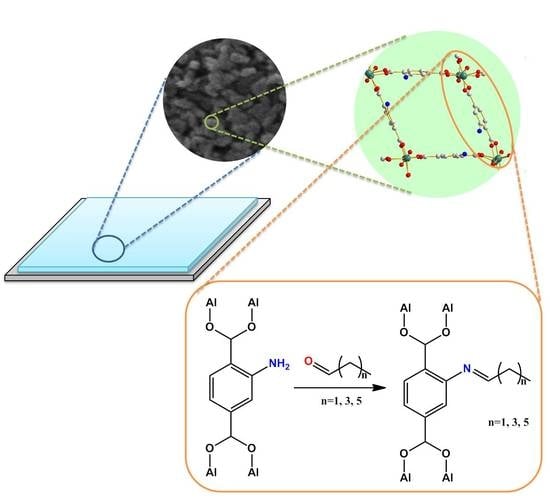
The Tuning of Optical Properties of Nanoscale MOFs-Based Thin Film through Post-Modification
Abstract
:1. Introduction
2. Experimental and Computational Method
2.1. Materials
2.2. Preparation of NH2-MIL-53(Al) Optical Thin Film
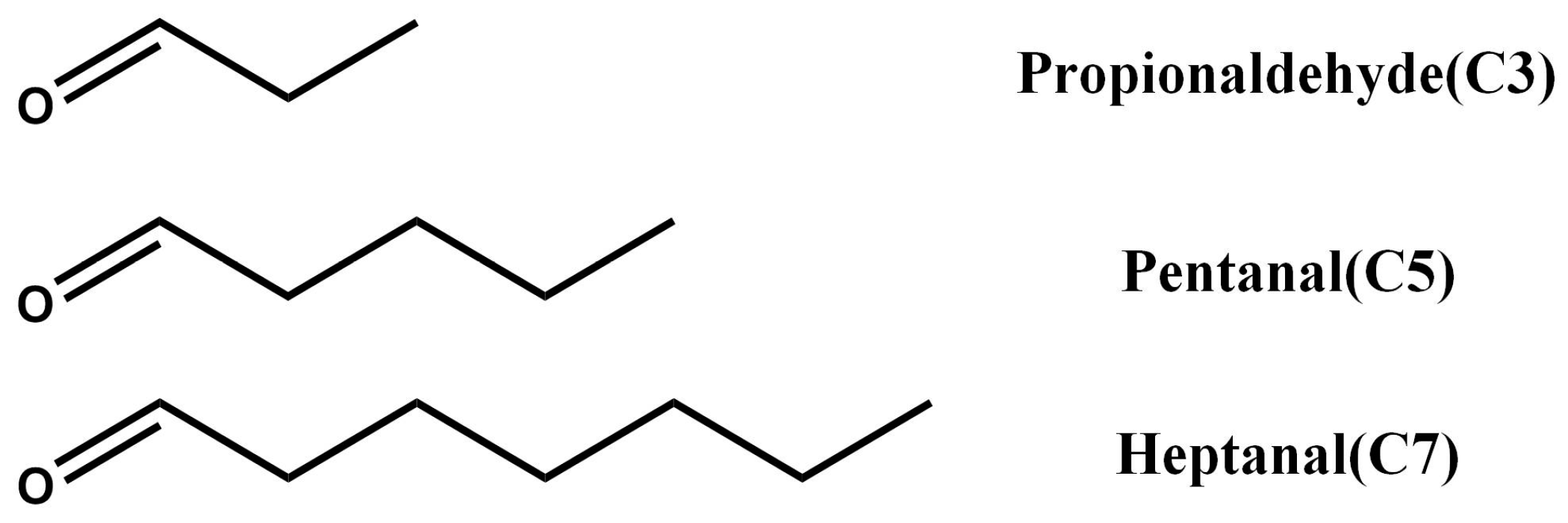
2.3. Post-Modification of NH2-MIL-53(Al) Optical Thin Film
2.4. Characterization
3. Results and Discussion
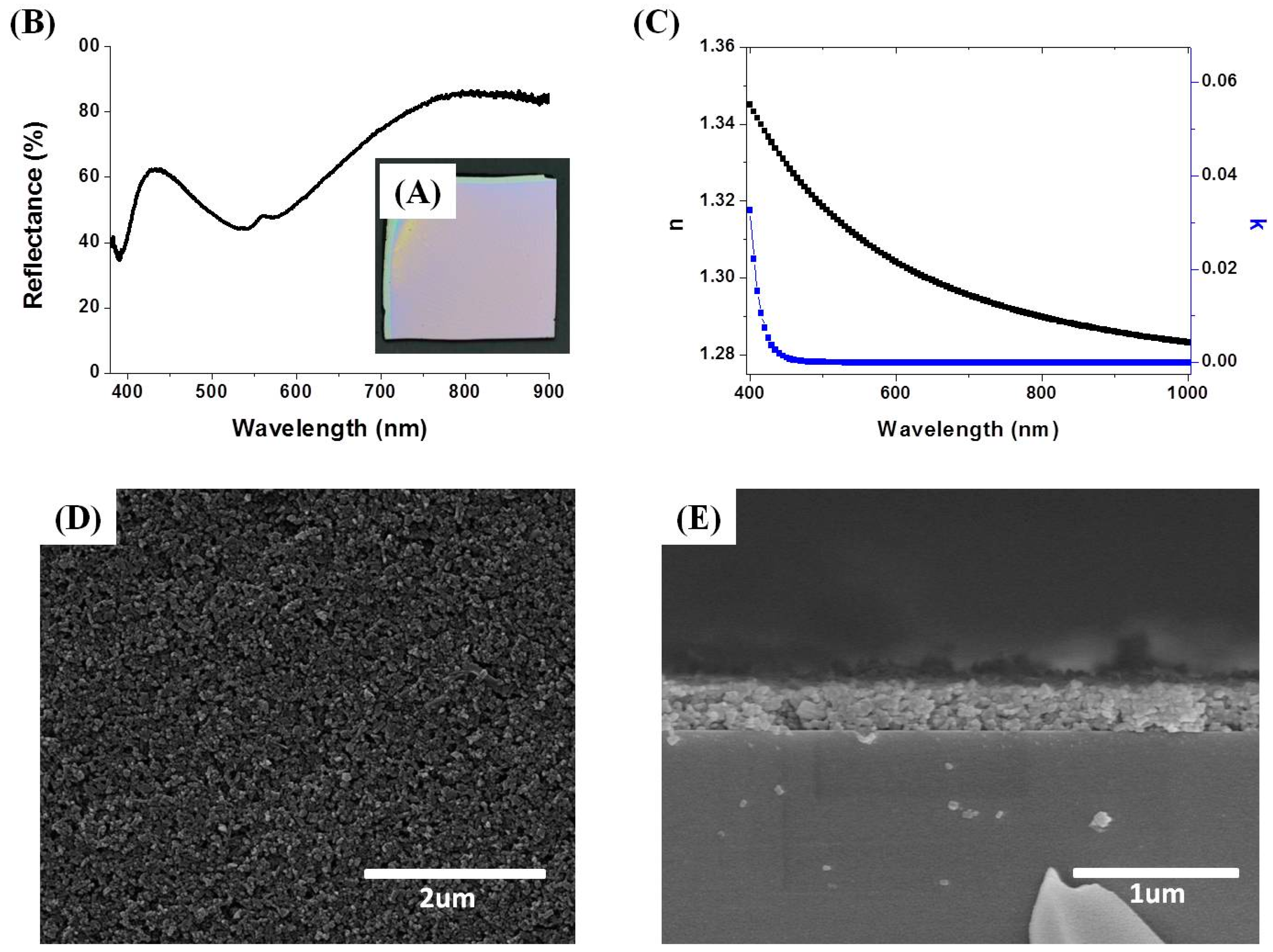
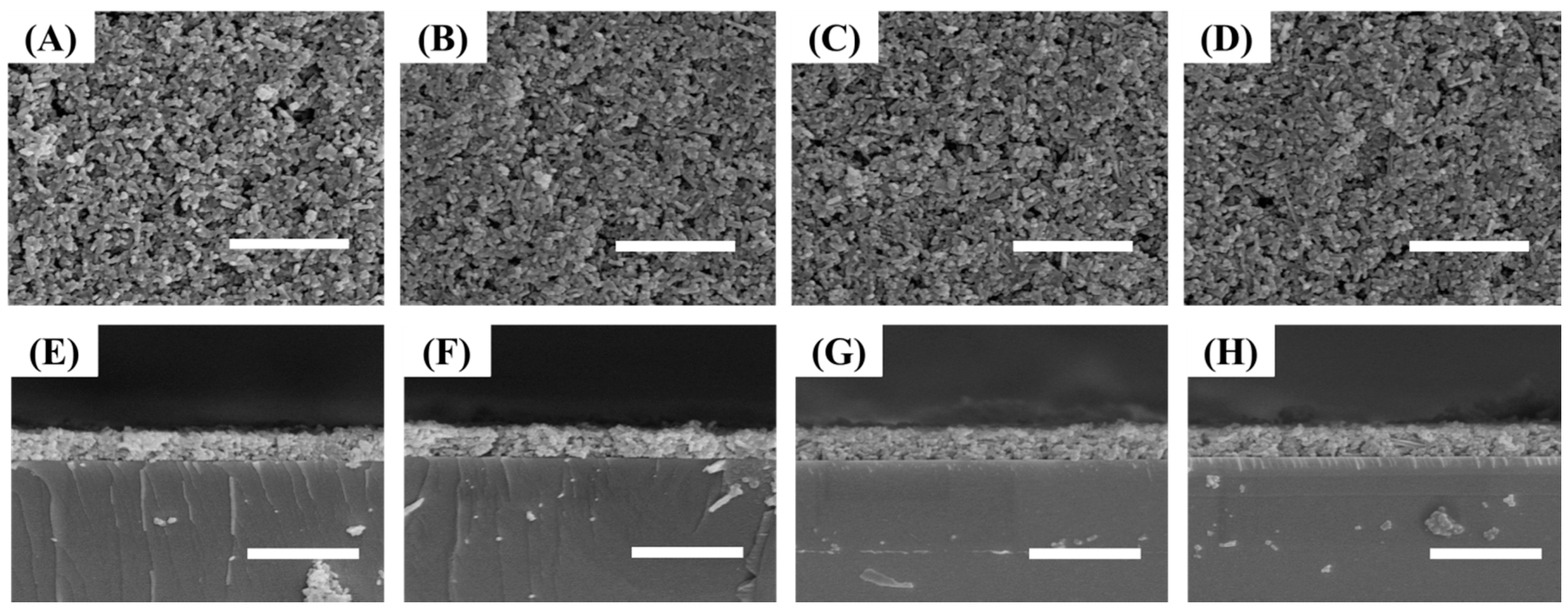
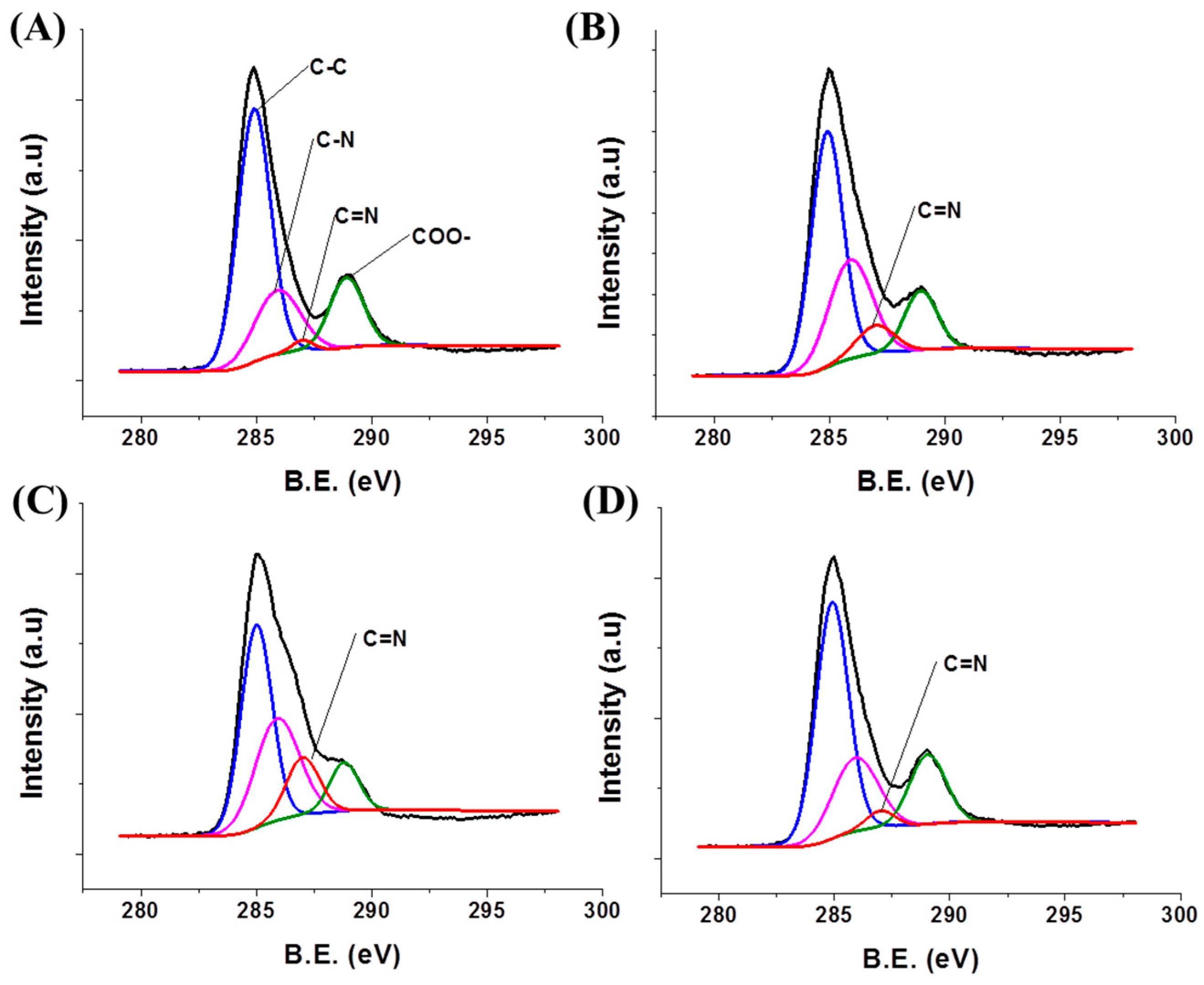
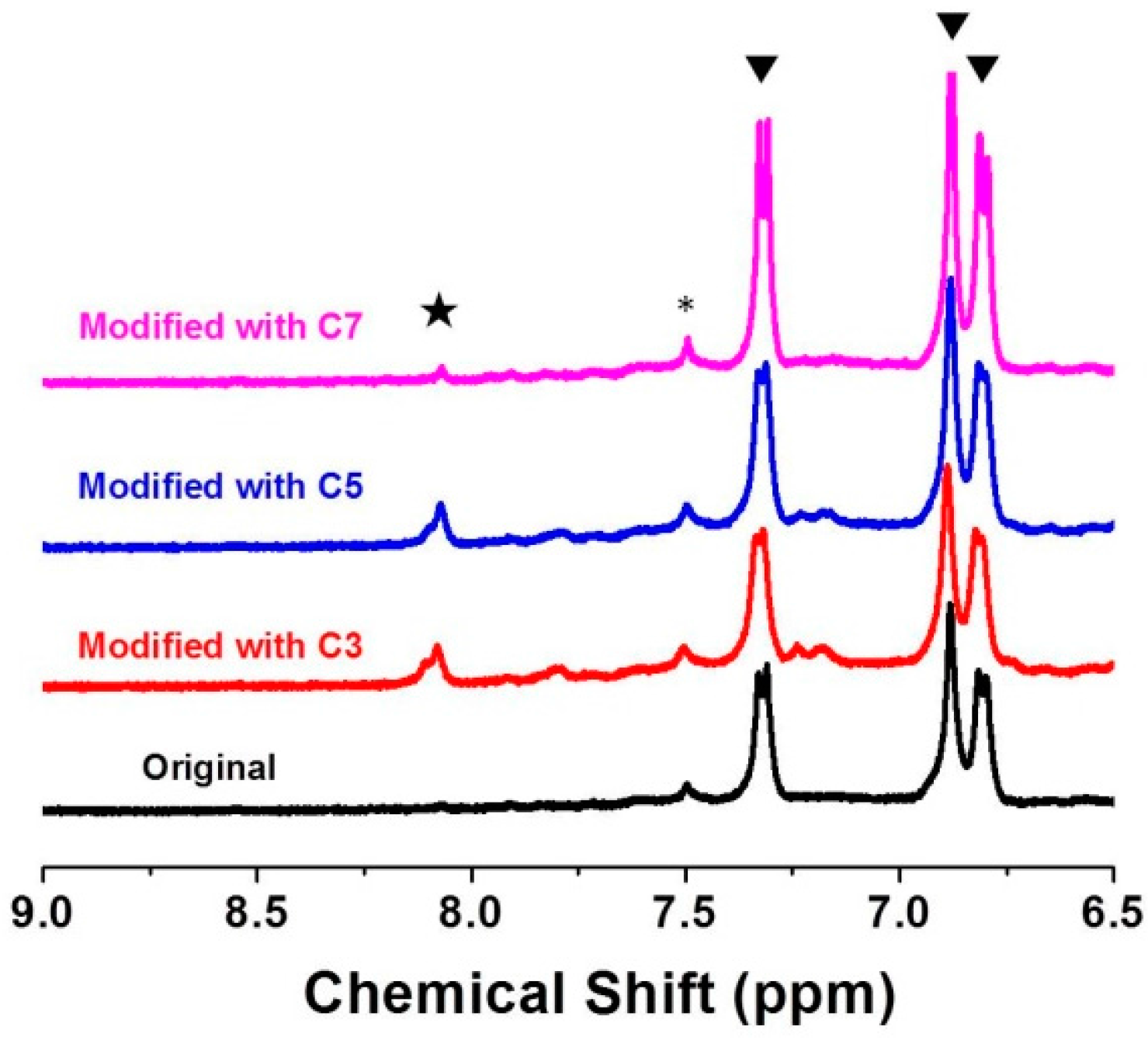
3.1. Preparation and Characterization NH2-MIL-53(Al) NRs
3.2. Preparation of NH2-MIL-53(Al) Optical Thin Film
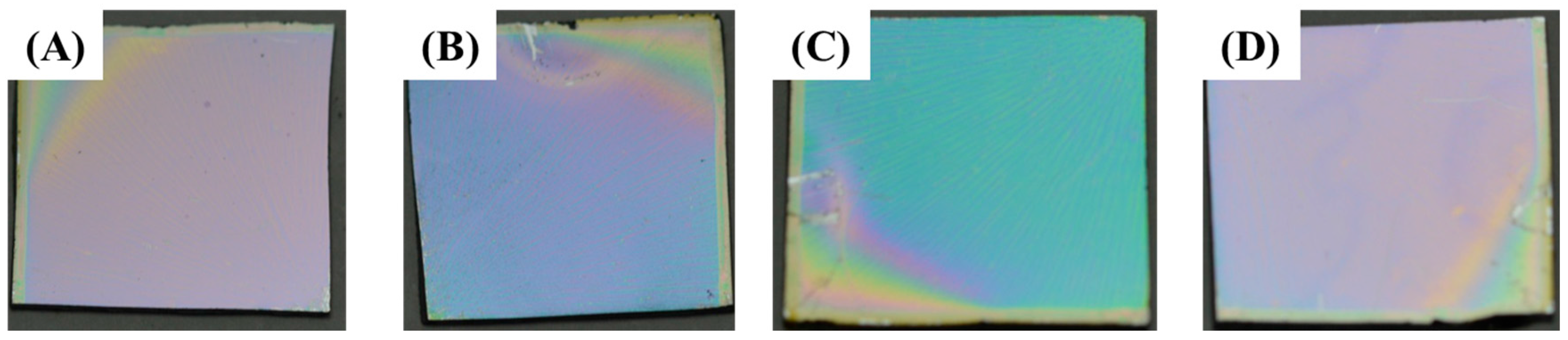
3.3. Tuning of Optical Properties of NH2-MIL-53(Al) Optical Thin Film
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hendon, C.H.; Rieth, A.J.; Korzyński, M.D.; Dincă, M. Grand challenges and future opportunities for metal-organic frameworks. ACS Cent. Sci. 2017, 3, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Sindoro, M.; Yanai, N.; Jee, A.Y.; Granick, S. Colloidal-sized metal-organic frameworks: Synthesis and applications. Acc. Chem. Res. 2014, 47, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Ko, N.; Yong, B.G.; Aratani, N.; Sang, B.C.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Ma, S.; Yuan, D.; Yoon, J.W.; Hwang, Y.K.; Chang, J.S.; Wang, X.; Jørgensen, M.R.; Chen, Y.S.; Zhou, H.C. A large-surface-area boracite-network-topology porous MOF constructed from a conjugated ligand exhibiting a high hydrogen uptake capacity. Inorg. Chem. 2009, 48, 7519–7521. [Google Scholar] [CrossRef] [PubMed]
- Marx, S.; Kleist, W.; Huang, J.; Maciejewski, M.; Baiker, A. Tuning functional sites and thermal stability of mixed-linker MOFs based on MIL-53(Al). Dalton Trans. 2010, 39, 3795–3798. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. Postsynthetic methods for the functionalization of metal-organic frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef] [PubMed]
- Mckinlay, A.C.; Xiao, B.; Wragg, D.S.; Wheatley, P.S.; Megson, I.L.; Morris, R.E. Exceptional behavior over the whole adsorption-storage-delivery cycle for no in porous metal organic frameworks. J. Am. Chem. Soc. 2008, 130, 10440–10444. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, L.; Amer, W.A.; Yu, H.; Ji, J.; Huang, L.; Shan, J.; Tong, R. Hydrogen storage in metal-organic frameworks. J. Inorg. Organomet. Polym. Mater. 2013, 23, 270–285. [Google Scholar] [CrossRef]
- Lykourinou, V.; Chen, Y.; Wang, X.S.; Meng, L.; Hoang, T.; Ming, L.J.; Musselman, R.L.; Ma, S. Immobilization of MP-11 into a mesoporous metal-organic framework, MP-11@mesoMOF: A new platform for enzymatic catalysis. J. Am. Chem. Soc. 2011, 133, 10382–10385. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Yang, Y.W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Gautier, R.; Jiang, T.; Faulques, E.; Latouche, C.; Paris, M.; Cario, L.; Bujoli-Doeuff, M.; Jobic, S. A p-type zinc-based metal–organic framework. Inorg. Chem. 2017, 56, 6208–6213. [Google Scholar] [CrossRef] [PubMed]
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An updated roadmap for the integration of metal-organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef] [PubMed]
- Hinterholzinger, F.M.; Ranft, A.; Feckl, J.M.; Rühle, B.; Bein, T.; Lotsch, B.V. One-dimensional metal-organic framework photonic crystals used as platforms for vapor sorption. J. Mater. Chem. 2012, 22, 10356–10362. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zheng, H.; Wei, X.; Lin, Z.; Guo, L.; Qiu, B.; Chen, G. Metal-organic framework (MOF): A novel sensing platform for biomolecules. Chem. Commun. 2012, 49, 1276–1278. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.N.; Li, F.; Zhu, W.; Cui, J.; Tao, C.A.; Lin, C.; Hannam, P.M.; Li, G. Metal-organic frameworks with a three-dimensional ordered macroporous structure: Dynamic photonic materials. Angew.Chem. Int. Ed. 2011, 50, 12518–12522. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Hupp, J.T. Metal-organic frameworks as sensors: A ZIF-8 based Fabry-Pérot device as a selective sensor for chemical vapors and gases. J. Am. Chem. Soc. 2010, 132, 7832–7833. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tao, C.; Wang, F.; Zou, X.; Wang, J. Flexible metal-organic framework-based one-dimensional photonic crystals. J. Mater. Chem. C 2015, 3, 211–216. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, H.; Chu, T.; Zhang, G.; Wang, Y.; Yang, Y. A lanthanide MOF thin-film fixed with Co3O4 nano-anchors as a highly efficient luminescent sensor for nitrofuran antibiotics. Chem. Eur. J. 2017, 23, 10293–10300. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fang, R.; Huang, C.; Li, Y. Dual amplifying fluorescence anisotropy for detection of respiratory syncytial virus DNA fragments with size-control synthesized metal–organic framework MIL-101. RSC Adv. 2015, 5, 46301–46306. [Google Scholar] [CrossRef]
- Li, D.J.; Gu, Z.G.; Vohra, I.; Kang, Y.; Zhu, Y.S.; Zhang, J. Epitaxial growth of oriented metalloporphyrin network thin film for improved selectivity of volatile organic compounds. Small 2017, 13, 1604035. [Google Scholar] [CrossRef] [PubMed]
- Zacher, D.; Shekhah, O.; Wöll, C.; Fischer, R.A. Thin films of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Bétard, A.; Fischer, R.A. Metal-organic framework thin films: From fundamentals to applications. Chem. Rev. 2012, 112, 1055–1083. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wöll, C. Surface-supported metal-organic framework thin films: Fabrication methods, applications, and challenges. Chem. Soc. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Wannapaibooon, S.; Sumida, K.; Dilchert, K.; Tu, M.; Kitagawa, S.; Furukawa, S.; Fischer, R.A. Enhanced properties of metal-organic framework thin-films fabricated via a coordination modulation-controlled layer-by-layer process. J. Mater. Chem. A 2017, 5, 13665–13673. [Google Scholar] [CrossRef]
- Fu, Z.; Xu, G. Crystalline, highly oriented mof thin film: The fabrication and application. Chem. Rec. 2017, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Grosso, D.; Boissière, C.; Perruchas, S.; Sanchez, C.; Férey, G. Colloidal route for preparing optical thin films of nanoporous metal–organic frameworks. Adv. Mater. 2010, 21, 1931–1935. [Google Scholar] [CrossRef]
- Demessence, A.; Horcajada, P.; Serre, C.; Boissière, C.; Grosso, D.; Sanchez, C.; Férey, G. Elaboration and properties of hierarchically structured optical thin films of MIL-101(Cr). Chem. Commun. 2009, 7149–7151. [Google Scholar] [CrossRef] [PubMed]
- Márquez, A.G.; Demessence, A.; Platero-Prats, A.E.; Heurtaux, D.; Horcajada, P.; Serre, C.; Chang, J.S.; Férey, G.; Peña-O’Shea, V.A.D.L.; Boissière, C. Green microwave synthesis of MIL-100 (Al, Cr, Fe) nanoparticles for thin-film elaboration. Eur. J. Inorg. Chem. 2012, 5165–5174. [Google Scholar] [CrossRef]
- Redel, E.; Wang, Z.; Walheim, S.; Liu, J.; Gliemann, H.; Wöll, C. On the dielectric and optical properties of surface-anchored metal-organic frameworks: A study on epitaxially grown thin films. Appl. Phys. Lett. 2013, 103, 199–204. [Google Scholar] [CrossRef]
- Ranft, A.; Niekiel, F.; Pavlichenko, I.; Stock, N.; Lotsch, B.V. Tandem MOF-based photonic crystals for enhanced analyte-specific optical detection. Chem. Mater. 2015, 27, 1961–1970. [Google Scholar] [CrossRef]
- Liu, J.; Redel, E.; Walheim, S.; Wang, Z.; Oberst, V.; Liu, J.; Heissler, S.; Welle, A.; Moosmann, M.; Scherer, T. Monolithic high performance surface anchored metal-organic framework bragg reflector for optical sensing. Chem. Mater. 2015, 27, 1991–1996. [Google Scholar] [CrossRef]
- Hu, Z.; Tao, C.; Liu, H.; Zou, X.; Zhu, H.; Wang, J. Fabrication of an NH2-MIL-88B photonic film for naked-eye sensing of organic vapors. J. Mater. Chem. 2014, 2, 14222–14227. [Google Scholar] [CrossRef]
- Müller, K.; Wadhwa, J.; Singh, M.J.; Schöttner, L.; Welle, A.; Schwartz, H. Photoswitchable nanoporous films by loading azobenzene in metal-organic frameworks of type HKUST-1. Chem. Commun. 2017, 53, 8070–8073. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.C.; Chen, E.Y.; Menon, A.G.; Tan, H.Y.; Hor, A.T.S.; Schreyer, M.K.; Xu, J. Tuning the aspect ratio of NH2-MIL-53(Al) microneedles and nanorods via coordination modulation. CrystEngComm 2012, 15, 654–657. [Google Scholar]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Férey, G. A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chemistry 2004, 10, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Seoane, B.; Téllez, C.; Coronas, J.; Staudt, C. NH2-MIL-53(Al) and NH2-MIL-101(Al) in sulfur-containing copolyimide mixed matrix membranes for gas separation. Sep. Purif. Technol. 2013, 111, 72–81. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, W.; Tao, C.-a.; Zou, X.; Wang, F.; Qu, T.; Wang, J. The Tuning of Optical Properties of Nanoscale MOFs-Based Thin Film through Post-Modification. Nanomaterials 2017, 7, 242. https://doi.org/10.3390/nano7090242
Yin W, Tao C-a, Zou X, Wang F, Qu T, Wang J. The Tuning of Optical Properties of Nanoscale MOFs-Based Thin Film through Post-Modification. Nanomaterials. 2017; 7(9):242. https://doi.org/10.3390/nano7090242
Chicago/Turabian StyleYin, Wenchang, Cheng-an Tao, Xiaorong Zou, Fang Wang, Tianlian Qu, and Jianfang Wang. 2017. "The Tuning of Optical Properties of Nanoscale MOFs-Based Thin Film through Post-Modification" Nanomaterials 7, no. 9: 242. https://doi.org/10.3390/nano7090242