A General Route for Growing Metal Sulfides onto Graphene Oxide and Exfoliated Graphite Oxide
Abstract
:1. Introduction
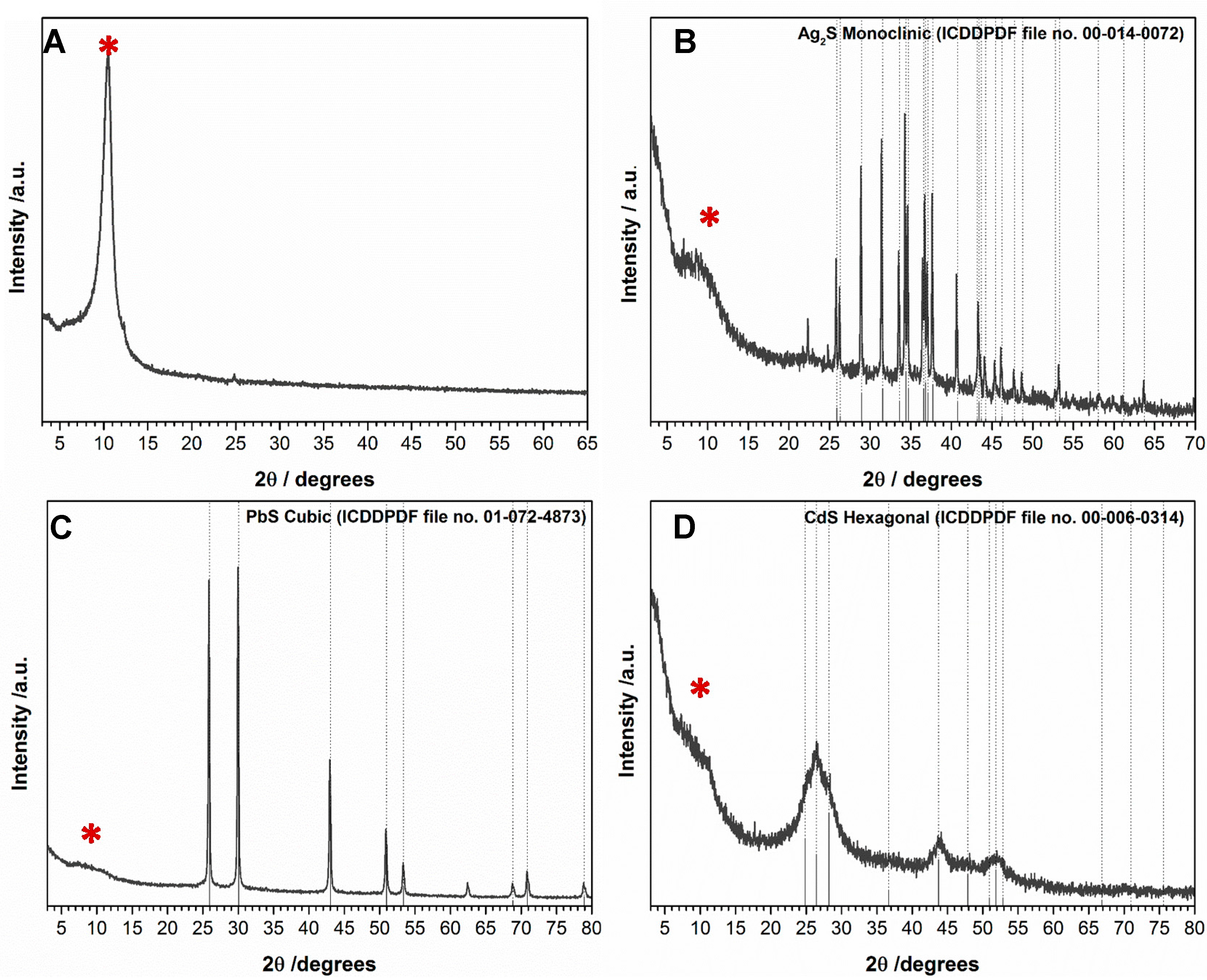
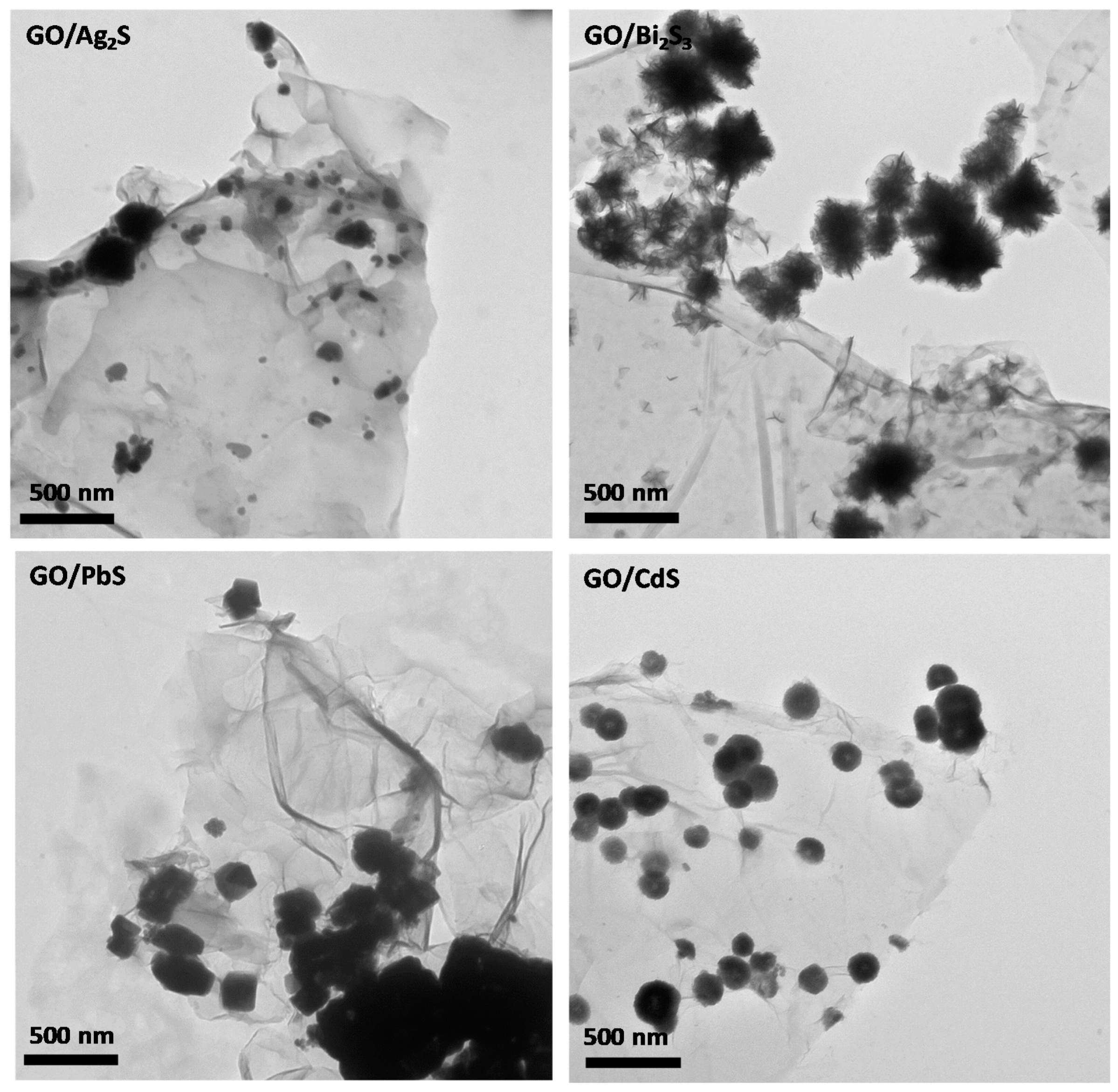
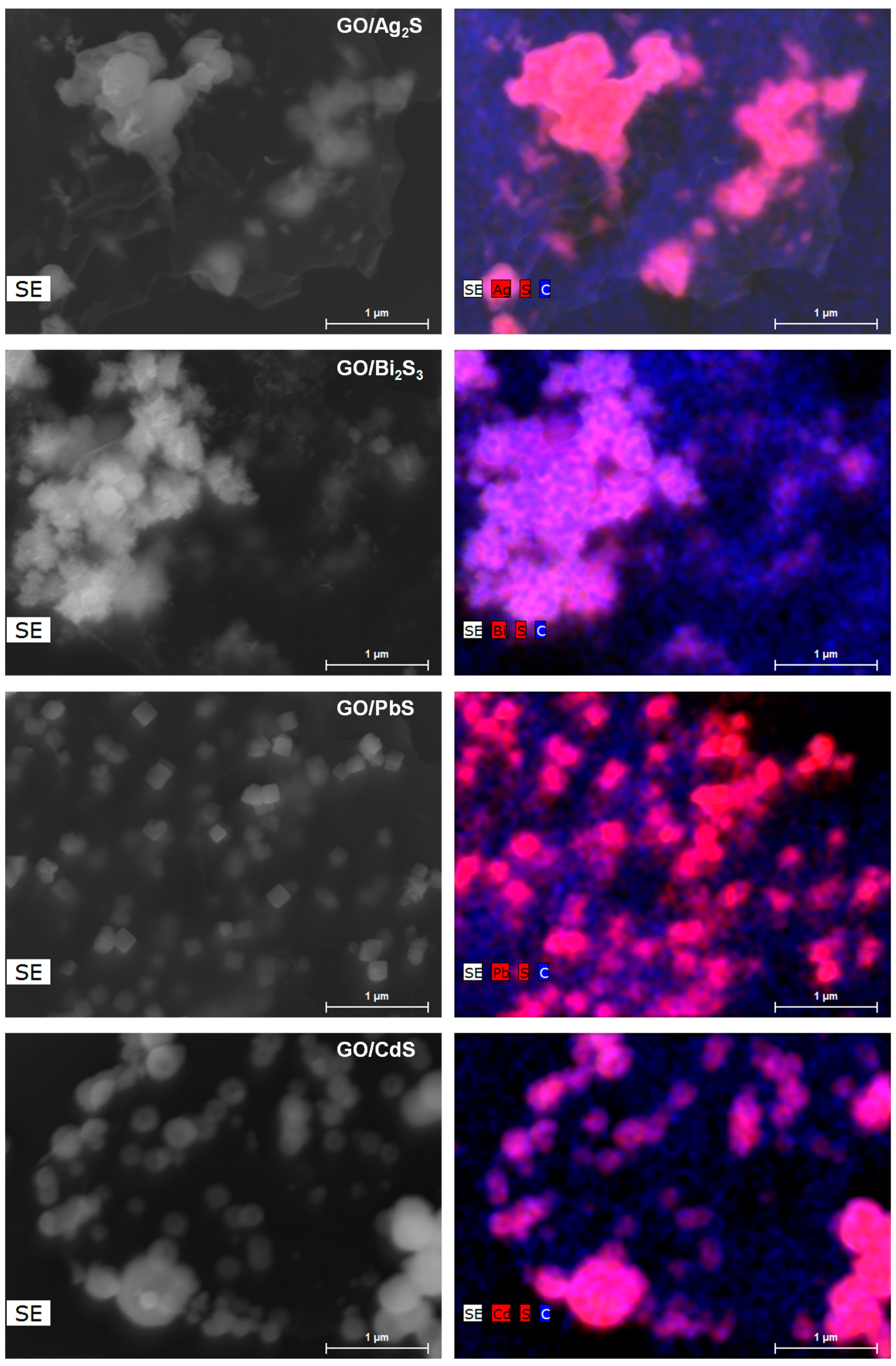
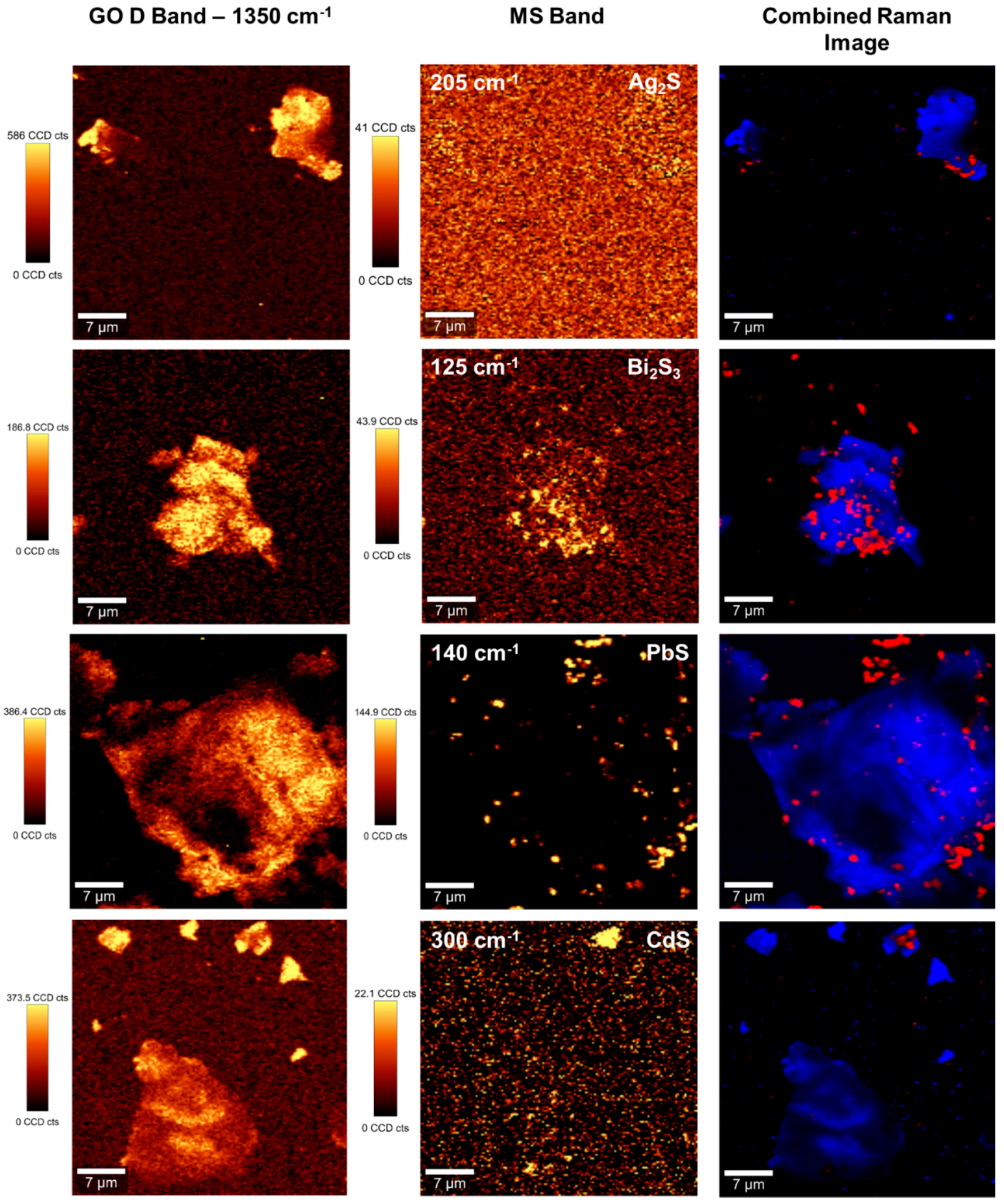
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Methods
3.2. Synthesis of Hybrid Carbon Nanostructures
3.3. Instrumentation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Seger, B.; Kamat, P.V. Electrocatalytically active graphene-platinum nanocomposites. Role of 2-D carbon support in PEM fuel cells. J. Phys. Chem. C 2009, 113, 7990–7995. [Google Scholar] [CrossRef]
- Yan, Q.; Huang, B.; Yu, J.; Zheng, F.; Zang, J.; Wu, J.; Gu, B.L.; Liu, F.; Duan, W. Intrinsic current–voltage characteristics of graphene nanoribbon transistors and effect of edge doping. Nano Lett. 2007, 7, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.J.; Kim, J.; Hosono, E.; Zhou, H.; Kudo, T.; Honma, I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Trindade, T.; Thomas, P.J. Solid-state materials, including ceramics and minerals. In Comprehensive Inorganic Chemistry II: From Elements to Applications, 2nd ed.; Reedijk, J., Poeppelmeter, K., Eds.; Elsevier: Oxford, MS, USA, 2013; Volume 4, pp. 343–369. [Google Scholar]
- Cao, A.; Liu, Z.; Chu, S.; Wu, M.; Ye, Z.; Cai, Z.; Chang, Y.; Wang, S.; Gong, Q.; Liu, Y. A facile one-step method to produce graphene-CdS quantum dot nanocomposites as promising optoelectronic materials. Adv. Mater. 2010, 22, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jiang, T.; Zhu, C.; Zhai, Y.; Wang, D.; Dong, S. One-step, solvothermal synthesis of graphene-CdS and graphene-ZnS quantum dot nanocomposites and their interesting photovoltaic properties. Nano Res. 2010, 3, 794–799. [Google Scholar] [CrossRef]
- Wu, J.; Bai, S.; Shen, X.; Jiang, L. Preparation and characterization of graphene/CdS nanocomposites. Appl. Surf. Sci. 2010, 257, 747–751. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Pan, X.; Fu, X.; Liu, S.; Xu, Y.-J. Assembly of CdS nanoparticles on the two-dimensional graphene scaffold as visible-light-driven photocatalyst for selective organic transformation under ambient conditions. J. Phys. Chem. C 2011, 115, 23501–23511. [Google Scholar] [CrossRef]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, X. One step synthesis and characterization of CdS nanorod/graphene nanosheet composite. Appl. Surf. Sci. 2011, 257, 10379–10383. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, N.; Wu, D.; Tao, W.; Xu, F.; Jiang, K. Graphene-CdS composite, synthesis and enhanced photocatalytic activity. Appl. Surf. Sci. 2012, 258, 2473–2478. [Google Scholar] [CrossRef]
- Pan, S.; Liu, X. CdS–Graphene nanocomposite: Synthesis, adsorption kinetics and high photocatalytic performance under visible light irradiation. New J. Chem. 2012, 36, 1781–1787. [Google Scholar] [CrossRef]
- Narayanam, P.K.; Singh, G.; Botcha, V.D.; Sutar, D.S.; Talwar, S.S.; Srinivasa, R.S.; Major, S.S. Growth of CdS nanocrystallites on graphene oxide Langmuir-Blodgett monolayers. Nanotechnology 2012, 23, 325605. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shao, X.; Wang, J.; Yang, S.; Li, H.; Meng, X.; Liu, X.; Wang, M. Solvothermal synthesis of graphene-CdS nanocomposites for highly efficient visible-light photocatalyst. J. Alloys Compd. 2013, 551, 327–332. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Delai, D.; Ng, W. Graphene oxide-CdS composite with high photocatalytic degradation and disinfection activities under visible light irradiation. J. Hazard. Mater. 2013, 250–251, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, M.Q.; Tang, Z.R.; Xu, Y.J. CdS-graphene nanocomposites as visible light photocatalyst for redox reactions in water: A green route for selective transformation and environmental remediation. J. Catal. 2013, 303, 60–69. [Google Scholar] [CrossRef]
- Lü, W.; Chen, J.; Wu, Y.; Duan, L.; Yang, Y.; Ge, X. Graphene-enhanced visible-light photocatalysis of large-sized CdS particles for wastewater treatment. Nanoscale Res. Lett. 2014, 9, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, X.; Gao, G. Bi2S3 microspheres grown on graphene sheets as low-cost counter-electrode materials for dye- sensitized solar cells. Nanoscale 2014, 6, 3283–3288. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.M.; Aashuri, H.; Simchi, A.; Kalytchuk, S.; Fan, Z. Quasi core/shell lead sulfide/graphene quantum dots for bulk heterojunction solar cells. J. Phys. Chem. C 2015, 119, 18886–18895. [Google Scholar] [CrossRef]
- Tayyebi, A.; Tavakoli, M.M.; Outokesh, M.; Shafiekhani, A.; Simchi, A. Supercritical synthesis and characterization of “graphene-PbS quantum dots” composite with enhanced photovoltaic properties. Ind. Eng. Chem. Res. 2015, 54, 7382–7392. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Lee, S.; Zhang, T.; Sun, D.D. High quality graphene oxide-CdS-Pt nanocomposites for efficient photocatalytic hydrogen evolution. J. Mater. Chem. 2012, 22, 2292–2298. [Google Scholar] [CrossRef]
- Chen, F.; Jia, D.; Jin, X.; Cao, Y.; Liu, A. A general method for the synthesis of graphene oxide-metal sulfide composites with improved photocatalytic activities. Dyes Pigment. 2016, 125, 142–150. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, J.; Xiao, F.-X.; Xiao, G. Revisiting the construction of graphene-CdS nanocomposites as efficient visible-light-driven photocatalysts for selective organic transformation. J. Mater. Chem. A 2014, 2, 5330–5339. [Google Scholar] [CrossRef]
- Estrada, A.C.; Mendoza, E.; Trindade, T. Decoration of carbon nanostructures with metal sulfides by sonolysis of single-molecule precursors. Eur. J. Inorg. Chem. 2014, 2014, 3184–3190. [Google Scholar] [CrossRef]
- Monteiro, O.C.; Esteves, A.C.C.; Trindade, T. The synthesis of SiO2@CdS nanocomposites using single-molecule precursors. Chem. Mater. 2002, 14, 2900–2904. [Google Scholar] [CrossRef] [Green Version]
- Sharon, M.; Sharon, M. Graphene: An Introduction to the Fundamentals and Industrial Applications, 1st ed.; Wiley-Scrivener: Hoboken, NJ, USA, 2015. [Google Scholar]
- Saikia, B.K.; Boruah, R.K.; Gogoi, P.K. A X-ray diffraction analysis on graphene layers of assam coal. J. Chem. Sci. 2009, 121, 103–106. [Google Scholar] [CrossRef]
- Pham, T.A.; Choi, B.C.; Jeong, Y.T. Facile covalent immobilization of cadmium sulfide quantum dots on graphene oxide nanosheets: Preparation, characterization, and optical properties. Nanotechnology 2010, 21, 465603. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, H.; Yang, Y.; Wang, H.; Wang, S.; Zheng, W.; Liu, Y. Reduced graphene oxide/CdS for Efficiently photocatalystic degradation of methylene blue. J. Alloys Compd. 2012, 524, 5–12. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cote, L.J.; Tung, V.C.; Tan, A.T.L.; Goins, P.E.; Wu, J.; Huang, J. Graphene oxide nanocolloids. J. Am. Chem. Soc. 2010, 132, 17667–17669. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, T. Fabrication and characterization of graphene oxide/zinc oxide nanorods hybrid. Appl. Surf. Sci. 2011, 257, 8950–8954. [Google Scholar] [CrossRef]
- Wu, J.; Shen, X.; Jiang, L.; Wang, K.; Chen, K. Solvothermal synthesis and characterization of sandwich-like graphene/ZnO nanocomposites. Appl. Surf. Sci. 2010, 256, 2826–2830. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Huang, Y.; Ma, Y.; Wang, Y.; Chen, Y. Size-controlled synthesis of graphene oxide sheets on a large scale using chemical exfoliation. Carbon 2009, 47, 3365–3380. [Google Scholar] [CrossRef]
- Brus, L. Quantum crystallites and nonlinear optics. Appl. Phys. A 1991, 53, 465–474. [Google Scholar] [CrossRef]
- Weller, H. Quantized semiconductor particles: A novel state of matter for materials science. Adv. Mater. 1993, 5, 88–95. [Google Scholar] [CrossRef]
- Neves, M.C.; Monteiro, O.C.; Hempelmann, R.; Silva, A.M.S.; Trindade, T. From single-molecule precursors to coupled Ag2S/TiO2 nanocomposites. Eur. J. Inorg. Chem. 2008, 2008, 4380–4386. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of grapheme. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl. Catal. B 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Beams, R.; Cançado, L.G.; Novotny, L. Raman characterization of defects and dopants in graphene. J. Phys. 2015, 27, 083002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rajaraman, B.R.S.; Liu, H.; Ramakrishna, S. Graphene’s potential in materials science and engineering. RSC Adv. 2014, 4, 28987–29011. [Google Scholar] [CrossRef]
- Lambert, T.N.; Chavez, C.A.; Hernandez-Sanchez, B.; Lu, P.; Bell, N.S.; Ambrosini, A.; Friedman, T.; Boyle, T.J.; Wheeler, D.R.; Huber, D.L. Synthesis and characterization of Titania-Graphene nanocomposites. J. Phys. Chem. C 2009, 113, 19812–19823. [Google Scholar] [CrossRef]
- Berciaud, S.; Li, X.; Htoon, H.; Brus, L.E.; Doorn, S.K.; Heinz, T.F. Intrinsic line shape of the raman 2D-mode in freestanding graphene monolayers. Nano Lett. 2013, 13, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Paz, F.A.; Rocha, J.; Klinowski, J.; Trindade, T.; Shi, S.; Mafra, L. Optimised hydrothermal synthesis of multi-dimensional hybrid coordination polymers containing flexible organic ligands. Prog. Solid State Chem. 2005, 33, 113–125. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Angelo, J.; Girão, A.V.; Trindade, T.; Andrade, L.; Mendes, L.A. N-doped carbon quantum dots/TiO2 composite with improved photocatalytic activity. Appl. Catal. B 2016, 193, 67–74. [Google Scholar] [CrossRef]
- Khan, U.; O’Neill, A.; Lotya, M.; De, S.; Coleman, J.N. High-concentration solvent exfoliation of graphene. Small 2010, 6, 864–871. [Google Scholar] [CrossRef] [PubMed]


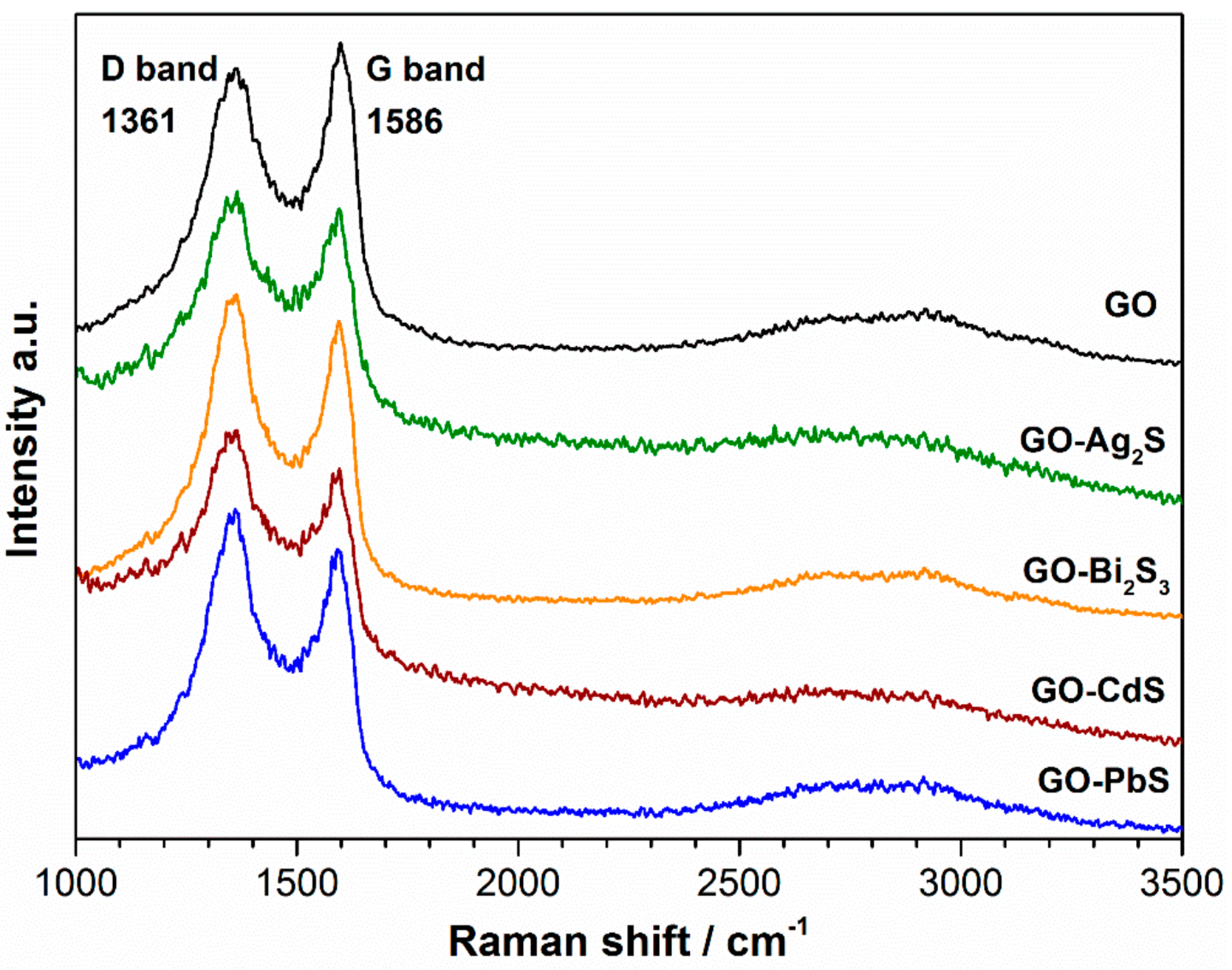

| Sample | Band Positions (cm−1) | ||
|---|---|---|---|
| D | G | ID/IG | |
| Graphene oxide (GO) | 1366.5 ± 1.2 | 1595.7 ± 0.7 | 0.92 |
| GO/Bi2S3 | 1359.5 ± 1.2 | 1587.9 ± 0.9 | 1.05 |
| GO/Ag2S | 1361.9 ± 1.1 | 1585.3 ± 0.7 | 1.02 |
| GO/CdS | 1361.4 ± 0.9 | 1580.1 ± 0.8 | 1.07 |
| GO/PbS | 1360.6 ± 1.1 | 1581.5 ± 1.0 | 1.07 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, J.L.; Estrada, A.C.; Fateixa, S.; Ferro, M.; Trindade, T. A General Route for Growing Metal Sulfides onto Graphene Oxide and Exfoliated Graphite Oxide. Nanomaterials 2017, 7, 245. https://doi.org/10.3390/nano7090245
Lopes JL, Estrada AC, Fateixa S, Ferro M, Trindade T. A General Route for Growing Metal Sulfides onto Graphene Oxide and Exfoliated Graphite Oxide. Nanomaterials. 2017; 7(9):245. https://doi.org/10.3390/nano7090245
Chicago/Turabian StyleLopes, Joana L., Ana C. Estrada, Sara Fateixa, Marta Ferro, and Tito Trindade. 2017. "A General Route for Growing Metal Sulfides onto Graphene Oxide and Exfoliated Graphite Oxide" Nanomaterials 7, no. 9: 245. https://doi.org/10.3390/nano7090245