TiO2 Nanobelt@Co9S8 Composites as Promising Anode Materials for Lithium and Sodium Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
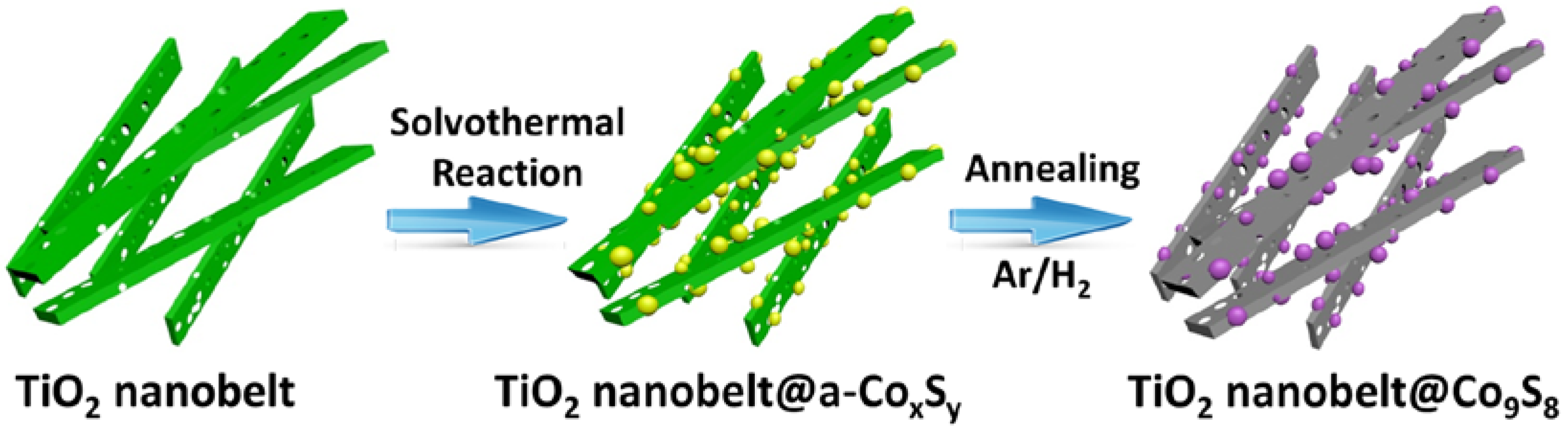
2.1. Synthesis of mixed Phase TiO2 Nanobelt@Co9S8 Composites
2.2. Sample Characterization
2.3. Electrochemical Measurements
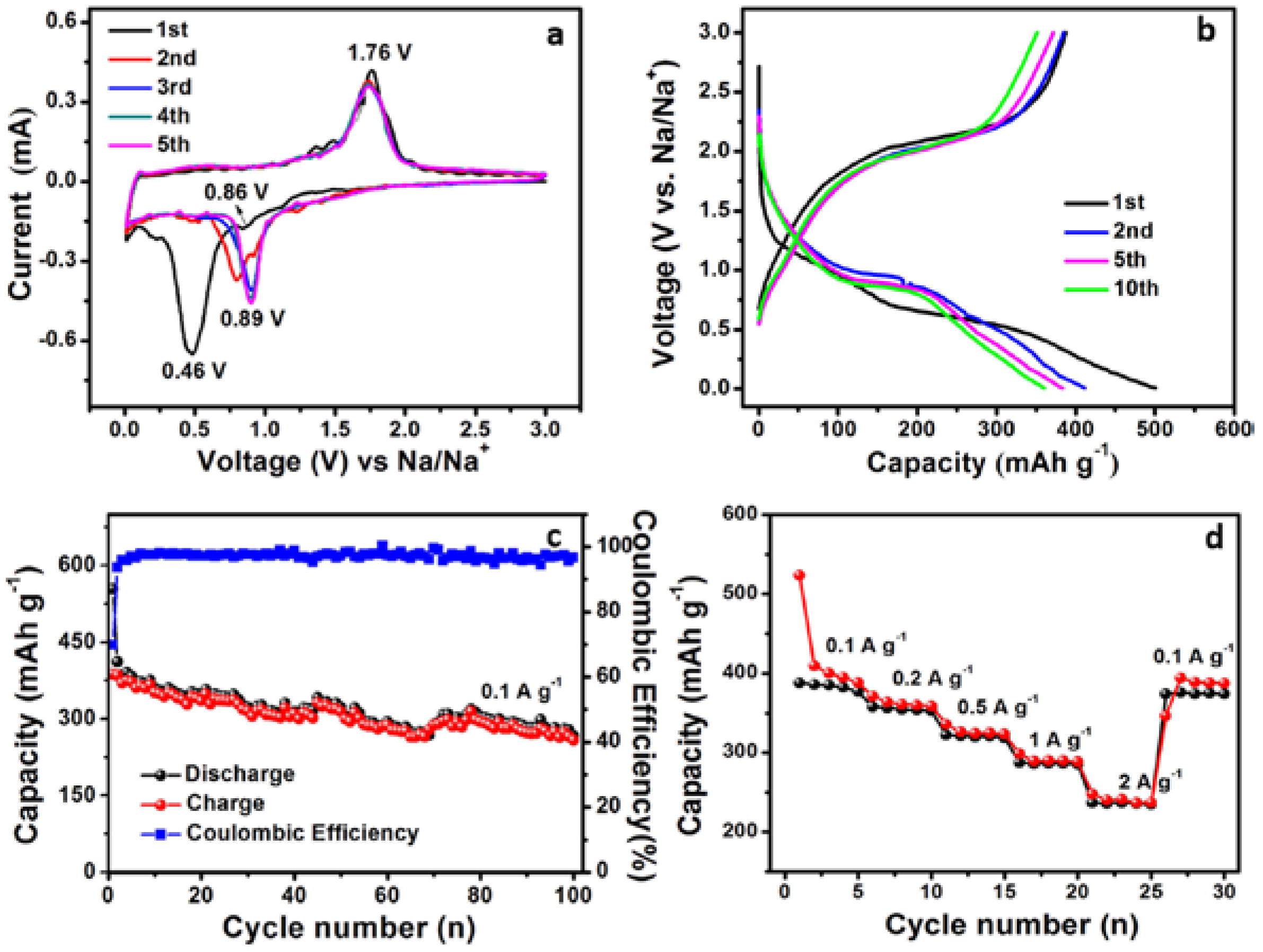
3. Results and Discussion
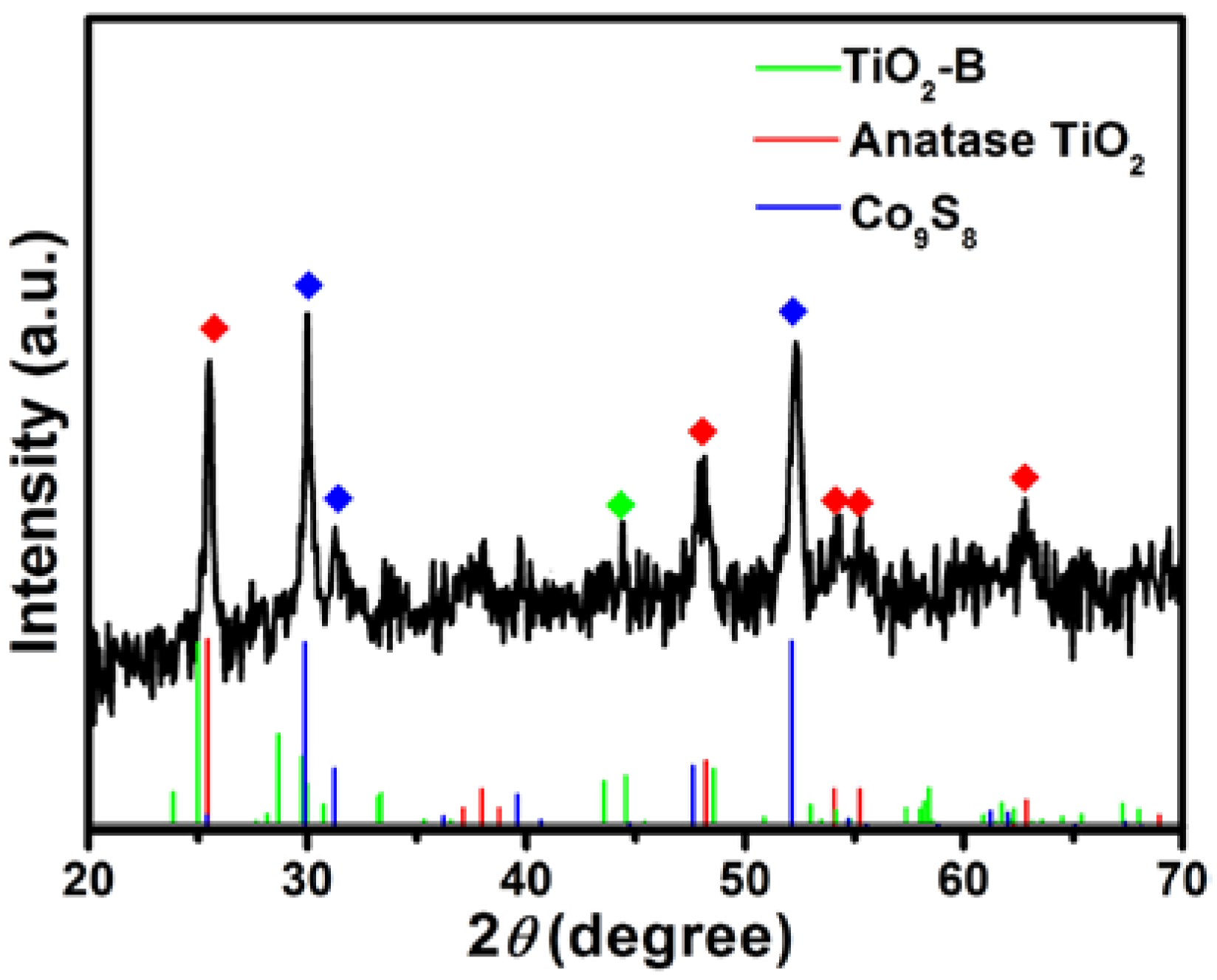
3.1. Characterization of Samples
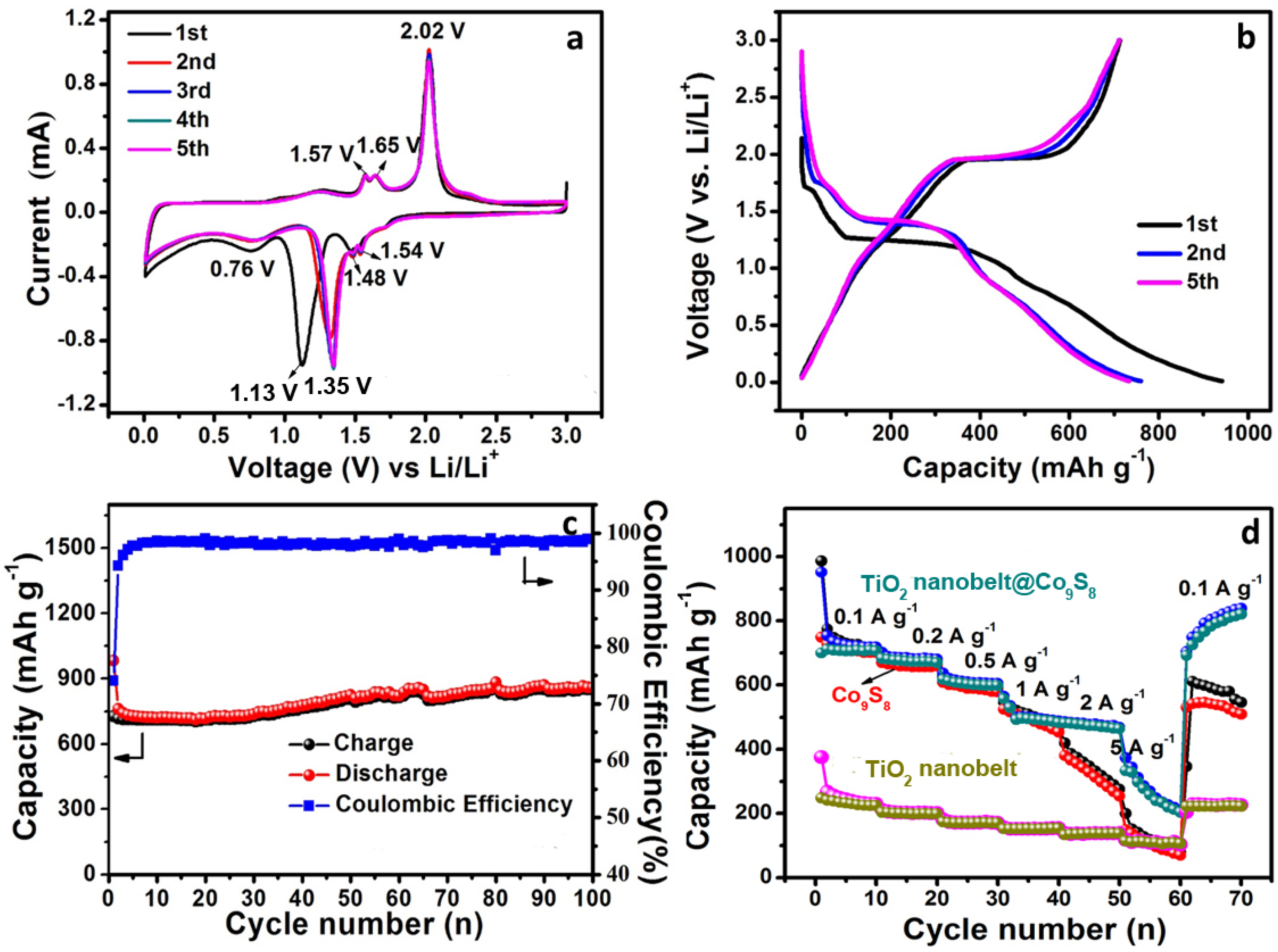
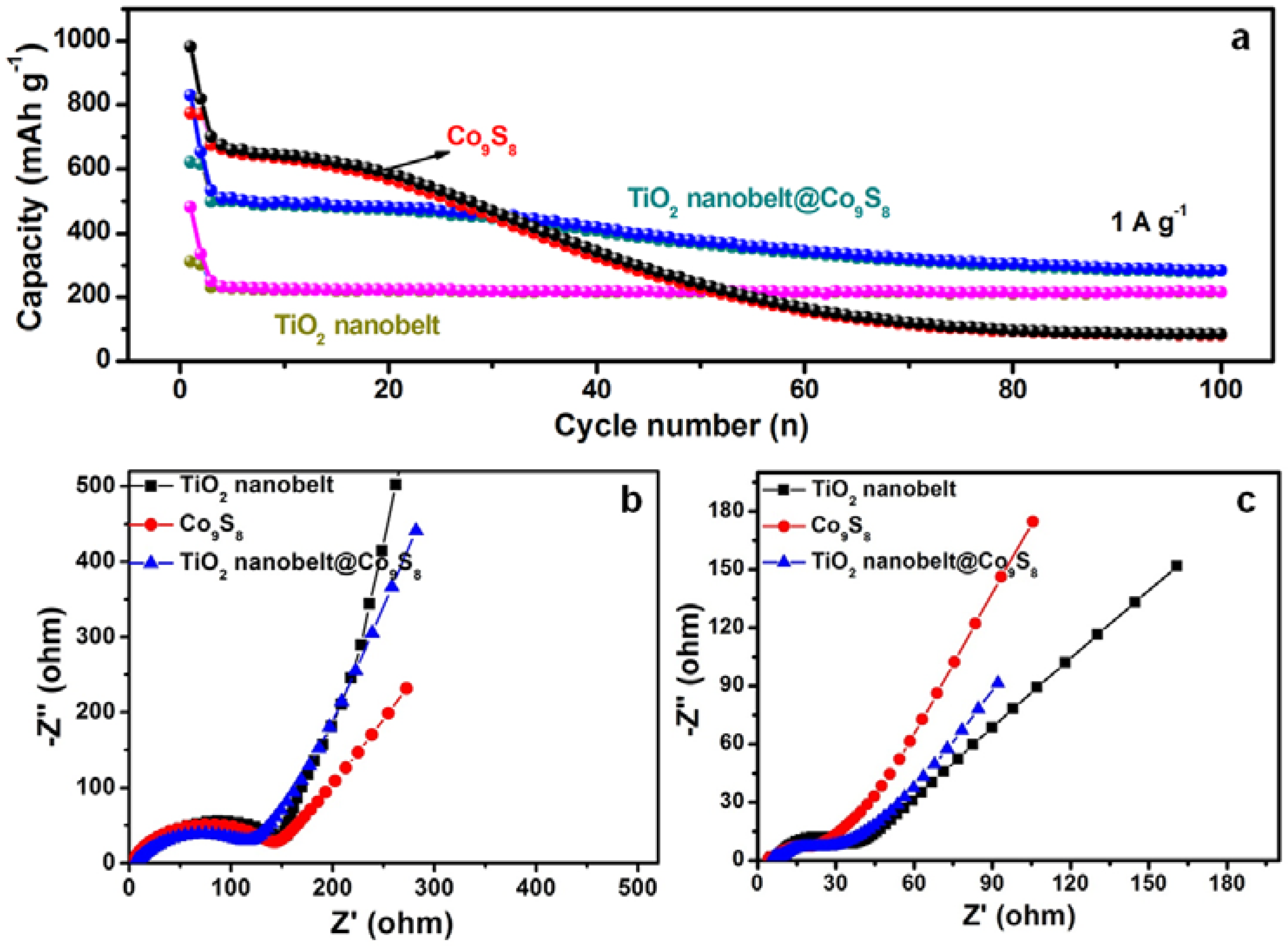
3.2. Electrochemical Performance of TiO2 Nanobelt@Co9S8 Composites for LIBs
3.3. Electrochemical Performance of TiO2 Nanobelt@Co9S8 Composites for SIBs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.P.; Yang, H.X. Multi-electron reaction materials for high energy density batteries. Energy Environ. Sci. 2010, 3, 174–189. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, L.; Shu, H.; Yang, X.; Wang, H.; Tan, J.; Zhou, Q.; Huang, Z.; Wang, X. A tightly integrated sodium titanate-carbon composite as an anode material for rechargeable sodium ion batteries. J. Power Sources 2015, 274, 8–14. [Google Scholar] [CrossRef]
- Liang, M.; Zhi, L. Graphene-based electrode materials for rechargeable lithium batteries. J. Mater. Chem. 2009, 19, 5871–5878. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, X.; Chen, L.; Yue, J.; Xu, H.; Yang, J.; Qian, Y. Novel mesoporous silicon nanorod as an anode material for lithium ion batteries. Electrochim. Acta 2014, 127, 252–258. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Liu, Y.; Wang, C. Electrochemical performance of porous carbon/tin composite anodes for sodium-ion and lithium-ion batteries. Adv. Energy Mater. 2013, 3, 128–133. [Google Scholar] [CrossRef]
- Zhu, C.; Mu, X.; Aken, P.A.; Yu, Y.; Maier, J. Single-layered ultrasmall nanoplates of MoS2 embedded in carbon nanofibers with excellent electrochemical performance for lithium and sodium storage. Angew. Chem. Int. Ed. 2014, 53, 2152–2156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D.; Xu, H.; Feng, J.; Jiang, X.; Yue, J.; Yang, J.; Qian, Y. Hollow nanospheres of mesoporous Co9S8 as a high-capacity and long-life anode for advanced lithium ion batteries. Nano Energy 2015, 12, 528–537. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, G.; Liu, W.; Quan, B.; Liang, X.; Shang, C.; Cheng, Y.; Du, Y. Thermal conversion of an Fe3O4@metal-organic framework: A new method for an efficient Fe-Co/nanoporous carbon microwave absorbing material. Nanoscale 2015, 7, 12932–12942. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wang, W.; Zeng, P.; Fang, Z. Effects of binders on electrochemical properties of the SnS2 nanostructured anode of the lithium-ion batteries. Langmuir 2017, 33, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.L.; Li, J.; Deshpande, R.D.; Subramanian, N.; Rankin, S.E.; Yang, F.; Cheng, Y.T. Aligned TiO2 nanotube arrays as durable lithium-ion battery negative electrodes. J. Phys. Chem. C 2012, 116, 18669–18677. [Google Scholar] [CrossRef]
- Han, H.; Song, T.; Lee, E.K.; Devadoss, A.; Jeon, Y.; Ha, J.; Chung, Y.C.; Choi, Y.M.; Jung, Y.G.; Paik, U. Dominant factors governing the rate capability of a TiO2 nanotube anode for high power lithium ion batteries. ACS Nano 2012, 6, 8308–8315. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Dou, S.; Wang, G. Anatase TiO2: Better anode material than amorphous and rutile phases of TiO2 for Na-ion batteries. Chem. Mater. 2015, 27, 6022–6029. [Google Scholar] [CrossRef]
- Bi, Z.; Paranthaman, M.P.; Menchhofer, P.A.; Dehoff, R.R.; Bridges, C.A.; Chi, M.; Guo, B.; Sun, X.G.; Dai, S. Self-organized amorphous TiO2 nanotube arrays on porous Ti foam for rechargeable lithium and sodium ion batteries. J. Power Sources 2013, 222, 461–466. [Google Scholar] [CrossRef]
- Qiu, J.; Li, S.; Gray, E.; Liu, H.; Gu, Q.F.; Sun, C.; Lai, C.; Zhao, H.; Zhang, S. Hydrogenation synthesis of blue TiO2 for high-performance lithium-ion batteries. J. Phys. Chem. C 2014, 118, 8824–8830. [Google Scholar] [CrossRef]
- Li, J.M.; Wan, W.; Zhou, H.H.; Li, J.J.; Xu, D.S. Hydrothermal synthesis of TiO2 (B) nanowires with ultrahigh surface area and their fast charging and discharging properties in Li-ion batteries. Chem. Commun. 2011, 47, 3439–3441. [Google Scholar] [CrossRef] [PubMed]
- Brutti, S.; Gentili, V.; Menard, H.; Scrosati, B.; Bruce, P.G. TiO2-(B) nanotubes as anodes for lithium batteries: Origin and mitigation of irreversible capacity. Adv. Energy Mater. 2012, 2, 322–327. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Nedelec, J.M.; Daniel, G.; Tiseanu, C.; Parvulescu, V.; Pol, V.G.; Abrego, L.; Kessler, V.G. Mesoporous anatase TiO2 nanorods as thermally robust anode materials for Li-ion batteries: Detailed insight into the formation mechanism. Chem. Eur. J. 2013, 19, 17439–17444. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.W.; Lee, D.K.; Cho, I.S.; Hong, K.S.; Kim, D.W. Facile hydrothermal synthesis of porous TiO2 nanowire electrodes with high-rate capability for Li ion batteries. Nanotechnology 2010, 21, 255706. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, C.; Wang, X.; Fan, H.J. A high energy and power Li-ion capacitor based on a TiO2 nanobelt array anode and a graphene hydrogel cathode. Small 2015, 11, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Wu, J.; Jiang, Y.; Yu, S.; Bai, J.; Cao, M.; Cui, J. Anatase TiO2 ultrathin nanobelts derived from room-temperature-synthesized titanates for fast and safe lithium storage. Sci. Rep. 2015, 5, 11804. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, Z.; Zhang, L.; Wu, H.B.; Lou, X.W. TiO2 nanotube arrays grafted with Fe2O3 hollow nanorods as integrated electrodes for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 122–127. [Google Scholar] [CrossRef]
- Mao, M.; Mei, L.; Guo, D.; Wu, L.; Zhang, D.; Li, Q.; Wang, T. High electrochemical performance based on the TiO2 nanobelt@few-layered MoS2 structure for lithium-ion batteries. Nanoscale 2014, 6, 12350–12353. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Fang, J.; Xia, Y.; Tao, X.; Gan, Y.; Du, J.; Zhu, W.; Zhang, W. Construction of sheet-belt hybrid nanostructures from one-dimensional mesoporous TiO2 (B) nanobelts and graphene sheets for advanced lithium-ion batteries. J. Mater. Chem. A 2013, 1, 2495–2500. [Google Scholar] [CrossRef]
- Huang, P.; Yuan, D.D.; Zhang, H.Z.; Cao, Y.L.; Li, G.R.; Yang, H.X.; Gao, X.P. Electrochemical sodium storage of TiO2 (B) nanotubes for sodium ion batteries. RSC Adv. 2013, 3, 12593–12597. [Google Scholar] [CrossRef]
- Wu, L.; Bresser, D.; Buchholz, D.; Passerini, S. Nanocrystalline TiO2 (B) as anode material for sodium-ion batteries. J. Electrochem. Soc. 2015, 162, A3052–A3058. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Liu, L.; Tan, J.; Zhou, Q.; Huang, Z.; Xia, D.; Shu, H.; Yang, X.; Wang, X. One-pot synthesis of bicrystalline titanium dioxide spheres with a core-shell structure as anode materials for lithium and sodium ion batteries. J. Power Sources 2014, 269, 37–45. [Google Scholar] [CrossRef]
- Zukalova, M.; Kalbac, M.; Kavan, L.; Exnar, I.; Graetzel, M. Pseudocapacitive lithium storage in TiO2 (B). Chem. Mater. 2005, 17, 1248–1255. [Google Scholar] [CrossRef]
- Liu, H.; Bi, Z.; Sun, X.G.; Unocic, R.R.; Paranthaman, M.P.; Dai, S.; Brown, G.M. Mesoporous TiO2-B microspheres with superior rate performance for lithium ion batteries. Adv. Mater. 2011, 23, 3450–3454. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, J.; Yang, X.; Lu, F.; He, S.; Yang, J.; Fan, H.J.; Wu, M. Ultrathin anatase TiO2 nanosheets embedded with TiO2-B nanodomains for lithium-ion storage: Capacity enhancement by phase boundaries. Adv. Energy Mater. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Xia, H.; Xiong, W.; Lim, C.K.; Yao, Q.; Wang, Y.; Xie, J. Hierarchical TiO2-B nanowire@α-Fe2O3 nanothorn core-branch arrays as superior electrodes for lithium-ion microbatteries. Nano Res. 2014, 7, 1797–1808. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, J.; Wang, T.; Zhang, H.; Wei, C.; Zhang, K.; Yue, Y. TiO2-B nanoribbons anchored with NiO nanosheets as hybrid anode materials for rechargeable Lithium Ion Batteries. CrystEngComm 2015, 17, 1710–1715. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.; Zhao, Z.; Zhou, W.; Wang, D.; Kang, X.; Liu, H.; Wang, J.; Chen, S.; Cai, H.; Huang, H. Enhanced photocatalytic performances of CeO2/TiO2 nanobelt heterostructures. Small 2013, 9, 3864–3872. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D.; Xu, H.; Liu, S.; Yang, J.; Qian, Y. Multiwalled carbon nanotube@a-C@Co9S8 nanocomposites: A high-capacity and long-life anode material for advanced lithium ion batteries. Nanoscale 2015, 7, 3520–3525. [Google Scholar] [CrossRef] [PubMed]
- Prochazka, J.; Kavan, L.; Zukalova, M.; Frank, O.; Kalbac, M.; Zukal, A.; Klementova, M.; Carbone, D.; Graetzel, M. Novel synthesis of the TiO2 (B) multilayer templated films. Chem. Mater. 2009, 21, 1457–1464. [Google Scholar] [CrossRef]
- Chen, C.; Hu, X.; Jiang, Y.; Yang, Z.; Hu, P.; Huang, Y. TiO2-B Nanosheets/Anatase Nanocrystals Co-Anchored on Nanoporous Graphene: In Situ Reduction-Hydrolysis Synthesis and Their Superior Rate Performance as an Anode Material. Chem. Eur. J. 2014, 20, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Tang, Y.; Lu, X.; Yang, C.; Qin, M.; Huang, F.; Li, X.; Zhang, X. Phase-controlled synthesis of cobalt sulfides for lithium ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 4246–4250. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.T.; Kim, D.W.; Yoo, P.J.; Chiang, C.Y.; Meethong, N.; Hammond, P.T.; Chiang, Y.M.; Belcher, A.M. Virus-enabled synthesis and assembly of nanowires for lithium ion battery electrodes. Science 2006, 312, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhu, J.; Rui, X.; Cao, X.; Chen, C.; Zhang, H.; Hng, H.H.; Yan, Q. Controlled synthesis of carbon-coated cobalt sulfide nanostructures in oil phase with enhanced Li storage performances. ACS Appl. Mater. Interfaces 2012, 4, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Campos, H.; Villarreal, J.; Alcoutlabi, M. A comparative study on the performance of binary SnO2/NiO/C and Sn/C composite nanofibers as alternative anode materials for lithium ion batteries. Electrochim. Acta 2017, 224, 608–621. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Garza, D.D.; Gallegos, L.; Pokhrel, M.; Alcoutlabi, M. Forcespinning: A new method for the mass production of Sn/Ccomposite nanofiber anodes for lithium ion batteries. Solid State Ion. 2016, 286, 72–82. [Google Scholar] [CrossRef]
- Yu, P.; Ritter, J.A.; White, R.E.; Popov, B.N. Ni-composite microencapsulated graphite as the negative electrode in lithium-ion batteries I. Initial irreversible capacity study. J. Electrochem. Soc. 2000, 147, 1280–1285. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Wang, J.; Zhang, F.; Wang, L. Highly uniform Co9S8 nanoparticles grown on graphene nanosheets as advanced anode materials for improved Li-storage performance. Appl. Surf. Sci. 2016, 390, 86–91. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Z.; Pourpoint, F.; Armstrong, A.R.; Grey, C.P.; Bruce, P.G. Nanoparticulate TiO2 (B): An anode for lithium-ion batteries. Angew. Chem. 2012, 124, 2206–2209. [Google Scholar] [CrossRef]
- Zuniga, L.; Agubra, V.; Flores, D.; Campos, H.; Villareal, J.; Alcoutlabi, M. Multichannel hollow structure for improved electrochemical performance of TiO2/Carbon composite nanofibers as anodes for lithium ion batteries. J. Alloys Compd. 2016, 686, 733–743. [Google Scholar] [CrossRef]
- Amaresh, S.; Karthikeyan, K.; Jang, I.C.; Lee, Y.S. Single-step microwave mediated synthesis of CoS2 anode material for high rate hybrid supercapacitors. J. Mater. Chem. A 2014, 2, 11099–11106. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Zhao, Y.; Dong, Y.; Fan, Q.; Kuang, Q. Synthesis of the carbon-coated nanoparticle Co9S8 and its electrochemical performance as an anode material for sodium-ion batteries. Langmuir 2016, 32, 12593–12602. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Tan, C.Y.J.; Gao, Z. Template-free formation of carbon nanotube-supported cobalt sulfide@carbon hollow nanoparticles for stable and fast sodium ion storage. J. Power Sources 2017, 339, 41–50. [Google Scholar] [CrossRef]
- Yang, H.; Duh, J.G. Aqueous sol-gel synthesized anatase TiO2 nanoplates with high-rate capabilities for lithium ion and sodium-ion batteries. RSC Adv. 2016, 6, 37160–37166. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhu, Q.; Tian, J.; Jiang, F. TiO2 Nanobelt@Co9S8 Composites as Promising Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials 2017, 7, 252. https://doi.org/10.3390/nano7090252
Zhou Y, Zhu Q, Tian J, Jiang F. TiO2 Nanobelt@Co9S8 Composites as Promising Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials. 2017; 7(9):252. https://doi.org/10.3390/nano7090252
Chicago/Turabian StyleZhou, Yanli, Qian Zhu, Jian Tian, and Fuyi Jiang. 2017. "TiO2 Nanobelt@Co9S8 Composites as Promising Anode Materials for Lithium and Sodium Ion Batteries" Nanomaterials 7, no. 9: 252. https://doi.org/10.3390/nano7090252




