Catalytic Behavior of Chromium Oxide Supported on Nanocasting-Prepared Mesoporous Alumina in Dehydrogenation of Propane
Abstract
:1. Introduction
2. Results
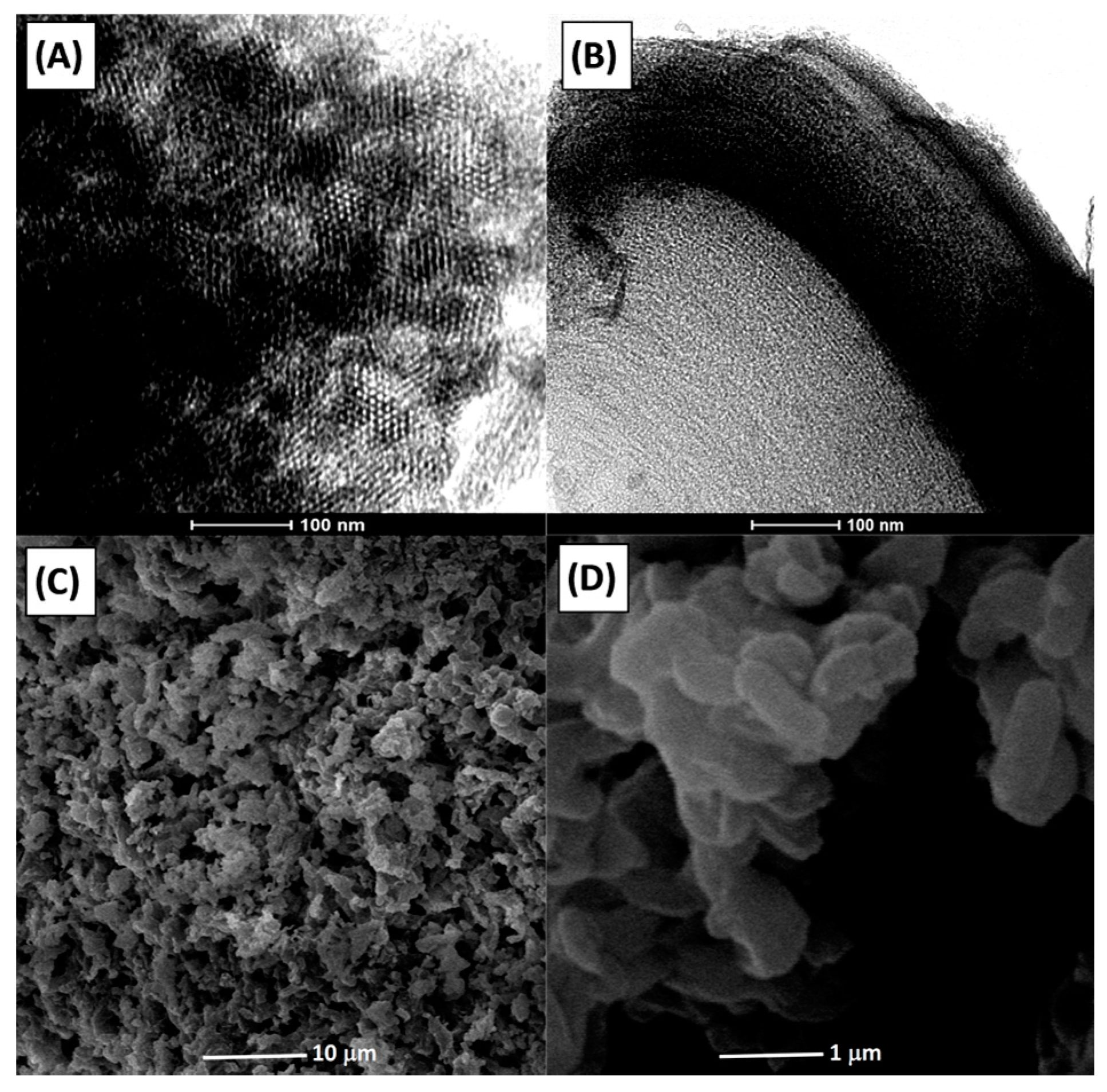
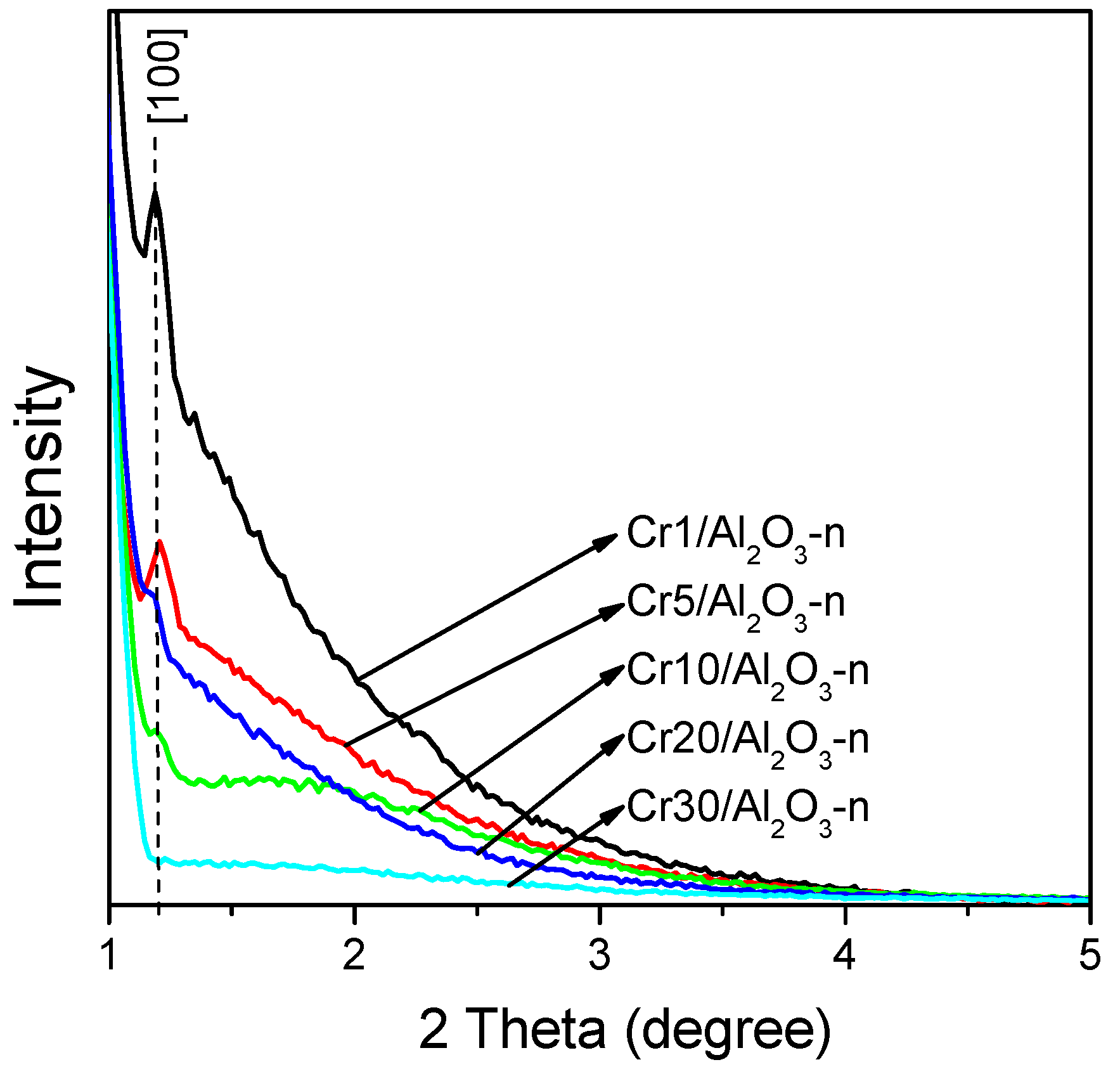
2.1. Characterization of Alumina Support
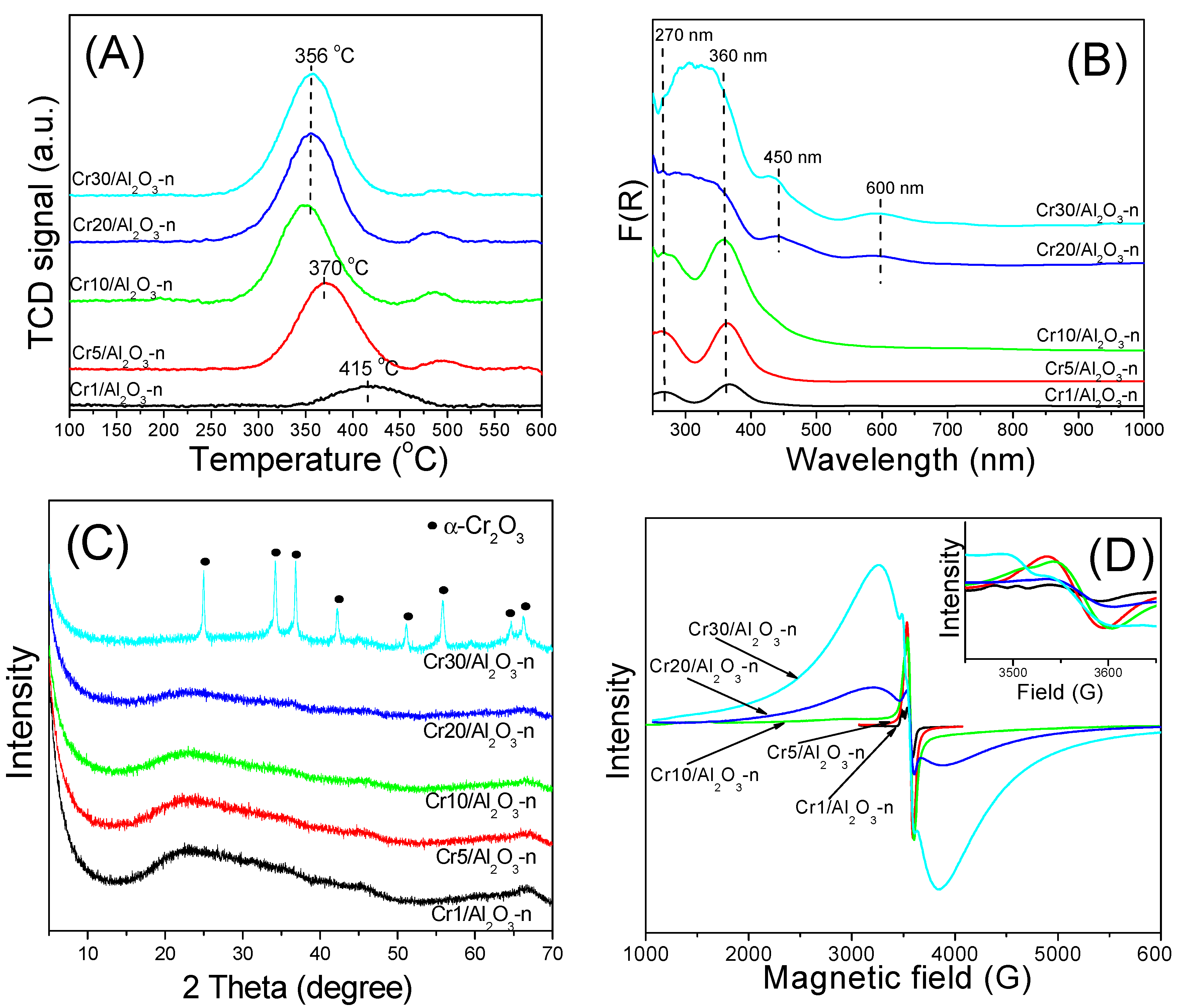
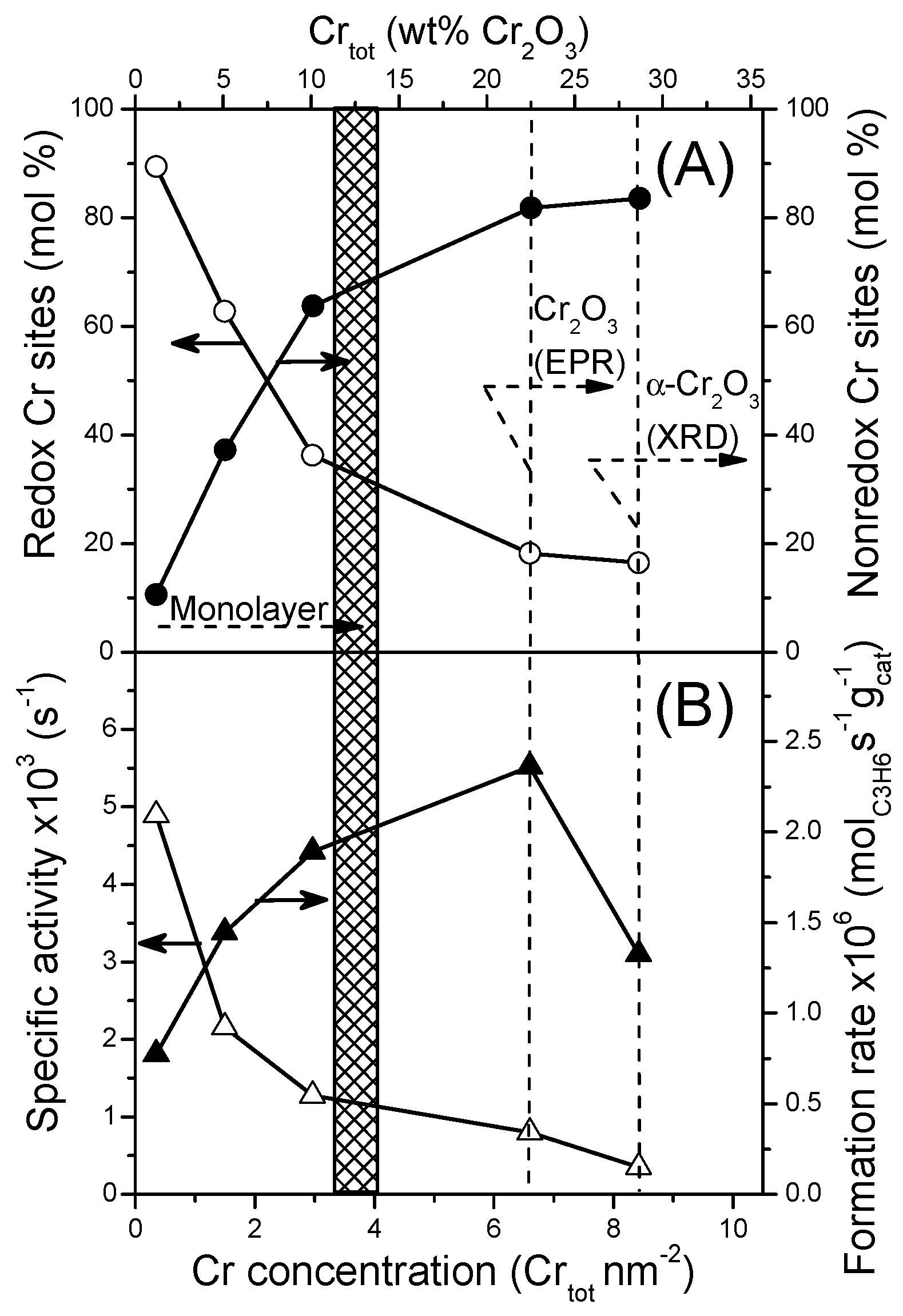
2.2. Characterization of Catalysts
2.3. Catalytic Properties of Cr-Containing Samples
2.3.1. Effect of the Nature of Cr
2.3.2. Effect of Support
2.3.3. Regeneration Behavior
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization Techniques
3.3. Catalytic Tests
3.4. Operando UV-Vis DRS
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhasin, M.M.; McCain, J.H.; Vora, B.V.; Imai, T.; Pujadó, P.R. Dehydrogenation and oxydehydrogenation of paraffins to olefins. Appl. Catal. A Gen. 2001, 221, 397–419. [Google Scholar] [CrossRef]
- Cavani, F.; Ballarini, N.; Cericola, A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation? Catal. Today 2007, 127, 113–131. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, D. Dehydrogenation of Paraffins; Key Technology for Petrochemicals and Fuels. Cattech 2000, 4, 56–73. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Kuśtrowski, P.; Chmielarz, L. Chromium oxide supported on MCM-41 as a highly active and selective catalyst for dehydrogenation of propane with CO2. Appl. Catal. A Gen. 2008, 349, 62–69. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Zhang, Y. Mesoporous silica-supported chromium catalyst: Characterization and excellent performance in dehydrogenation of propane to propylene with carbon dioxide. Catal. Commun. 2007, 8, 565–570. [Google Scholar] [CrossRef]
- Botavina, M.A.; Martra, G.; Agafonov, Y.A.; Gaidai, N.A.; Nekrasov, N.V.; Trushin, D.V.; Coluccia, S.; Lapidus, A.L. Oxidative dehydrogenation of C3-C4 paraffins in the presence of CO2 over CrOx/SiO2 catalysts. Appl. Catal. A Gen. 2008, 347, 126–132. [Google Scholar] [CrossRef]
- Ohishi, Y.; Kawabata, T.; Shishido, T.; Takaki, K.; Zhang, Q.; Wang, Y.; Takehira, K. Dehydrogenation of ethylbenzene with CO2 over Cr-MCM-41 catalyst. J. Mol. Catal. A Chem. 2005, 230, 49–58. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Niemczyk, M. Investigation of catalytic activity of CrSBA-1 materials obtained by direct method in the dehydrogenation of propane with CO2. Appl. Catal. A Gen. 2010, 374, 142–149. [Google Scholar] [CrossRef]
- Bai, P.; Ma, Z.; Li, T.; Tian, Y.; Zhang, Z.; Zhong, Z.; Xing, W.; Wu, P.; Liu, X.; Yan, Z. Relationship between Surface Chemistry and Catalytic Performance of Mesoporous γ-Al2O3 Supported VOX Catalyst in Catalytic Dehydrogenation of Propane. ACS Appl. Mater. Interfaces 2016, 8, 25979–25990. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, S.; Stoyanova, M.; Rodemerck, U.; Linke, D.; Kondratenko, E.V. Effect of support on selectivity and on-stream stability of surface VOx species in non-oxidative propane dehydrogenation. Catal. Sci. Technol. 2014, 4, 1323–1332. [Google Scholar] [CrossRef]
- Sokolov, S.; Bychkov, V.Y.; Stoyanova, M.; Rodemerck, U.; Bentrup, U.; Linke, D.; Tyulenin, Y.P.; Korchak, V.N.; Kondratenko, E.V. Effect of VOx species and support on coke formation and catalyst stability in nonoxidative propane dehydrogenation. ChemCatChem 2015, 7, 1691–1700. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J. Dehydrogenation of propane to propene over gallium oxide in the presence of CO2. Appl. Catal. A Gen. 2003, 251, 425–433. [Google Scholar] [CrossRef]
- Zheng, B.; Hua, W.; Yue, Y.; Gao, Z. Dehydrogenation of propane to propene over different polymorphs of gallium oxide. J. Catal. 2005, 232, 143–151. [Google Scholar] [CrossRef]
- Tan, S.; Hu, B.; Kim, W.G.; Pang, S.H.; Moore, J.S.; Liu, Y.; Dixit, R.S.; Pendergast, J.G.; Sholl, D.S.; Nair, S.; et al. Propane Dehydrogenation over Alumina-Supported Iron/Phosphorus Catalysts: Structural Evolution of Iron Species Leading to High Activity and Propylene Selectivity. ACS Catal. 2016, 6, 5673–5683. [Google Scholar] [CrossRef]
- Michorczyk, P.; Kuśtrowski, P.; Chmielarz, L.; Ogonowski, J. Influence of redox properties on the activity of iron oxide catalysts in dehydrogenation of propane with CO2. React. Kinet. Catal. Lett. 2004, 82, 121–130. [Google Scholar] [CrossRef]
- Yun, Y.; Araujo, J.R.; Melaet, G.; Baek, J.; Archanjo, B.S.; Oh, M.; Alivisatos, A.P.; Somorjai, G.A. Activation of Tungsten Oxide for Propane Dehydrogenation and Its High Catalytic Activity and Selectivity. Catal. Lett. 2017, 147, 622–632. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Gao, Y.; He, H.-Y.; Fan, K.-N.; Zhuang, J.-H. Dehydrogenation of propane over In2O3–Al2O3 mixed oxide in the presence of carbon dioxide. J. Catal. 2010, 272, 101–108. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Brito, J.L.F.; Dieguez, L.C. Characterization of chromium species in catalysts for dehydrogenation and polymerization. J. Mol. Catal. A Chem. 2003, 203, 251–266. [Google Scholar] [CrossRef]
- Mentasty, L.R.; Gorriz, O.F.; Cadus, L.E. Chromium Oxide Supported on Different Al2O3 Supports: Catalytic Propane Dehydrogenation. Ind. Eng. Chem. Res. 1999, 38, 396–404. [Google Scholar] [CrossRef]
- Cherian, M.; Rao, M.S.; Hirt, A.M.; Wachs, I.E.; Deo, G. Oxidative dehydrogenation of propane over supported chromia catalysts: Influence of oxide supports and chromia loading. J. Catal. 2002, 211, 482–495. [Google Scholar] [CrossRef]
- Airaksinen, S.M.K.; Krause, A.O.I. Effect of catalyst prereduction on the dehydrogenation of isobutane over chromia/alumina. Ind. Eng. Chem. Res. 2005, 44, 3862–3868. [Google Scholar] [CrossRef]
- Santhosh Kumar, M.; Hammer, N.; Rønning, M.; Holmen, A.; Chen, D.; Walmsley, J.C.; Øye, G. The nature of active chromium species in Cr-catalysts for dehydrogenation of propane: New insights by a comprehensive spectroscopic study. J. Catal. 2009, 261, 116–128. [Google Scholar] [CrossRef]
- Puurunen, R. Spectroscopic Study on the Irreversible Deactivation of Chromia/Alumina Dehydrogenation Catalysts. J. Catal. 2002, 210, 418–430. [Google Scholar] [CrossRef]
- Cherian, M.; Someswara, M.; Yang, W.; Jehng, J.; Hirt, A.M.; Deo, G. Oxidative dehydrogenation of propane over Cr2O3/Al2O3 and Cr2O3 catalysts: Effects of loading, precursor and surface area. Appl. Catal. A Gen. 2002, 233, 21–33. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Dieguez, L.C. Distribution of chromium species in catalysts supported on ZrO2/Al2O3 and performance in dehydrogenation. J. Catal. 2003, 220, 309–316. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Tinnemans, S.J.; Visser, T.; Weckhuysen, B.M. Towards real-time spectroscopic process control for the dehydrogenation of propane over supported chromium oxide catalysts. Chem. Eng. Sci. 2004, 59, 5487–5492. [Google Scholar] [CrossRef]
- Michorczyk, P.; Pietrzyk, P.; Ogonowski, J. Preparation and characterization of SBA-1-supported chromium oxide catalysts for CO2 assisted dehydrogenation of propane. Microporous Mesoporous Mater. 2012, 161, 56–66. [Google Scholar] [CrossRef]
- De Rossi, S.; Casaletto, M.P.; Ferraris, G.; Cimino, A.; Minelli, G. Chromia/zirconia catalysts with Cr content exceeding the monolayer. A comparison with chromia/alumina and chromia/silica for isobutane dehydrogenation. Appl. Catal. A Gen. 1998, 167, 257–270. [Google Scholar] [CrossRef]
- Hakuli, A.; Kytökivi, A.; Krause, A.O.I. Dehydrogenation of i-Butane on CrOx/Al2O3 Catalysts Prepared by ALE and Impregnation Techniques. Appl. Catal. A Gen. 2000, 190, 219–232. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, Y.; Gao, Z. Chromium oxide supported on mesoporous SBA-15 as propane dehydrogenation and oxidative dehydrogenation catalysts. Catal. Lett. 2002, 83, 19–25. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Zeńczak, K. Activity of chromium oxide deposited on different silica supports in the dehydrogenation of propane with CO2—A comparative study. J. Mol. Catal. A Chem. 2011, 349, 1–12. [Google Scholar] [CrossRef]
- Vaudry, F.; Khodabandeh, S.; Davis, M.E. Synthesis of Pure Alumina Mesoporous Materials. Chem. Mater. 1996, 8, 1451–1464. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, A.; Xu, J.; Zhang, Y.; Wang, X.; Zhang, T. Preparation of ordered mesoporous crystalline alumina replicated by mesoporous carbon. Microporous Mesoporous Mater. 2008, 116, 461–468. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, A.; Wang, X.; Zhang, T. Ordered crystalline alumina molecular sieves synthesized via a nanocasting route. Chem. Mater. 2006, 18, 5153–5155. [Google Scholar] [CrossRef]
- Haffer, S.; Weinberger, C.; Tiemann, M. Mesoporous Al2O3 by nanocasting: Relationship between crystallinity and mesoscopic order. Eur. J. Inorg. Chem. 2012, 3283–3288. [Google Scholar] [CrossRef]
- Michorczyk, P.; Kuśtrowski, P.; Kolak, A.; Zimowska, M. Ordered mesoporous Ga2O3 and Ga2O3-Al2O3 prepared by nanocasting as effective catalysts for propane dehydrogenation in the presence of CO2. Catal. Commun. 2013, 35, 95–100. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Verberckmoes, A.A.; De Baets, A.R.; Schoonheydt, R.A. Diffuse reflectance spectroscopy of supported chromium oxide catalysts: A self-modeling mixture analysis. J. Catal. 1997, 166, 160–171. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Wachs, I.E.; Schoonheydt, R.A. Surface Chemistry and Spectroscopy of Chromium in Inorganic Oxides. Chem. Rev. 1996, 96, 3327–3350. [Google Scholar] [CrossRef] [PubMed]
- Wachs, I.E.; Deo, G.; Vuurman, M.A.; Hu, H.; Kim, D.S.; Jehng, J.-M. Molecular design of supported metal oxide catalysts: An initial step to theoretical models. J. Mol. Catal. 1993, 82, 443–455. [Google Scholar] [CrossRef]
- Hakuli, A.; Harlin, M.E.; Backman, L.B.; Krause, A.O.I. Dehydrogenation of i-Butane on CrOx/SiO2 Catalysts. J. Catal. 1999, 184, 349–356. [Google Scholar] [CrossRef]
- Węgrzyniak, A.; Jarczewski, S.; Wach, A.; Hędrzak, E.; Kuśtrowski, P.; Michorczyk, P. Catalytic behaviour of chromium oxide supported on CMK-3 carbon replica in the dehydrogenation propane to propene. Appl. Catal. A Gen. 2015, 508, 1–9. [Google Scholar] [CrossRef]
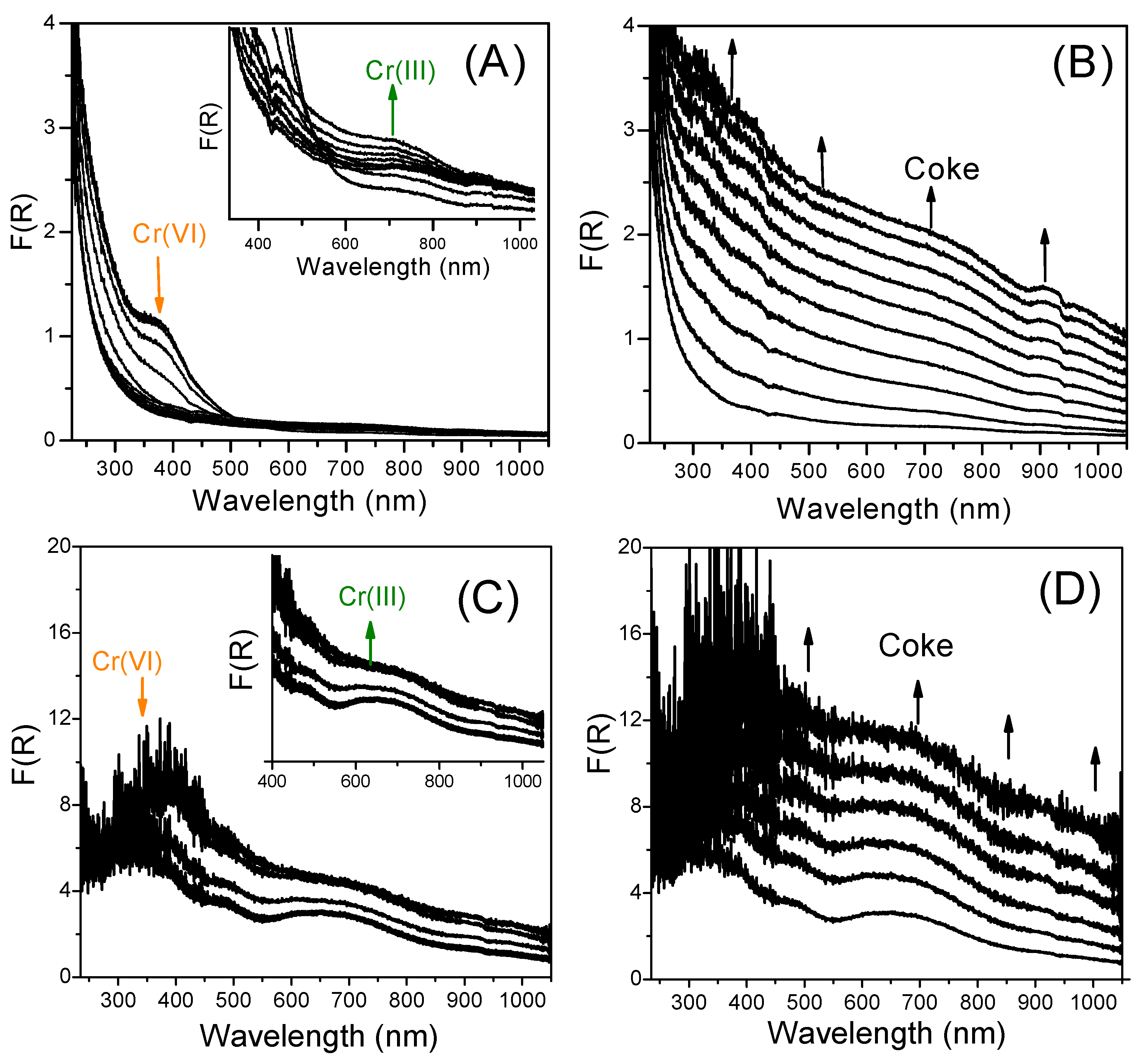
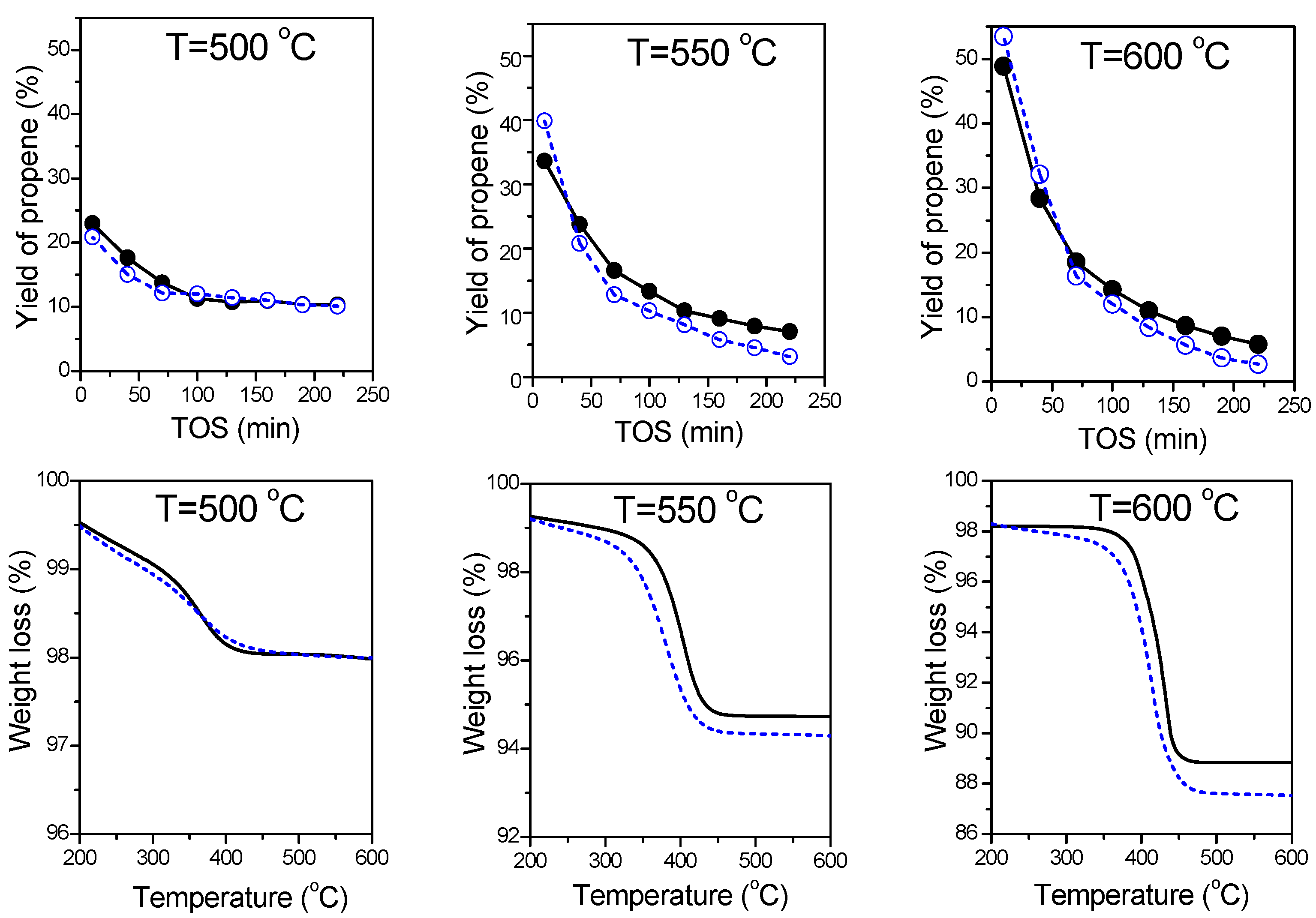
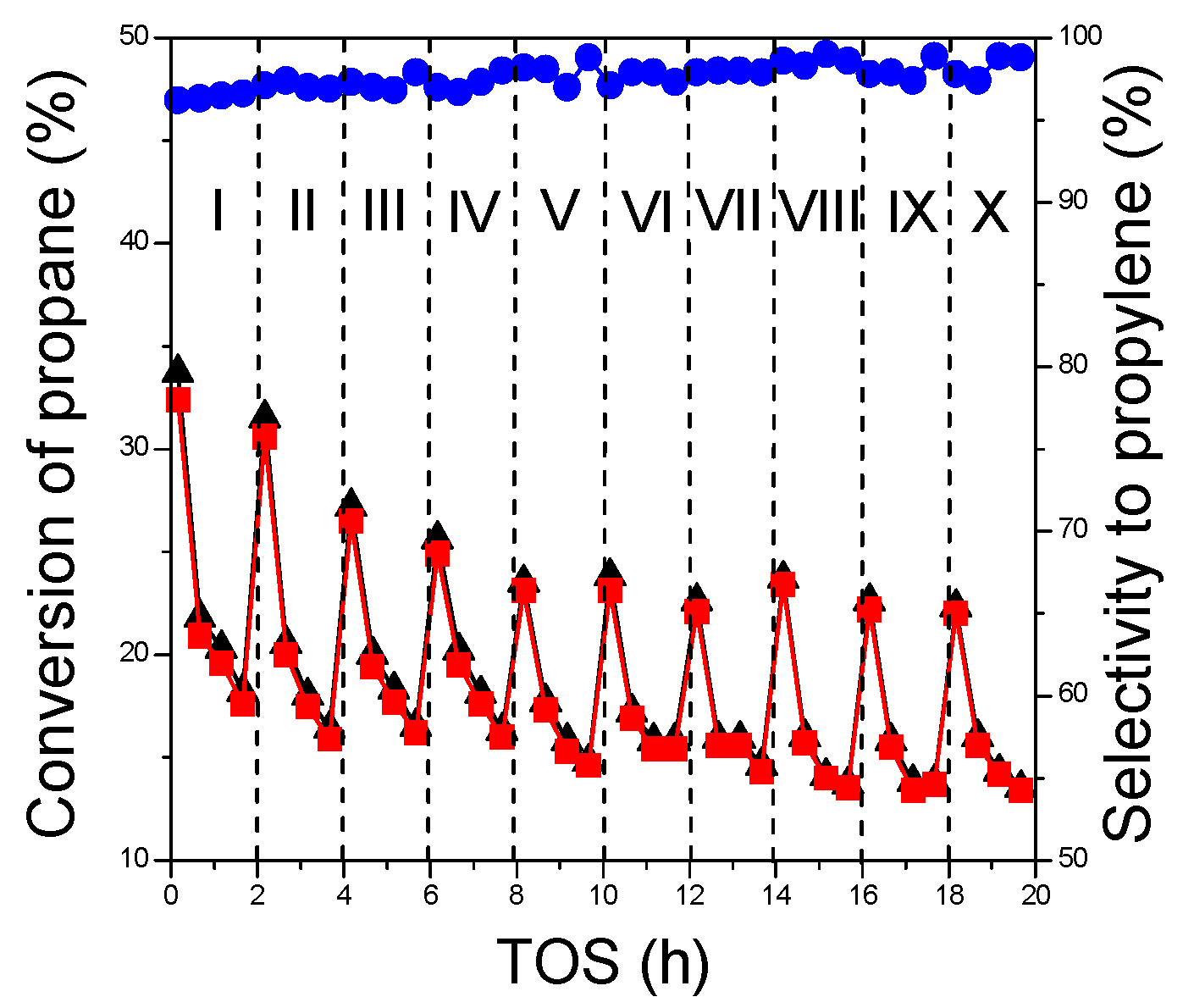
| Sample | Calcination/Pretreatment Temperature (°C) | Phase Composition | SBET (m2∙g−1) | Vmicro (cm3∙g−1) | Vmeso (cm3∙g−1) | Vtotal (cm3∙g−1) |
|---|---|---|---|---|---|---|
| SBA-15 a | 550 | - | 756 | 0.01 | 0.83 | 0.84 |
| CMK-3 a | 800 | - | 1411 | 0.10 | 1.22 | 1.23 |
| Al2O3-n | 600 | - | 259 | 0.01 | 0.32 | 0.33 |
| 700 | - | 270 | 0.00 | 0.41 | 0.41 | |
| 800 | γ-Al2O3 | 254 | 0.00 | 0.44 | 0.44 | |
| 900 | γ-Al2O3 | 178 | 0.02 | 0.35 | 0.37 |
| Sample | Crtot Content a (wt % of Cr2O3) | H2-TPR | NH3-TPD (μmol NH3∙m−2) b | SBET (m2∙g−1) | Vtotal (cm3∙g−1) | |||
|---|---|---|---|---|---|---|---|---|
| H2 mmol·g−1 | H2/Crtot | Weak | Medium-Strong | Total | ||||
| Cr1/Al2O3-n | 1.2 | 0.16 | 1.34 | 0.26 | 1.31 | 1.57 | 232 | 0.36 |
| Cr5/Al2O3-n | 5.1 | 0.67 | 0.94 | 0.43 | 1.93 | 2.36 | 175 | 0.23 |
| Cr10/Al2O3-n | 10.1 | 1.33 | 0.54 | 0.31 | 2.02 | 2.33 | 170 | 0.12 |
| Cr20/Al2O3-n | 22.5 | 2.96 | 0.27 | 0.63 | 2.23 | 2.87 | 148 | 0.13 |
| Cr30/Al2O3-n | 28.7 | 3.78 | 0.25 | 0.59 | 1.31 | 1.90 | 129 | 0.12 |
| Sample | Temp. (°C) | Conversion (%) | Yield (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|---|
| C3H8 | C3H6 | C3H6 | C2H6 | C2H4 | CH4 | ||
| Cr1/Al2O3-n | 550 | 13.0 | 10.4 | 79.9 | 1.4 | 12.1 | 6.6 |
| Cr5/Al2O3-n | 550 | 21.9 | 19.4 | 88.8 | 2.3 | 5.1 | 3.9 |
| Cr10/Al2O3n | 550 | 24.6 | 22.8 | 92.3 | 1.6 | 2.9 | 3.2 |
| Cr20/Al2O3-n | 500 | 12.9 | 11.3 | 87.7 | 1.8 | 5.2 | 5.3 |
| 550 | 33.8 | 31.7 | 94.0 | 1.4 | 2.1 | 2.4 | |
| 600 | 41.8 | 37.5 | 89.8 | 2.3 | 3.9 | 3.9 | |
| Cr30/Al2O3-n | 550 | 17.8 | 17.5 | 98.2 | 0.3 | 0.4 | 0.2 |
| Cr20/SBA-15 | 550 | 25.7 | 22.1 | 86.0 | 4.8 | 2.7 | 5.6 |
| Cr20/MCM-41 | 550 | 28.9 | 24.9 | 86.0 | 4.0 | 4.6 | 5.4 |
| Cr20/CMK-3 b | 550 | 47.4 | 40.1 | 84.7 | 3.3 | 3.8 | 8.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Węgrzyniak, A.; Jarczewski, S.; Węgrzynowicz, A.; Michorczyk, B.; Kuśtrowski, P.; Michorczyk, P. Catalytic Behavior of Chromium Oxide Supported on Nanocasting-Prepared Mesoporous Alumina in Dehydrogenation of Propane. Nanomaterials 2017, 7, 249. https://doi.org/10.3390/nano7090249
Węgrzyniak A, Jarczewski S, Węgrzynowicz A, Michorczyk B, Kuśtrowski P, Michorczyk P. Catalytic Behavior of Chromium Oxide Supported on Nanocasting-Prepared Mesoporous Alumina in Dehydrogenation of Propane. Nanomaterials. 2017; 7(9):249. https://doi.org/10.3390/nano7090249
Chicago/Turabian StyleWęgrzyniak, Adam, Sebastian Jarczewski, Adam Węgrzynowicz, Barbara Michorczyk, Piotr Kuśtrowski, and Piotr Michorczyk. 2017. "Catalytic Behavior of Chromium Oxide Supported on Nanocasting-Prepared Mesoporous Alumina in Dehydrogenation of Propane" Nanomaterials 7, no. 9: 249. https://doi.org/10.3390/nano7090249






