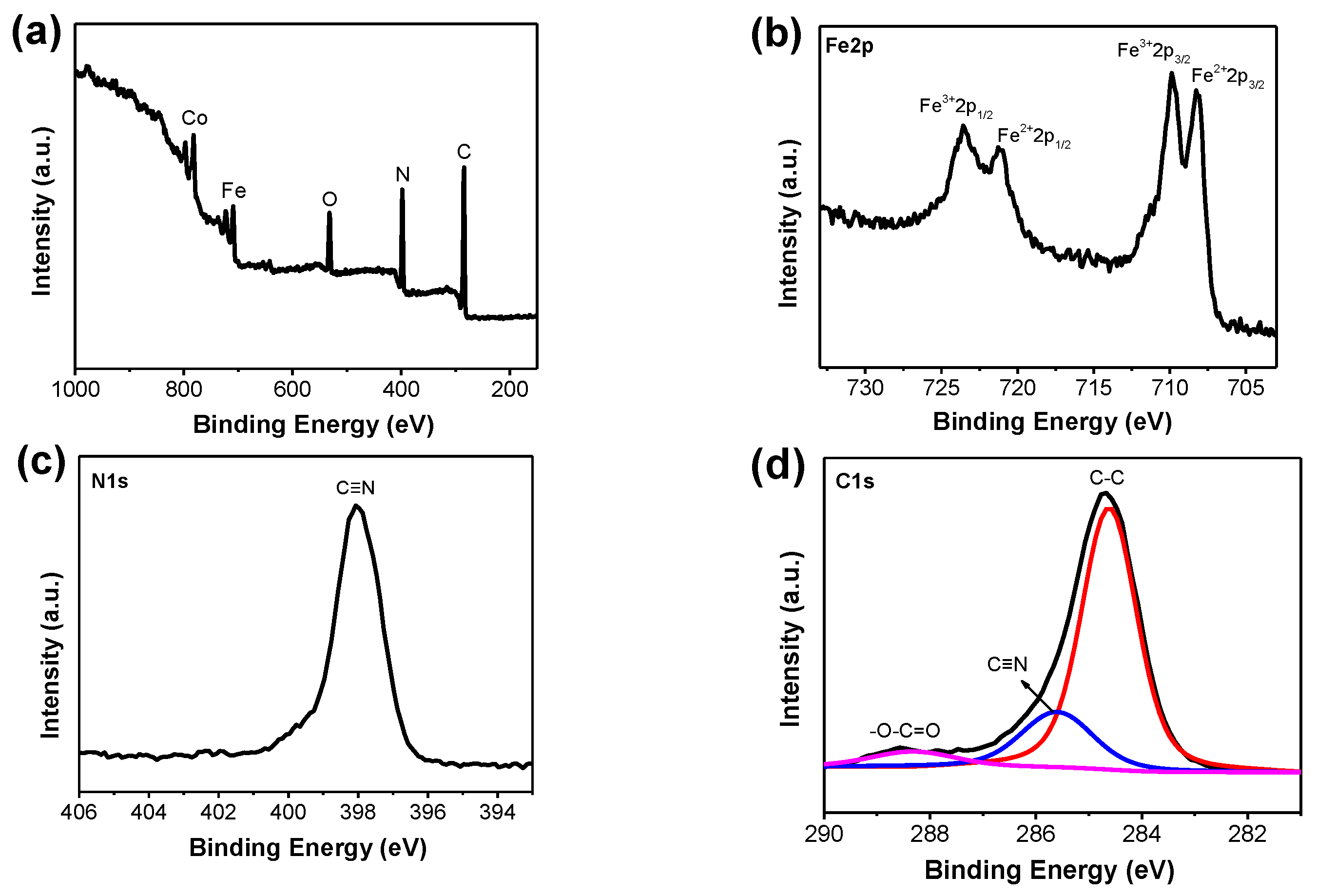
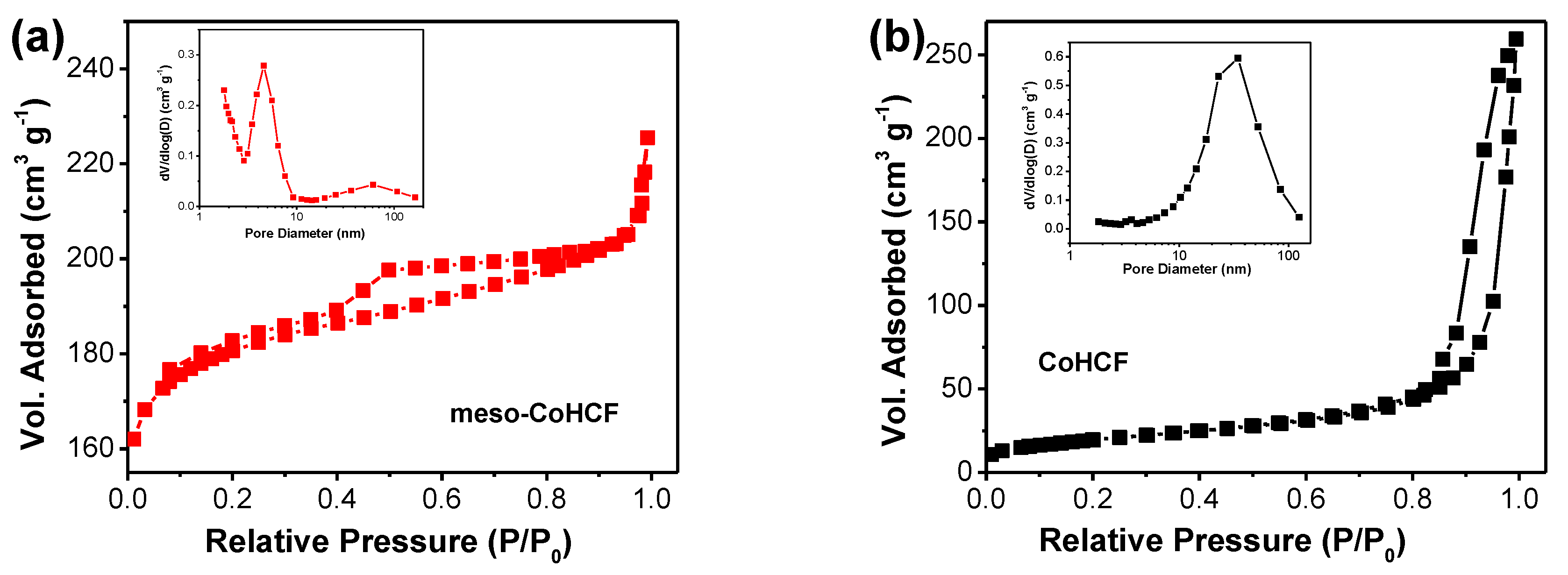
2.1. Characterization of Meso–CoHCF
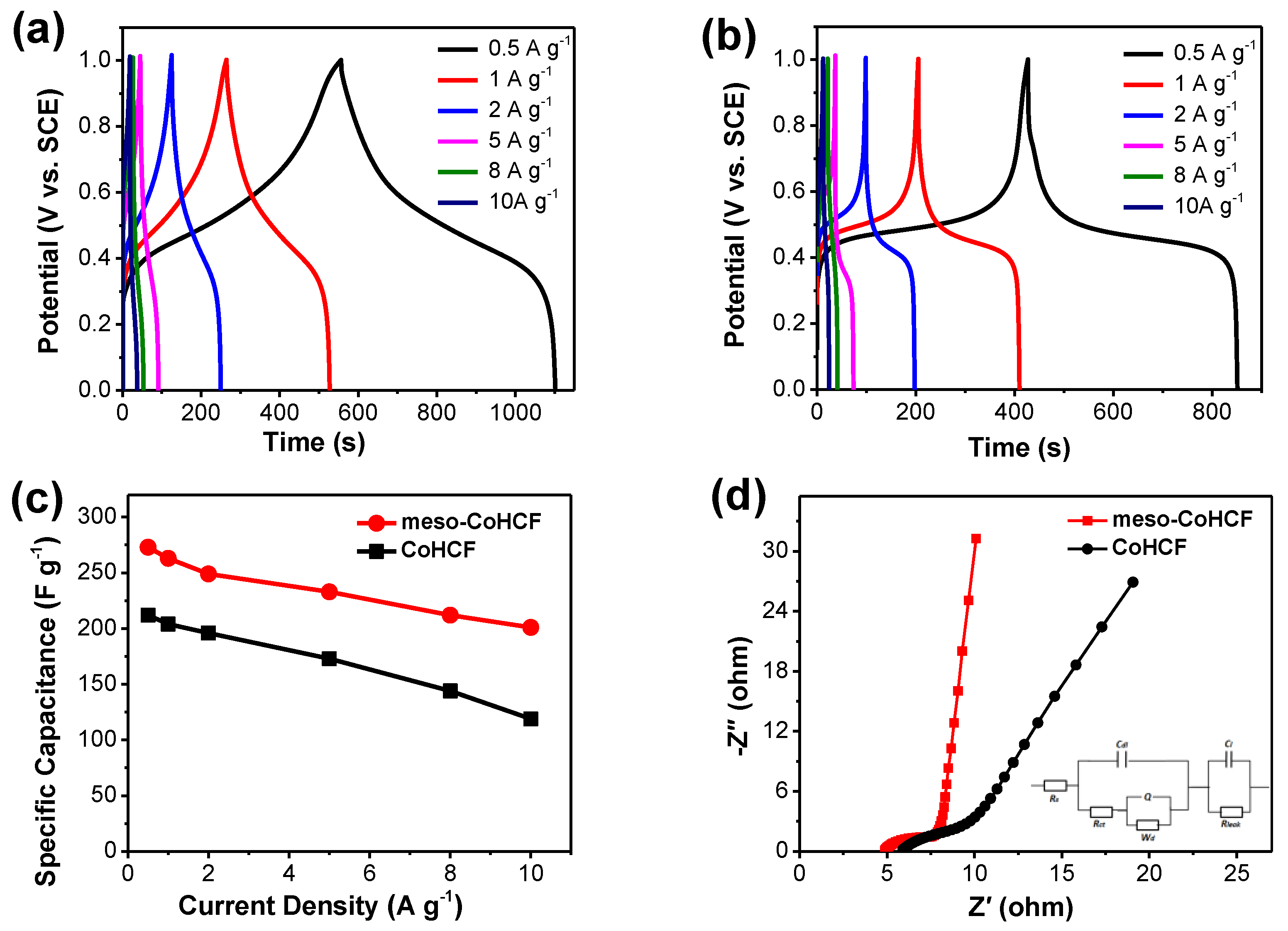
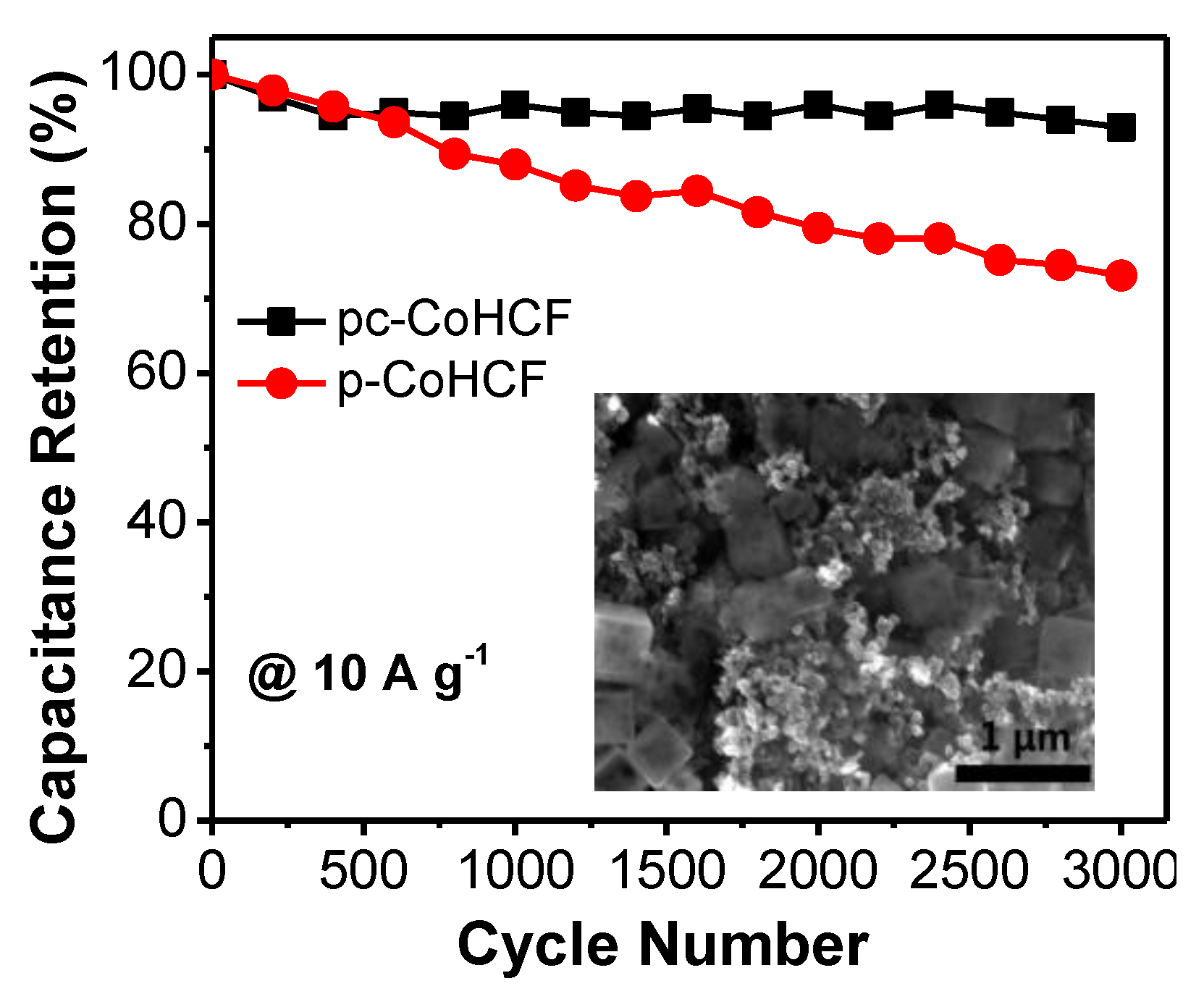
2.2. Electrochemical Performance
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.; Kang, F.; Wei, B. Engineering of MnO2-based nanocomposites for high-performance supercapacitors. Prog. Mater. Sci. 2015, 74, 51–124. [Google Scholar] [CrossRef]
- Li, H.; Yu, M.; Wang, F.; Liu, P.; Liang, Y.; Xiao, J.; Wang, C.; Tong, Y.; Yang, G. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat. Commun. 2013, 4, 1894. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Nitrogen-doped activated carbon for a high energy hybrid supercapacitor. Energ. Environ. Sci. 2016, 9, 102–106. [Google Scholar] [CrossRef]
- Masarapu, C.; Zeng, H.; Wei, B. Effect of temperature on the capacitance of carbon nanotube supercapacitors. ACS Nano 2009, 3, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-G.; Zhang, C.; Jin, D.; Xie, K.; Wei, B. Synthesis of ultralong MnO/C coaxial nanowires as freestanding anodes for high-performance lithium ion batteries. J. Mater. Chem. A 2015, 3, 13699–13705. [Google Scholar] [CrossRef]
- Peng, Z.; Lin, J.; Ye, R.; Samuel, E.; Tour, J. Flexible and stackable laser-induced graphene supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3414–3419. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-G.; Jin, D.; Zhou, R.; Shen, C.; Xie, K.; Wei, B. One-step synthesis of NiCo2S4 ultrathin nanosheets on conductive substrates as advanced electrodes for high-efficient energy storage. J. Power Sources 2016, 306, 100–106. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.; Chen, M.; Yan, Y.; Wu, L. Nickel-cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials. Adv. Funct. Mater. 2014, 24, 934–942. [Google Scholar] [CrossRef]
- Yue, Y.; Binder, A.; Guo, B.; Zhang, Z.; Qiao, Z.; Tian, C.; Dai, S. Mesoporous prussian blue analogues: Template-free synthesis and sodium-ion battery applications. Angew. Chem. Int. Edit. 2014, 53, 3134–3137. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-G.; Jin, D.; Liu, H.; Zhang, C.; Zhou, R.; Shen, C.; Xie, K.; Wei, B. All-manganese-based Li-ion batteries with high rate capability and ultralong cycle life. Nano Energy 2016, 22, 524–532. [Google Scholar] [CrossRef]
- Liu, S.; Pan, G.; Li, G.; Gao, X. Copper hexacyanoferrate nanoparticles as cathode material for aqueous Al-ion batteries. J. Mater. Chem. A 2015, 3, 959–962. [Google Scholar] [CrossRef]
- Su, D.; McDonagh, A.; Qiao, S.; Wang, G. High-capacity aqueous potassium-ion batteries for large-scale energy storage. Adv. Mater. 2017, 29, 1604007. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Selomulya, C.; Zheng, G.; Zhao, D. New faces of porous prussian blue: Interfacial assembly of integrated hetero-structures for sensing applications. Chem. Soc. Rev. 2015, 44, 7997–8018. [Google Scholar] [CrossRef] [PubMed]
- Shiba, F.; Fujishiro, R.; Kojima, T.; Okawa, Y. Preparation of monodisperse cobalt(II) hexacyanoferrate(III) nanoparticles using cobalt ions released from a citrate complex. J. Phys. Chem. C 2012, 116, 3394–3399. [Google Scholar] [CrossRef]
- Subramani, K.; Jeyakumar, D.; Sathish, M. Manganese hexacyanoferrate derived Mn3O4 nanocubes-reduced graphene oxide nanocomposites and their charge storage characteristics in supercapacitors. Phys. Chem. Chem. Phys. 2014, 16, 4952–4961. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Okubo, M.; Hosono, E.; Kudo, T.; Oh-ishi, K.; Okazawa, A.; Kojima, N.; Kurono, R.; Nishimura, S.; Yamada, A. Electrochemical Mg2+ intercalation into a bimetallic CuFe prussian blue analog in aqueous electrolytes. J. Mater. Chem. A 2013, 1, 13055–13059. [Google Scholar] [CrossRef]
- You, Y.; Wu, X.; Yin, Y.; Guo, Y. High-quality prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energy Rnviron. Sci. 2014, 7, 1643–1647. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Q.; Xiang, C.; She, Z.; Chu, H.; Qiu, S.; Xu, F.; Liu, S.; Tang, C.; Sun, L. One-pot synthesis of ternary polypyrrole–prussian-blue–graphene-oxide hybrid composite as electrode material for high-performance supercapacitors. Electrochim. Acta 2016, 188, 126–134. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Liu, X.; Wei, B. Facile synthesis of cobalt hexacyanoferrate/graphene nanocomposites for high-performance supercapacitor. Electrochim. Acta 2017, 235, 114–121. [Google Scholar] [CrossRef]
- Moo Lee, K.; Tanaka, H.; Ho Kim, K.; Kawamura, M.; Abe, Y.; Kawamoto, T. Improvement of redox reactions by miniaturizing nanoparticles of zinc prussian blue analog. Appl.Phys.Lett. 2013, 102, 141901–141903. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, H.; Hu, L.; Yan, N.; Hu, H.; Chen, Q. Manganese hexacyanoferrate/MnO2 composite nanostructures as a cathode material for supercapacitors. J. Mater. Chem. A 2013, 1, 2621–2630. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q. Dual-Layer-Structured nickel hexacyanoferrate/MnO2 composite as a high-energy supercapacitive material based on the complementarity and interlayer concentration enhancement effect. ACS Appl. Mater. Interfaces 2014, 6, 6196–6201. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Zhang, Z.; Binder, A. Hierarchically superstructured prussian blue analogues: Spontaneous assembly synthesis and applications as pseudocapacitive materials. ChemSusChem 2015, 8, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, Y.; Xu, X.; Liu, Y.; Song, R.; Lu, G.; Li, Y. Cobalt hexacyanoferrate nanoparticles as a high-rate and ultra-stable supercapacitor electrode material. ACS Appl. Mater. Interfaces. 2014, 6, 11007–11012. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Fulvio, P.F.; Dai, S. Hierarchical Metal–Organic Framework Hybrids: Perturbation-Assisted Nanofusion Synthesis. Acc. Chem. Res. 2015, 48, 3044–3052. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Song, B.; Gao, X.; Dai, H.; Zhang, J.; Ma, H. High-energy cobalt hexacyanoferrate and carbon micro-spheres aqueous sodium-ion capacitors. J. Power Sources 2016, 303, 347–353. [Google Scholar] [CrossRef]
- Wang, J.-G.; Zhang, Z.; Zhang, X.; Yin, X.; Li, X.; Liu, X.; Kang, F.; Wei, B. Cation exchange formation of prussian blue analogue submicroboxes for high-performance Na-ion hybrid supercapacitors. Nano Energy 2017, 39, 647–653. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Y.; Sun, M.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Low-defect prussian blue nanocubes as high capacity and long life cathodes for aqueous Na-ion batteries. Nano Energy 2015, 13, 117–123. [Google Scholar] [CrossRef]
- Luo, X.; Pan, J.; Pan, K.; Yu, Y.; Zhong, A.; Wei, S.; Li, J.; Shi, J.; Li, X. An electrochemical sensor for hydrazine and nitrite based on graphene–cobalt hexacyanoferrate nanocomposite: Toward environment and food detection. J. Electroanal. Chem. 2015, 745, 80–87. [Google Scholar] [CrossRef]
- Luo, M.; Dou, Y.; Kang, H.; Ma, Y.; Ding, X.; Liang, B.; Ma, B.; Li, L. A novel interlocked prussian blue/reduced graphene oxide nanocomposites as high-performance supercapacitor electrodes. J. Solid State Electrochem. 2015, 19, 1621–1631. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Huang, Z.; Kang, F. Coaxial carbon nanofibers/MnO2 nanocomposites as freestanding electrodes for high-performance electrochemical capacitors. Electrochim. Acta 2011, 56, 9240–9247. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Huang, Z. A high-performance asymmetric supercapacitor based on carbon and carbon–MnO2 nanofiber electrodes. Carbon 2013, 61, 190–199. [Google Scholar] [CrossRef]
- Wang, J.; Jin, D.; Zhou, R.; Li, X.; Liu, X.; Shen, C.; Xie, K.; Li, B.; Kang, F.; Wei, B. Highly flexible graphene/Mn3O4 nanocomposite membrane as advanced anodes for Li-ion batteries. ACS Nano 2016, 10, 6227–6234. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Chu, D.; Li, S. Ethanol-directed morphological evolution of hierarchical CeOx architectures as advanced electrochemical capacitors. J. Mater. Chem. A 2015, 3, 13970–13977. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, J.-G.; Wei, B. Facile synthesis of Mesoporouscobalt Hexacyanoferrate Nanocubes for High-Performance Supercapacitors. Nanomaterials 2017, 7, 228. https://doi.org/10.3390/nano7080228
Zhang Z, Wang J-G, Wei B. Facile synthesis of Mesoporouscobalt Hexacyanoferrate Nanocubes for High-Performance Supercapacitors. Nanomaterials. 2017; 7(8):228. https://doi.org/10.3390/nano7080228
Chicago/Turabian StyleZhang, Zhiyong, Jian-Gan Wang, and Bingqing Wei. 2017. "Facile synthesis of Mesoporouscobalt Hexacyanoferrate Nanocubes for High-Performance Supercapacitors" Nanomaterials 7, no. 8: 228. https://doi.org/10.3390/nano7080228




