Pro-Inflammatory Versus Anti-Inflammatory Effects of Dendrimers: The Two Faces of Immuno-Modulatory Nanoparticles
Abstract
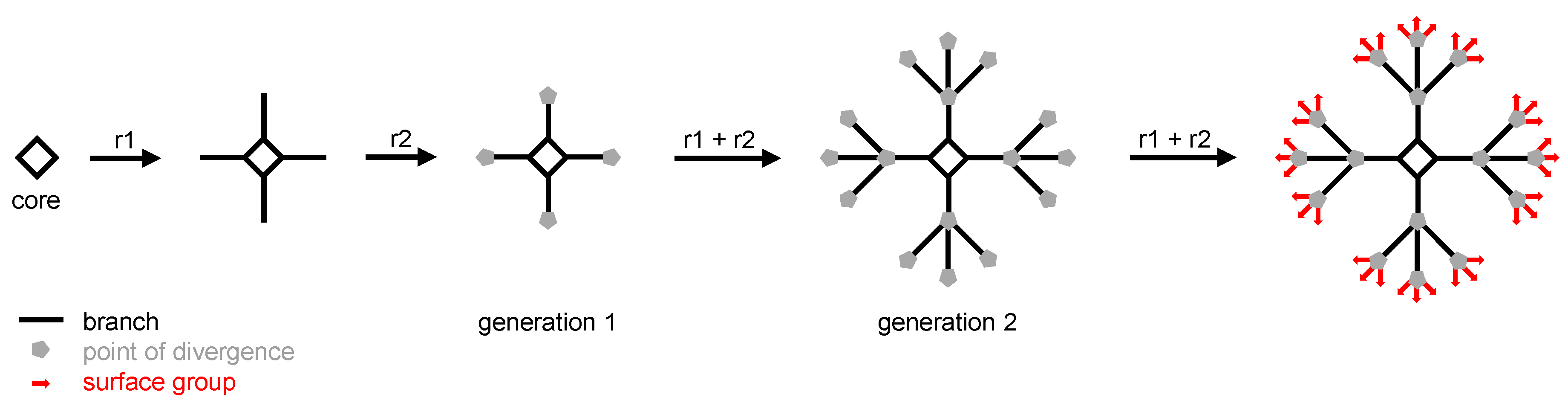
:1. Introduction
2. Review of Articles Dealing with Intrinsically Anti-Inflammatory Dendrimers
2.1. PolyAMidoAMine (PAMAM) Dendrimers
2.2. From PAMAM to PolypropylETherIMine (PETIM) Dendrimers
2.3. To Finish with PAMAM Dendrimers
2.4. PolyEthylene Oxide (PEO)/PolyEthylene Glycol (PEG) Dendrimers
2.5. Phosphorus-Based Poly(PhosphorHydrazone) (PPH) Dendrimers
3. Immunological Breaking News: Pro-Inflammatory Dendrimers Do Exist
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vögtle, F.; Weber, E. Octopus molecules. Angew. Chem. Int. Ed. 1974, 13, 814–816. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vögtle, F. Cascade-chain-like and nonskid-chain-like syntheses of molecular cavity topologies. Synthesis 1978, 2, 155–158. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Dendritic macromolecules: Synthesis of starbust dendrimers. Macromolecules 1986, 19, 2466–2468. [Google Scholar] [CrossRef]
- Lim, J.; Kostiainen, M.; Maly, J.; da Costa, V.C.P.; Annunziata, O.; Pavan, G.M.; Simanek, E.E. Synthesis of large dendrimers with the dimensions of small viruses. J. Am. Chem. Soc. 2013, 135, 4660–4663. [Google Scholar] [CrossRef] [PubMed]
- Varner, C.T.; Rosen, T.; Martin, J.T.; Kane, R.S. Recent advances in engineering polyvalent biological interactions. Biomacromolecules 2015, 16, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, N. Multivalent ligand presentation as a central concept to study intricate carbohydrate-protein interactions. Chem. Soc. Rev. 2009, 38, 3463–3483. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2754–2794. [Google Scholar] [CrossRef]
- Gestwicki, J.E.; Cairo, C.W.; Strong, L.E.; Oetjen, K.A.; Kiessling, L.L. Influencing receptor-ligand binding mechanisms with multivalent ligand-architecture. J. Am. Chem. Soc. 2002, 124, 14922–14933. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.; Legiest, B.; Geys, J.; Nemery, B. Do nanomedicines require novel safety assessments to ensure their safety for long-term human use? Drug Saf. 2009, 32, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Hamburg, M.A. FDA’s approach to regulation of products of nanotechnology. Science 2012, 336, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, F.; Sakai-Kato, K.; Duncan, R.; Hernán Pérez de la Ossa, D.; Pita, R.; Vidal, J.M.; Kohli, A.; Tothfalusi, L.; Sanh, A.; Tinton, S.; et al. Next-generation nanomedicines and nanosimilars: EU regulators’ initiatives relating to the development and evaluation of nanomedicines. Nanomedicine 2013, 8, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Pita, R.; Ehmann, F.; Papaluca, M. Nanomedicines in the EU—Regulatory overview. AAPS J. 2016, 18, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- FDA Nanotechnology Regulatory Science Research Plan. Available online: http://www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/ucm273325.htm (accessed on 1 September 2017).
- Nanomedicine Initiative Project. Available online: http://www.pmda.go.jp/english/rs-sb-std/standards-development/cross-sectional-project/0008.html#r=s&r=s (accessed on 1 September 2017).
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Peng, L. Dendrimer nanovectors for SiRNA delivery. Methods Mol. Biol. 2016, 1364, 127–142. [Google Scholar] [PubMed]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Fruchon, S.; Fournié, J.J.; Poupot, M.; Poupot, R. Anti-inflammatory properties of dendrimers per se. Sci. World J. 2011, 11, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Shaunak, S. Perspective: Dendrimer drugs for infection and inflammation. Biochem. Biophys. Res. Commun. 2015, 468, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Shaunak, S.; Thomas, S.; Gianasi, E.; Godwin, A.; Jones, E.; Teo, I.; Mireskandari, K.; Luthert, P.; Duncan, R.; Patterson, S.; et al. Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat. Biotechnol. 2004, 8, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Teo, I.; Toms, S.M.; Marteyn, B.; Barata, T.S.; Simpson, P.; Johnston, K.A.; Schnupf, P.; Puhar, A.; Bell, T.; Tang, C.; et al. Preventing acute gut wall damage in infectious diarrhoeas with glycosylated dendrimers. EMBO Mol. Med. 2012, 4, 866–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barata, T.S.; Shaunak, S.; Teo, I.; Zloh, M.; Brocchini, S. Structural studies of biologically active glycosylated polyamidoamine (PAMAM) dendrimers. J. Mol. Model. 2011, 17, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Barata, T.S.; Teo, I.; Lalwani, S.; Simanek, E.; Zloh, M.; Shaunak, S. Computational design principles for bioactive dendrimer based constructs as antagonists of the TLR4-MD-2-LPS complex. Biomaterials 2011, 32, 8702–8711. [Google Scholar] [CrossRef] [PubMed]
- Barata, T.S.; Teo, I.; Brocchini, S.; Zloh, M.; Shaunak, S. Partially glycosylated dendrimers block MD-2 and prevent TLR4-MD-2-LPS complex mediated cytokine responses. PLoS Comput. Biol. 2011, 7, e1002095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, D.; Lombardini, E.; Ruamsap, N.; Imerbsin, R.; Khantapura, P.; Teo, I.; Neesanant, P.; Gonwong, S.; Yongvanitchit, K.; Swierczewski, B.E.; et al. Controlling the cytokine storm in severe bacterial diarrhoea with an oral Toll-like receptor 4 antagonist. Immunology 2015, 147, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S.; Diwan, P.V.; Jain, N.K.; Tomalia, D.A. Unexpected in vivo anti-inflammatory activity observed for simple, surface functionalized poly(amidoamine) dendrimers. Biomacromolecules 2009, 10, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Han, Y.; Liu, L.; Shen, W.; Zhang, H.; Wang, Y.; Cui, X.; Wang, Y.; Liu, G.; Qi, R. Protective effects and mechanisms of G5 PAMAM dendrimers against acute pancreatitis induced by caerulein in mice. Biomacromolecules 2015, 16, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Rele, S.M.; Cui, W.; Wang, L.; Hou, S.; Barr-Zarse, G.; Tatton, D.; Gnanou, Y.; Esko, J.D.; Chaikof, E.L. Dendrimer-like PEO glycopolymers exhibit anti-inflammatory properties. J. Am. Chem. Soc. 2005, 127, 10132–10133. [Google Scholar] [CrossRef] [PubMed]
- Dernedde, J.; Rausch, A.; Weinhart, M.; Enders, S.; Tauber, R.; Licha, K.; Schirner, M.; Zügel, U.; von Bonin, A.; Haag, R. Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 19679–19684. [Google Scholar] [CrossRef] [PubMed]
- Ledall, J.; Fruchon, S.; Garzoni, M.; Pavan, G.M.; Caminade, A.M.; Turrin, C.O.; Blanzat, M.; Poupot, R. Interaction studies reveal specific recognition of an anti-inflammatory polyphosphorhydrazone dendrimer by human monocytes. Nanoscale 2015, 7, 17672–17684. [Google Scholar] [CrossRef] [PubMed]
- Fruchon, S.; Poupot, M.; Martinet, L.; Turrin, C.O.; Majoral, J.P.; Fournié, J.J.; Caminade, A.M.; Poupot, R. Anti-inflammatory and immunosuppressive activation of human monocytes by a bioactive dendrimer. J. Leukoc. Biol. 2009, 85, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Rolland, O.; Turrin, C.O.; Bacquet, G.; Poupot, R.; Poupot, M.; Caminade, A.M.; Majoral, J.P. Efficient synthesis of phosphorus-containing dendrimers capped with isosteric functions of amino-bis(methylene) phosphonic acids. Tetrahedron Lett. 2009, 50, 2078–2082. [Google Scholar] [CrossRef]
- Caminade, A.M.; Fruchon, S.; Turrin, C.O.; Poupot, M.; Ouali, A.; Maraval, A.; Garzoni, M.; Maly, M.; Furer, V.; Kovalenko, V.; et al. The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nat. Commun. 2015, 6, 7722. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournié, J.J.; Cantagrel, A.; et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Poupot, M.; Baron, M.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournié, J.J.; Cantagrel, A.; Poupot, R.; et al. Frequency and route of administration in the treatment of experimental arthritis by phosphorus-based dendrimer. Ann. Rheum. Dis. 2012, 71. [Google Scholar] [CrossRef]
- Hayder, M.; Varilh, M.; Turrin, C.O.; Saoudi, A.; Caminade, A.M.; Poupot, R.; Liblau, R.S. Phosphorus-based dendrimer ABP treats neuroinflammation by promoting IL-10-producing CD4+ T cells. Biomacromolecules 2015, 16, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- Degboé, Y.; Fruchon, S.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Poupot, R.; Cantagrel, A.; Davignon, J.L. Modulation of pro-inflammatory activation of monocytes and dendritic cells by aza-bis-phosphonate dendrimer as an experimental therapeutic agent. Arthritis Res. Ther. 2014, 16, R98. [Google Scholar] [CrossRef] [PubMed]
- Portevin, D.; Poupot, M.; Rolland, O.; Turrin, C.O.; Fournié, J.J.; Majoral, J.P.; Caminade, A.M.; Poupot, R. Regulatory activity of azabisphosphonate-capped dendrimers on human CD4+ T cell proliferation enhances ex-vivo expansion of NK cells from PBMCs for immunotherapy. J. Transl. Med. 2009, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Fruchon, S.; Caminade, A.M.; Abadie, C.; Davignon, J.L.; Combette, J.M.; Turrin, C.O.; Poupot, R. An azabisphosphonate-capped poly(phosphorhydrazone) dendrimer for the treatment of endotoxin-induced uveitis. Molecules 2013, 18, 9305–9316. [Google Scholar] [CrossRef] [PubMed]
- Blattes, E.; Vercellone, A.; Eutamène, H.; Turrin, C.O.; Théodorou, V.; Majoral, J.P.; Caminade, A.M.; Prandi, J.; Nigou, J.; Puzo, G. Mannodendrimers prevent acute lung inflammation by inhibiting neutrophil recruitment. Proc. Natl. Acad. Sci. USA 2013, 110, 8795–8800. [Google Scholar] [CrossRef] [PubMed]
- Durocher, I.; Girard, D. In vivo proinflammatory activity of generations 0–3 (G0–G3) polyamidoamine (PAMAM) nanoparticles. Inflamm. Res. 2016, 65, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Fruchon, S.; Mouriot, S.; Thiollier, T.; Grandin, C.; Caminade, A.M.; Turrin, C.O.; Contamin, H.; Poupot, R. Repeated intravenous injections in non-human primates demonstrate preclinical safety of an anti-inflammatory phosphorus-based dendrimer. Nanotoxicology 2015, 9, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Germolec, D.R.; Weaver, J.L. Evaluation of nanoparticle immunotoxicity. Nat. Nanotechnol. 2009, 4, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. Br. J. Pharmacol. 2014, 171, 3988–4000. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fruchon, S.; Poupot, R. Pro-Inflammatory Versus Anti-Inflammatory Effects of Dendrimers: The Two Faces of Immuno-Modulatory Nanoparticles. Nanomaterials 2017, 7, 251. https://doi.org/10.3390/nano7090251
Fruchon S, Poupot R. Pro-Inflammatory Versus Anti-Inflammatory Effects of Dendrimers: The Two Faces of Immuno-Modulatory Nanoparticles. Nanomaterials. 2017; 7(9):251. https://doi.org/10.3390/nano7090251
Chicago/Turabian StyleFruchon, Séverine, and Rémy Poupot. 2017. "Pro-Inflammatory Versus Anti-Inflammatory Effects of Dendrimers: The Two Faces of Immuno-Modulatory Nanoparticles" Nanomaterials 7, no. 9: 251. https://doi.org/10.3390/nano7090251




