Construction of Hierarchical CuO/Cu2O@NiCo2S4 Nanowire Arrays on Copper Foam for High Performance Supercapacitor Electrodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
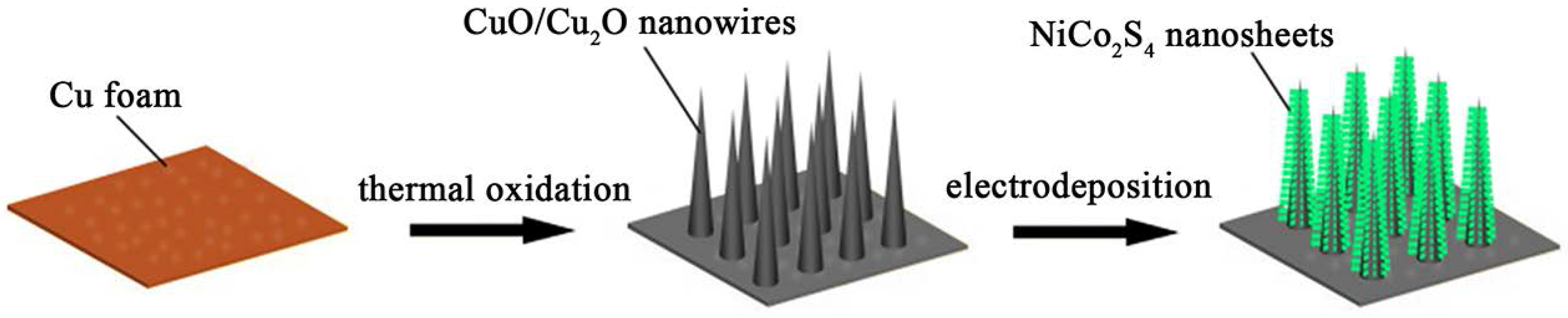
2.2. Fabrication of CuO/Cu2O Nanowire Arrays on Cu Foam
2.3. Synthesis of CuO/Cu2O@NiCo2S4 Core-shell Nanostructures
2.4. Material Characterization
2.5. Electrochemical Measurements
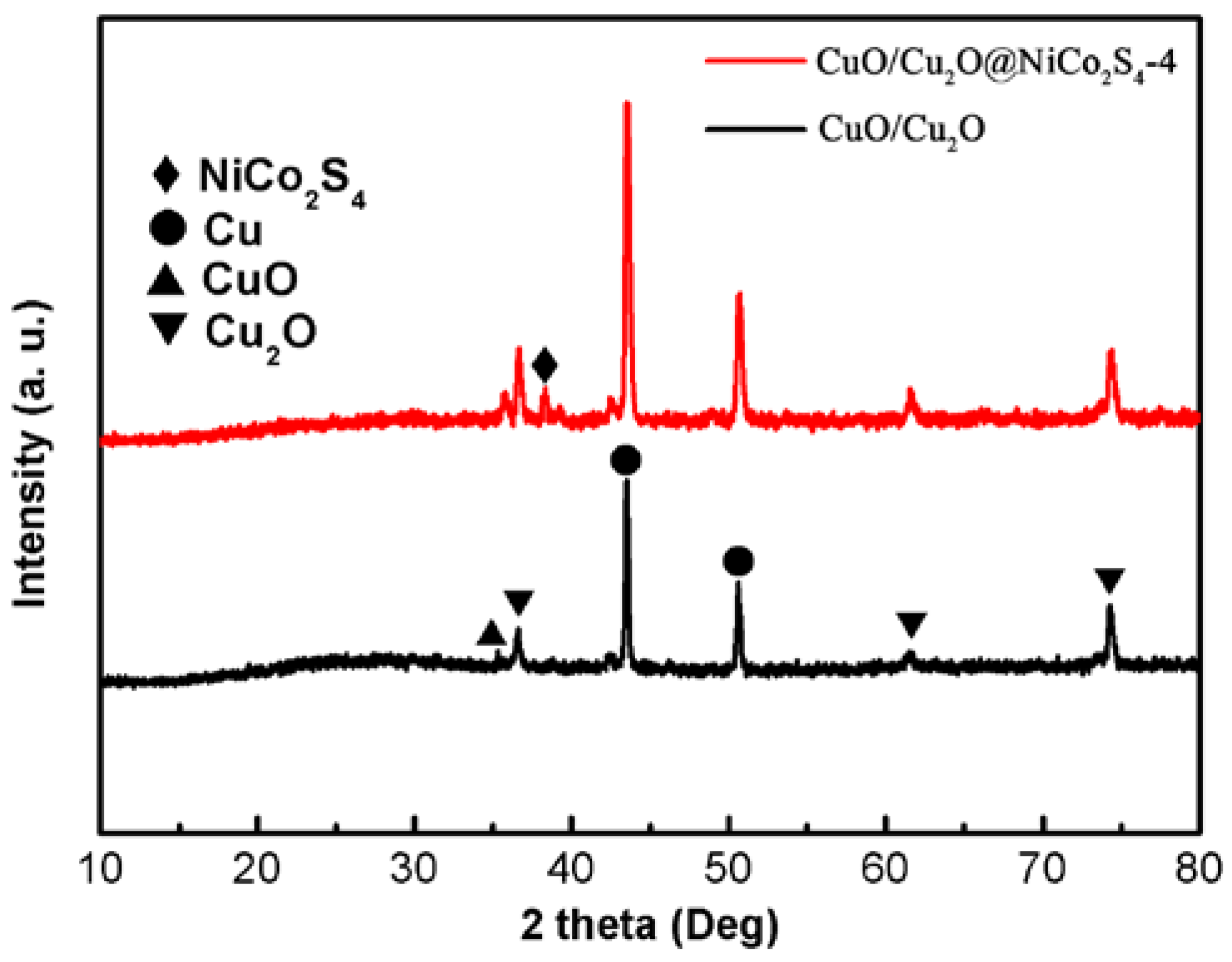
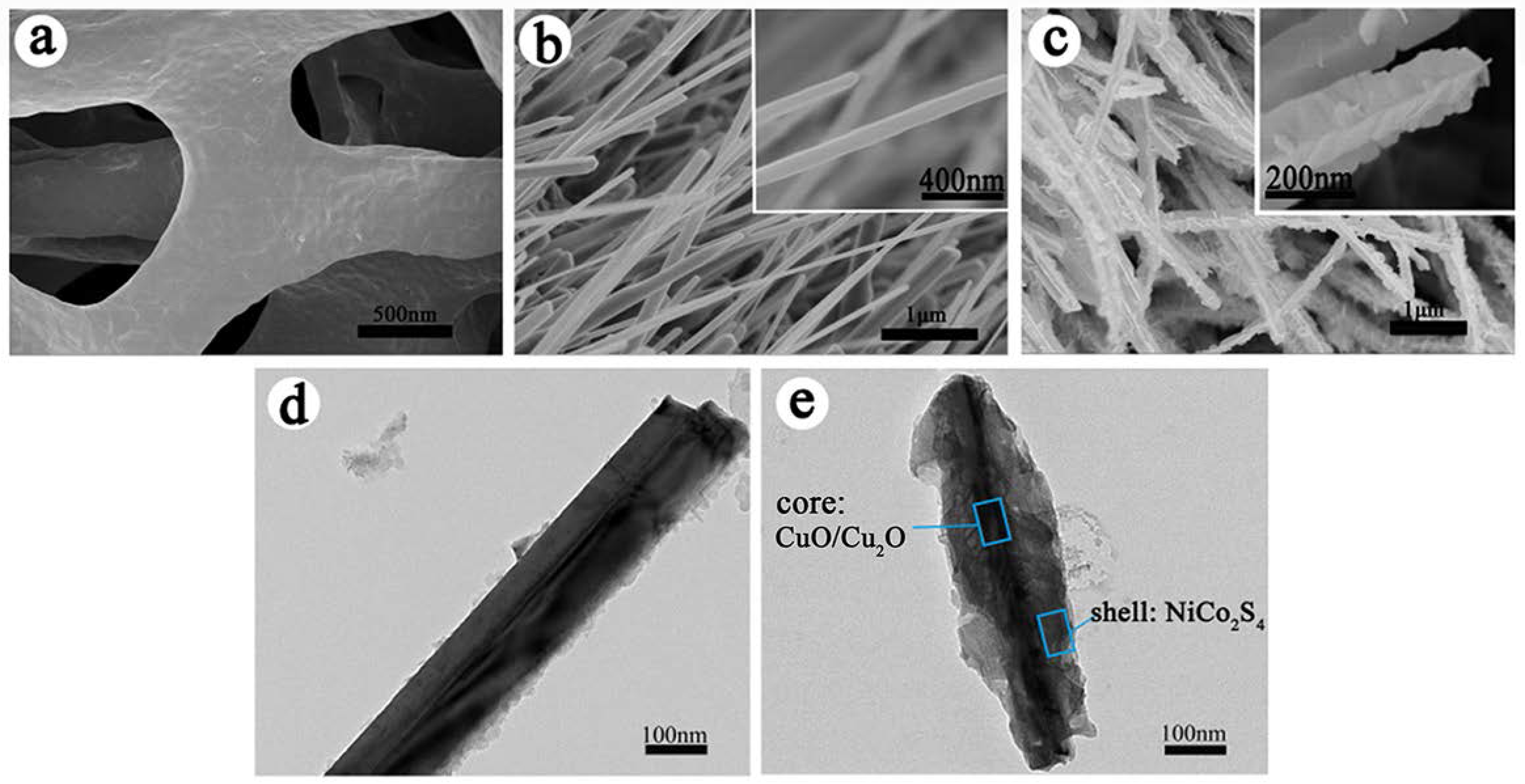
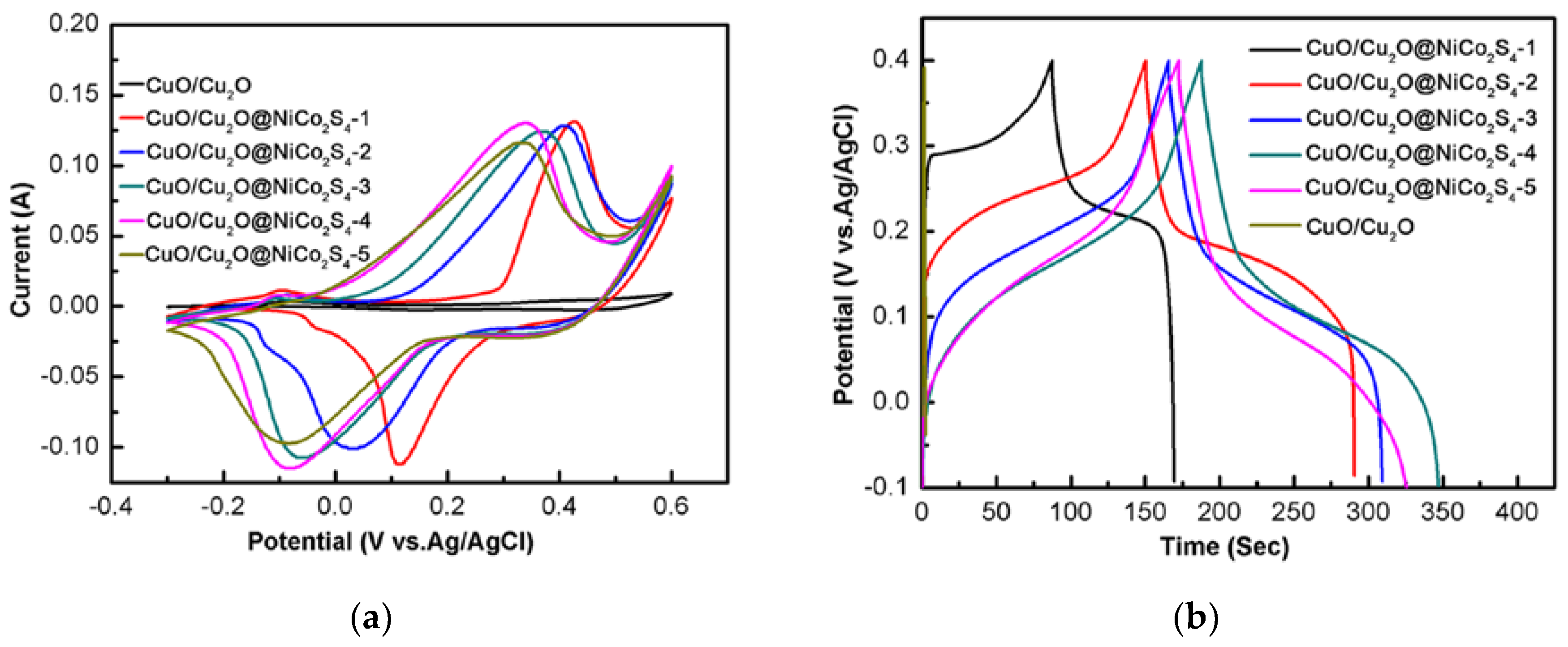
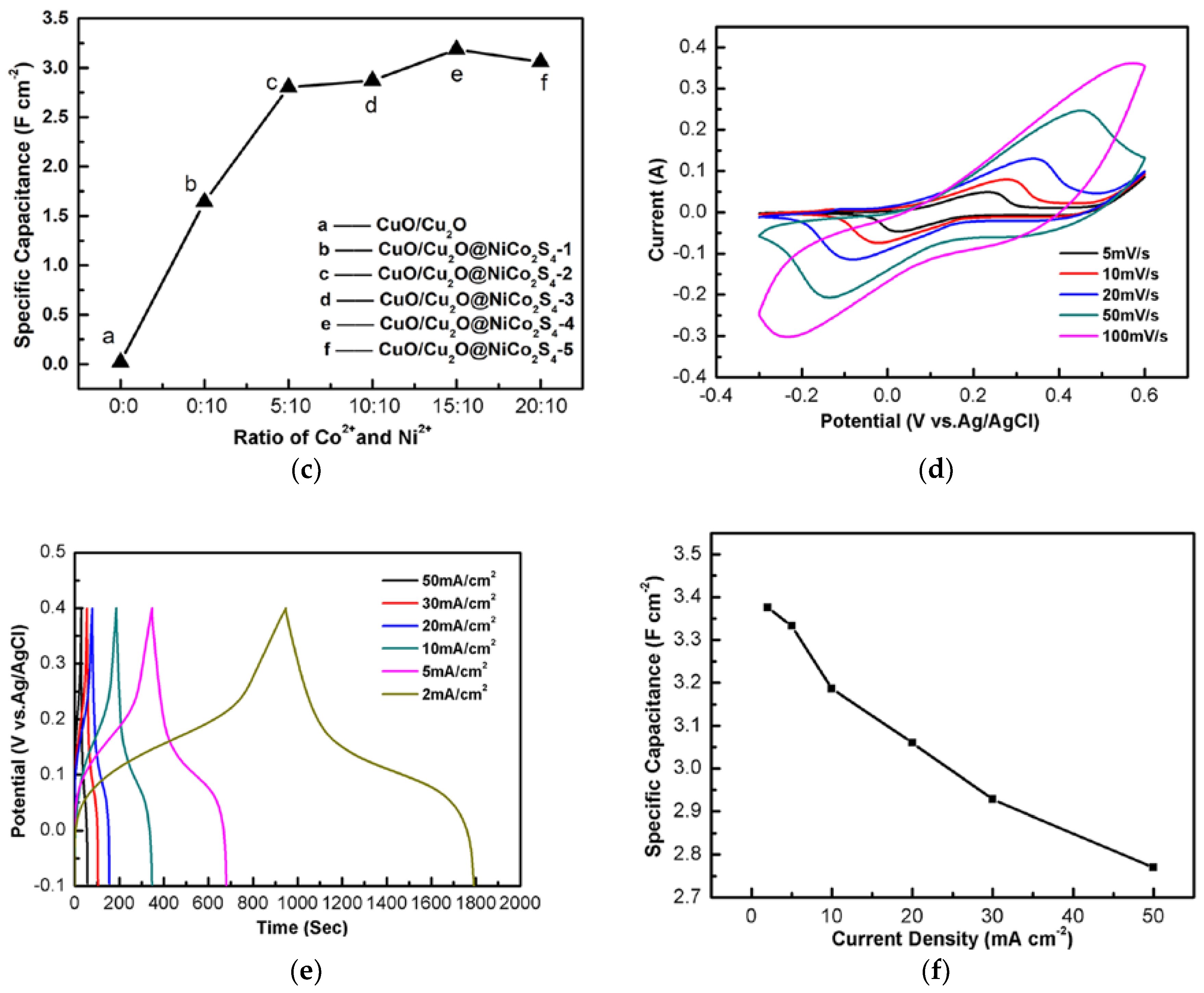
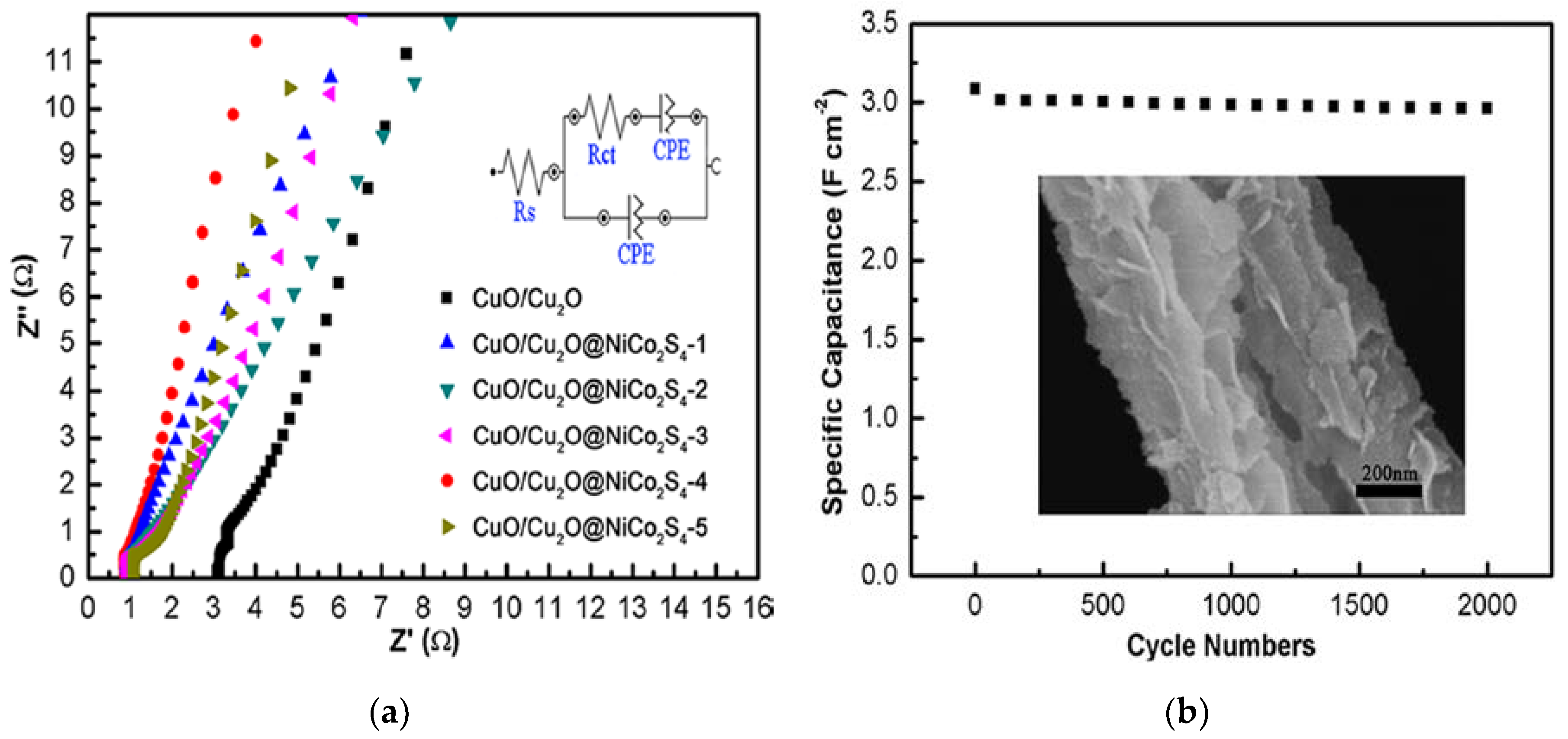
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Duong, B.; Abbitt, D.; Thomas, J. Highly ordered MnO2 nanopillars for enhanced supercapacitor performance. Adv. Mater. 2013, 25, 3302–3306. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; McDonagh, A.; Qiao, S.; Wang, G. High-capacity aqueous potassium-ion batteries for large-scale energy storage. Adv. Mater. 2017, 29, 1604007. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Chen, Q.; Gerhardt, M.; Tong, L.; Kim, S.; Eisenach, L.; Valle, A.; Hardee, D.; Gordon, R.; Aziz, M.; et al. Alkaline quinone flow battery. Science 2015, 349, 1529–1532. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Tao, J.; Meng, W.; Zhu, M.; Huang, Y.; Fu, Y.; Gao, Y.; Zhi, C. Super-high rate stretchable polypyrrole-based supercapacitors with excellent cycling stability. Nano Energy 2015, 11, 518–525. [Google Scholar] [CrossRef]
- Chen, T.; Hao, R.; Peng, H.; Dai, L. High-performance, stretchable, wire-shaped supercapacitors. Angew. Chem. Int. Ed. 2015, 54, 618–622. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Lai, P.M.; Cheng, H. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Nitrogen-doped activated carbon for a high energy hybrid supercapacitor. Energy Environ. Sci. 2016, 9, 102–106. [Google Scholar] [CrossRef]
- Gawli, Y.; Banerjee, A.; Dhakras, D.; Deo, M.; Bulani, D.; Wadgaonkar, P.; Shelke, M.; Ogale, S. 3D polyaniline architecture by concurrent inorganic and organic acid doping for superior and robust high rate supercapacitor performance. Sci. Rep. 2016, 6, 21002. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y.; Yang, G. A flexible, transparent and super-long-life supercapacitor based on ultrafine Co3O4 nanocrystal electrodes. Nanoscale 2016, 8, 4227–4235. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; He, P.; Wang, D.; Zhang, Q.; Chen, T.; Fisher, T. Hierarchical Ni–Co hydroxide petals on mechanically robust graphene petal poam for high-energy asymmetric supercapacitors. Adv. Funct. Mater. 2016, 26, 5460–5470. [Google Scholar] [CrossRef]
- Dai, Z.; Zang, X.; Yang, J.; Sun, C.; Si, W.; Huang, W.; Dong, X. Template synthesis of shape-tailorable NiS2 hollow prisms as high-performance supercapacitor materials. ACS Appl. Mater. Interfaces 2015, 7, 25396–25401. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guan, G.; Gui, Y.; Blackwood, D. Rational design of self-supported Ni3S2 nanosheets array for advanced asymmetric supercapacitor with a superior energy density. ACS Appl. Mater. Interfaces 2017, 9, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mao, C.; Niu, Y.; Yi, F.; Hou, J.; Lu, S.; Jiang, J.; Xu, M.; Li, C. Facile synthesis of novel networked ultralong cobalt sulde nanotubes and its application in supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 25568–25573. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Li, Z.; Farha, O.; Hupp, J. Atomically precise growth of catalytically active cobalt sulde on flat surfaces and within a metal-organic framework via atomic layer deposition. ACS Nano 2015, 9, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Yu, L.; Wang, X.; Song, S.; Lou, X. Formation of onion-like NiCo2S4 particles via sequential ion-exchange for hybrid supercapacitors. Adv. Mater. 2017, 29, 1605051. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Bao, R.; Chen, Z.; Rehan, M.; Tong, L.; Pang, G.; Yuan, C. Comparative investigation of hollow mesoporous NiCo2S4 ellipsoids with enhanced pseudo-capacitances towards high-performance asymmetric supercapacitors. Electrochim. Acta 2016, 214, 76–84. [Google Scholar] [CrossRef]
- Kong, W.; Lu, C.; Zhang, W.; Pu, J.; Wang, Z. Homogeneous core-shell NiCo2S4 nanostructures supported on nickel foam for supercapacitors. J. Mater. Chem. A 2015, 3, 12452–12460. [Google Scholar] [CrossRef]
- Xiao, J.; Zeng, X.; Chen, W.; Xiao, F.; Wang, S. High electrocatalytic activity of self-standing hollow NiCo2S4 single crystalline nanorod arrays towards sulfide redox shuttles in quantum dot-sensitized solar cells. Chem. Commun. 2013, 49, 11734–11736. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-G.; Jin, D.; Zhou, R.; Shen, C.; Xie, K.; Wei, B. One-step synthesis of NiCo2S4 ultrathin nanosheets on conductive substrates as advanced electrodes for high-efficient energy storage. J. Power Sources 2016, 306, 100–106. [Google Scholar] [CrossRef]
- Wang, J.-G.; Zhou, R.; Jin, D.; Xie, K.; Wei, B. Controlled synthesis of NiCo2S4 nanostructures on nickel foams for high-performance supercapacitors. Energy Storage Mater. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Xu, G.; Li, H.; Dou, H.; Zhang, X. NiCo2S4 Nanosheets grown on nitrogen-doped carbon foams as an advanced electrode for supercapacitors. Adv. Energy Mater. 2015, 5, 1400977. [Google Scholar] [CrossRef]
- Niu, L.; Wang, Y.; Ruan, F.; Shen, C.; Shan, S.; Xu, M.; Sun, Z.; Li, C.; Liu, X.; Gong, Y. In situ growth of NiCo2S4@Ni3V2O8 on Ni foam as binder-free electrode for asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 5669–5677. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, C.; Han, W.; Liu, Y.; Zhao, H.; Ma, Y.; Xie, E. Cobalt sulfide nanosheets coated on NiCo2S4 nanotube arrays as electrode materials for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 10492–10497. [Google Scholar] [CrossRef]
- Xu, W.; Dai, S.; Liu, G.; Xi, Y.; Hu, C.; Wang, X. CuO nanoflowers growing on carbon fiber fabric for flexible high-performance supercapacitors. Electrochim. Acta 2016, 203, 1–8. [Google Scholar] [CrossRef]
- Xu, P.; Liu, J.; Liu, T.; Ye, K.; Cheng, K.; Yin, J.; Cao, D.; Wang, G.; Li, Q. Preparation of binder-free CuO/Cu2O/Cu composites: A novel electrode material for supercapacitor applications. RSC Adv. 2016, 6, 28270–28278. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Luo, W.; Sun, Y.; Jin, C.; Zhang, W. Graphitic carbon coated CuO hollow nanospheres with penetrated mesochannels for high-performance asymmetric supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 105–111. [Google Scholar] [CrossRef]
- Yang, Y.; Pei, L.; Xu, X.; Xu, J.; Shen, J.; Ye, M. In-situ growth of self-assembled 3D Cu2O@Cu foam with enhanced electrochemical properties. Electrochim. Acta 2016, 221, 56–61. [Google Scholar] [CrossRef]
- Ruan, J.; Huo, Y.; Hu, B. Three-dimensional Ni(OH)2/Cu2O/CuO porous cluster grown on nickel foam for high performance supercapacitor. Electrochim. Acta 2016, 215, 108–113. [Google Scholar] [CrossRef]
- Li, Z.; Shao, M.; Zhou, L.; Zhang, R.; Zhang, C.; Han, J.; Wei, M.; Evans, D.; Duan, X. A flexible all-solid-state micro-supercapacitor based on hierarchical CuO@layered double hydroxide core–shell nanoarrays. Nano Energy 2016, 20, 294–304. [Google Scholar] [CrossRef]
- Zhao, J.; Shu, X.; Wang, Y.; Yu, C.; Zhang, J.; Cui, J.; Qin, Y.; Zheng, H.; Liu, J.; Zhang, Y.; et al. Construction of CuO/Cu2O@CoO core-shell nanowire arrays for high-performance supercapacitors. Surf. Coat. Technol. 2016, 299, 15–21. [Google Scholar] [CrossRef]
- Filipic, G.; Cvelbar, U. Copper oxide nanowires: A review of growth. Nanotechnology 2012, 23, 194001. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Myung, Y.; Banerjee, P. Unravelling transient phases during thermal oxidation of copper for dense CuO nanowire growth. CrystEngComm 2014, 16, 3264–3267. [Google Scholar] [CrossRef]
- Lamberti, A.; Fontana, M.; Bianco, S.; Tresso, E. Flexible solid-state CuxO-based pseudo-supercapacitor by thermal oxidation of copper foils. Int. J. Hydrog. Energy 2016, 41, 11700–11708. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhang, L.; Wan, H.; Qi, T.; Xia, D. Highly conductive NiCo2S4 urchin-like nanostructures for high-rate pseudocapacitors. Nanoscale 2013, 5, 8879–8883. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, X.; Song, X.; Liang, S.; Wang, L.; Yang, Z. Bottom-up assembly of hierarchical Cu2O nanospheres: Controllable synthesis, formation mechanism and enhanced photochemical activities. CrystEngComm 2012, 14, 3545–3553. [Google Scholar] [CrossRef]
- Li, B.; Liu, T.; Hu, L.; Wang, Y. A facile one-pot synthesis of Cu2O/RGO nanocomposite for removal of organic pollutant. J. Phys. Chem. Solids 2013, 74, 635–640. [Google Scholar] [CrossRef]
- Dubal, D.; Gund, G.; Holze, R.; Lokhande, C. Mild chemical strategy to grow micro-roses and micro-woolen like arranged CuO nanosheets for high performance supercapacitors. J. Power Sources 2013, 242, 687–698. [Google Scholar] [CrossRef]
- Hu, W.; Chen, R.; Xie, W.; Zou, L.; Qin, N.; Bao, D. CoNi2S4 nanosheet arrays supported on nickel foams with ultrahigh capacitance for aqueous asymmetric supercapacitor applications. ACS Appl. Mater. Interfaces 2014, 6, 19318–19326. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Qin, D.; Huang, S.; Guo, X.; Li, D.; Luo, Y.; Men, Q. Dye-sensitized solar cells with NiS counter electrodes electrodeposited by a potential reversal technique. Energy Environ. Sci. 2011, 4, 2630–2637. [Google Scholar] [CrossRef]
- Pu, J.; Cui, F.; Chu, S.; Wang, T.; Sheng, E.; Wang, Z. Preparation and electrochemical characterization of hollow hexagonal NiCo2S4 nanoplates as pseudocapacitor materials. ACS Sustain. Chem. Eng. 2014, 2, 809–815. [Google Scholar] [CrossRef]
- Legrand, D.; Nesbitt, H.; Bancroft, G. X-ray photoelectron spectroscopic study of a pristine millerite (NiS) surface and the effect of air and water oxidation. Am. Mineral. 1998, 83, 1256–1265. [Google Scholar] [CrossRef]
- Chen, W.; Xia, C.; Alshareef, H. One-step electrodeposited nickel cobalt sulfide nanosheet arrays for high-performance asymmetric supercapacitors. ACS Nano 2014, 8, 9531–9541. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Wang, T.; Wang, H.; Tong, Y.; Lu, C.; Kong, W.; Wang, Z. Direct growth of NiCo2S4 nanotube arrays on nickel foam as high-performance binder-free electrodes for supercapacitors. ChemPlusChem 2014, 45, 577–583. [Google Scholar]
- Wan, H.; Jiang, J.; Yu, J.; Xu, K.; Miao, L.; Zhang, L.; Chen, K.; Ruan, Y. NiCo2S4 porous nanotubes synthesis via sacrificial templates: High-performance electrode materials of supercapacitors. CrystEngComm 2013, 15, 7649–7651. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Zhang, X.; Shen, L.; Lou, X. Ultrathin mesoporous NiCo2O4 nanosheets supported on Ni foam as advanced electrodes for supercapacitors. Adv. Funct. Mater. 2012, 22, 4592–4597. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhang, L.; Xia, D.; Zhao, Y.; Guo, D.; Qi, T.; Wan, H. In situ growth of NiCo2S4 nanotube arrays on Ni foam for supercapacitors: Maximizing utilization efficiency at high mass loading to achieve ultrahigh areal pseudocapacitance. J. Power Sources 2014, 254, 249–257. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Huang, Z.; Yu, Y. High-performance NiCo2O4@Ni3S2 core/shell mesoporous nanothorn arrays on Ni foam for supercapacitors. J. Mater. Chem. A 2014, 2, 17595–17601. [Google Scholar] [CrossRef]
- Nguyen, V.; Lamiel, C.; Shim, J. 3D hierarchical mesoporous NiCo2S4@Ni(OH)2 core-shell nanosheet arrays for high performance supercapacitors. New J. Chem. 2016, 40, 4810–4817. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; He, Y.; Jia, C.; Pavlinek, V.; Saha, P.; Cheng, Q. Construction of Hierarchical CuO/Cu2O@NiCo2S4 Nanowire Arrays on Copper Foam for High Performance Supercapacitor Electrodes. Nanomaterials 2017, 7, 273. https://doi.org/10.3390/nano7090273
Zhou L, He Y, Jia C, Pavlinek V, Saha P, Cheng Q. Construction of Hierarchical CuO/Cu2O@NiCo2S4 Nanowire Arrays on Copper Foam for High Performance Supercapacitor Electrodes. Nanomaterials. 2017; 7(9):273. https://doi.org/10.3390/nano7090273
Chicago/Turabian StyleZhou, Luoxiao, Ying He, Congpu Jia, Vladimir Pavlinek, Petr Saha, and Qilin Cheng. 2017. "Construction of Hierarchical CuO/Cu2O@NiCo2S4 Nanowire Arrays on Copper Foam for High Performance Supercapacitor Electrodes" Nanomaterials 7, no. 9: 273. https://doi.org/10.3390/nano7090273



