Acetylene Gas-Sensing Properties of Layer-by-Layer Self-Assembled Ag-Decorated Tin Dioxide/Graphene Nanocomposite Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
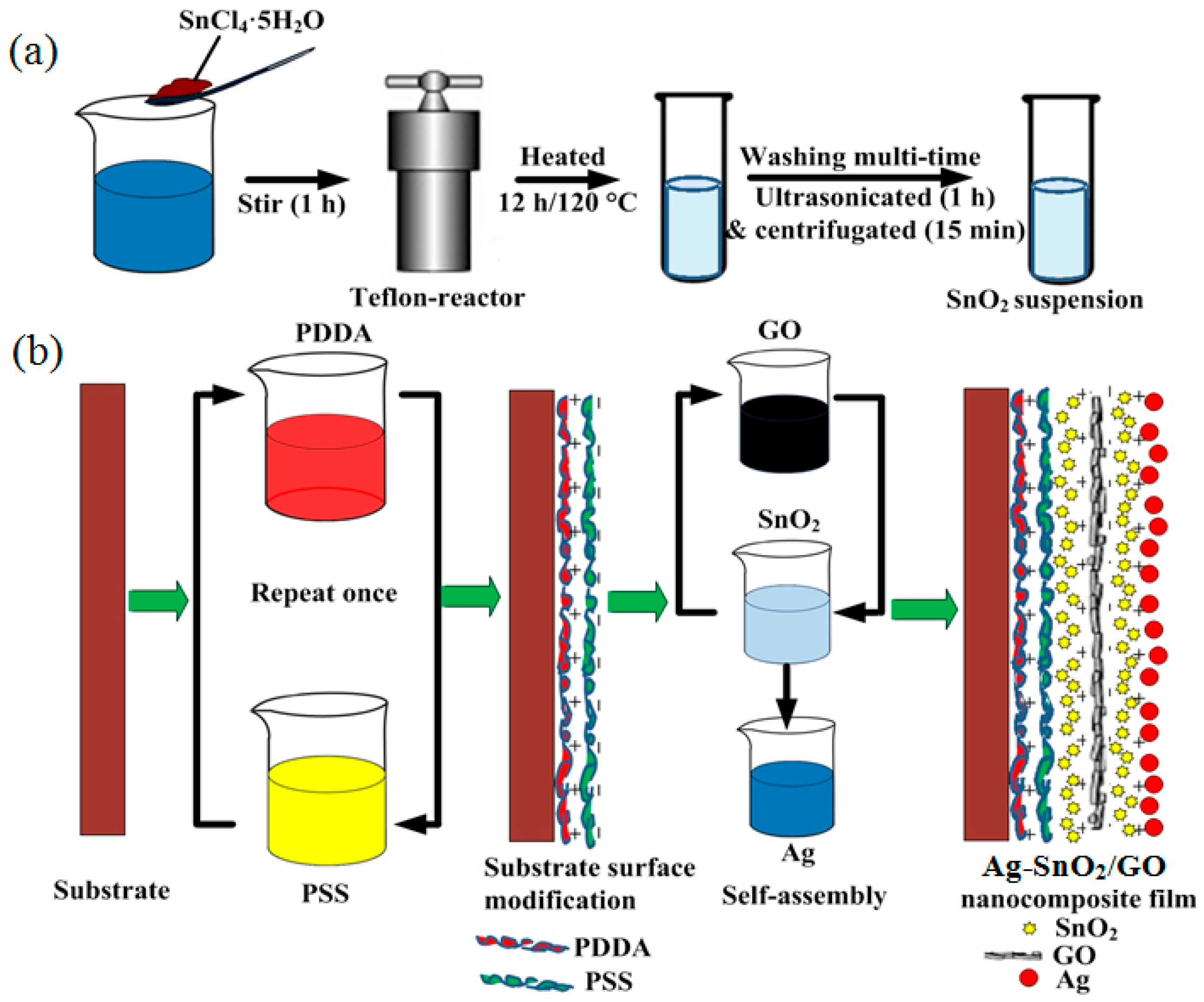
2.2. Preparation of the Ag–SnO2/rGO Nanocomposite
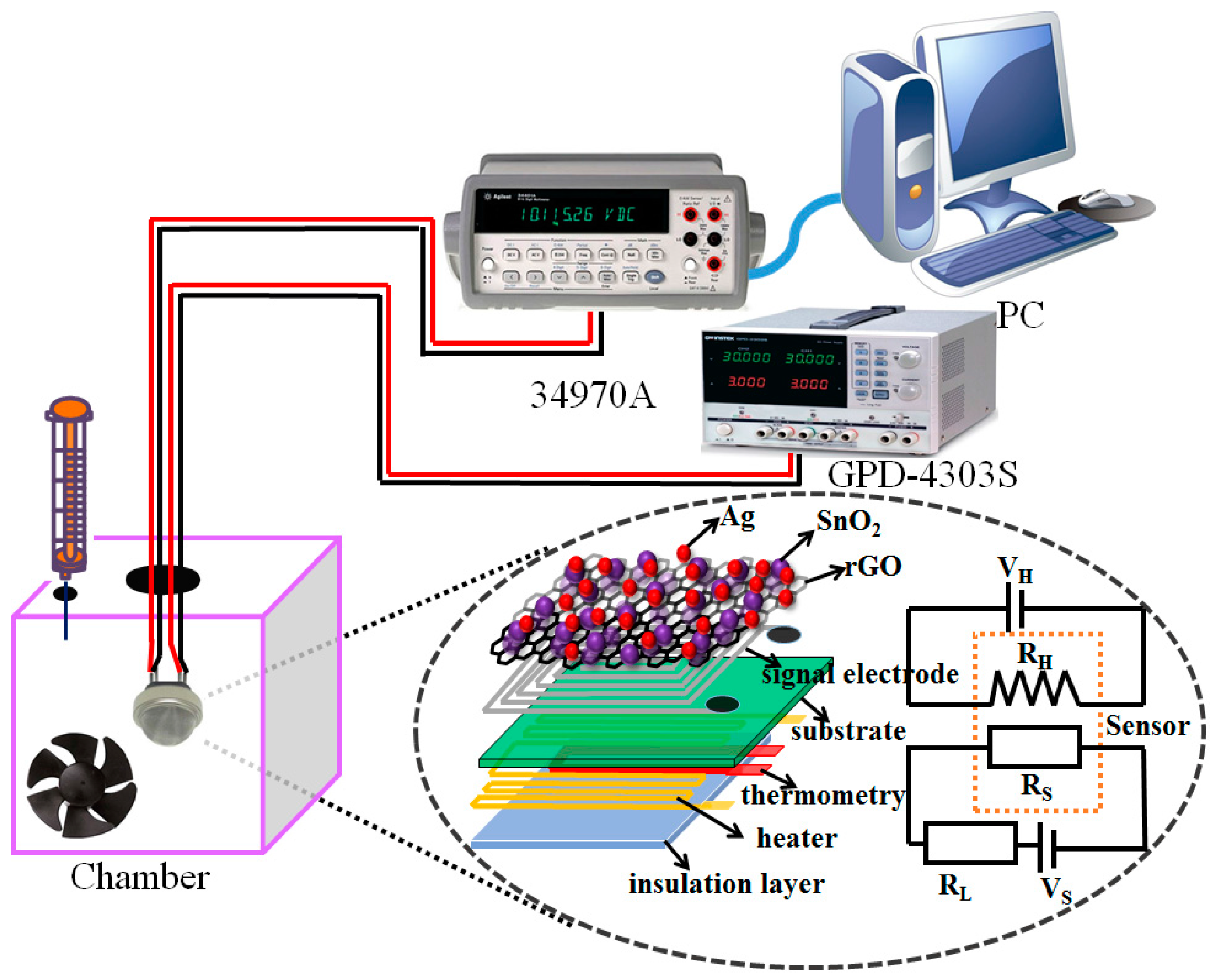
2.3. Instruments and Analysis
3. Results and Discussion
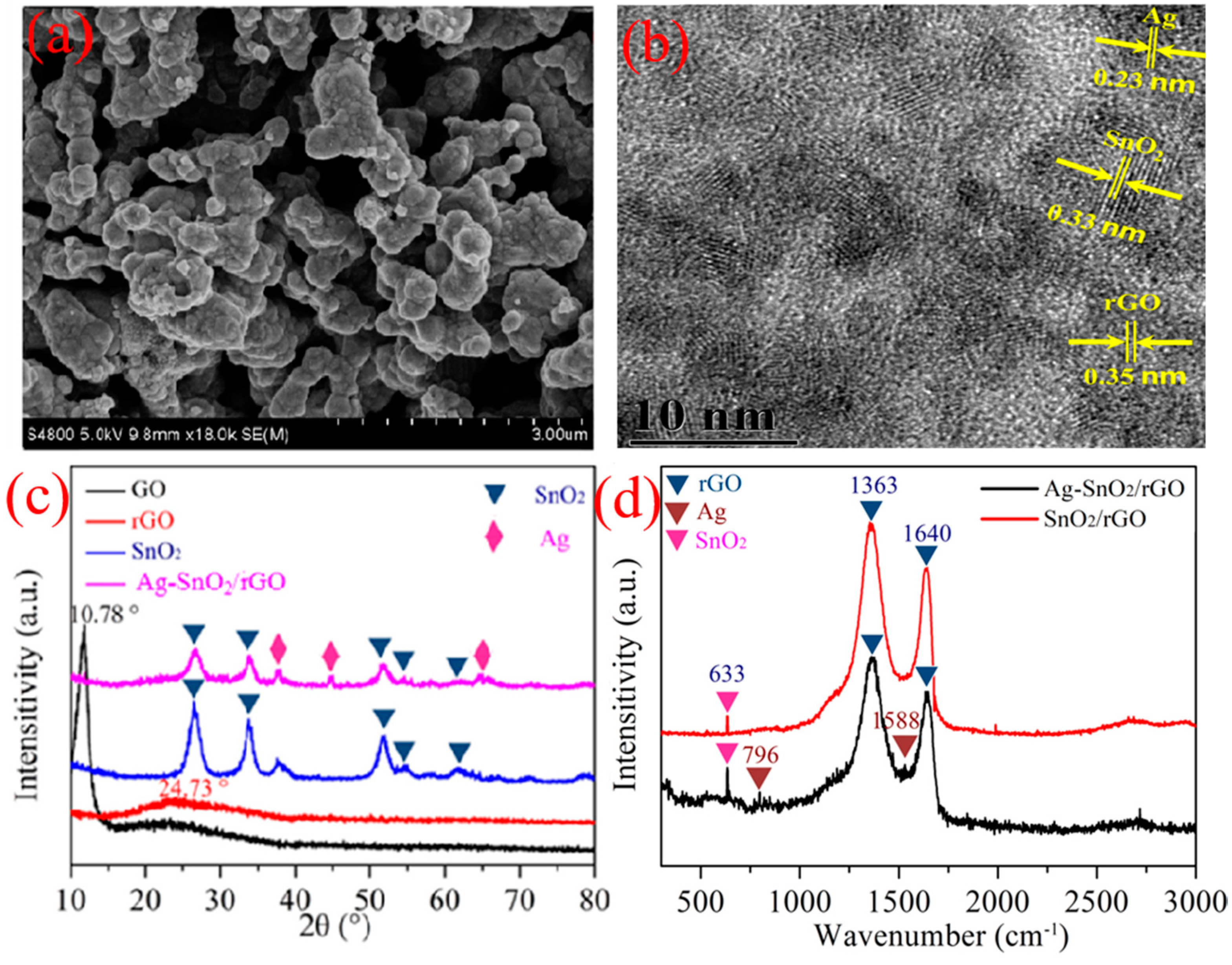
3.1. Sample Characterization
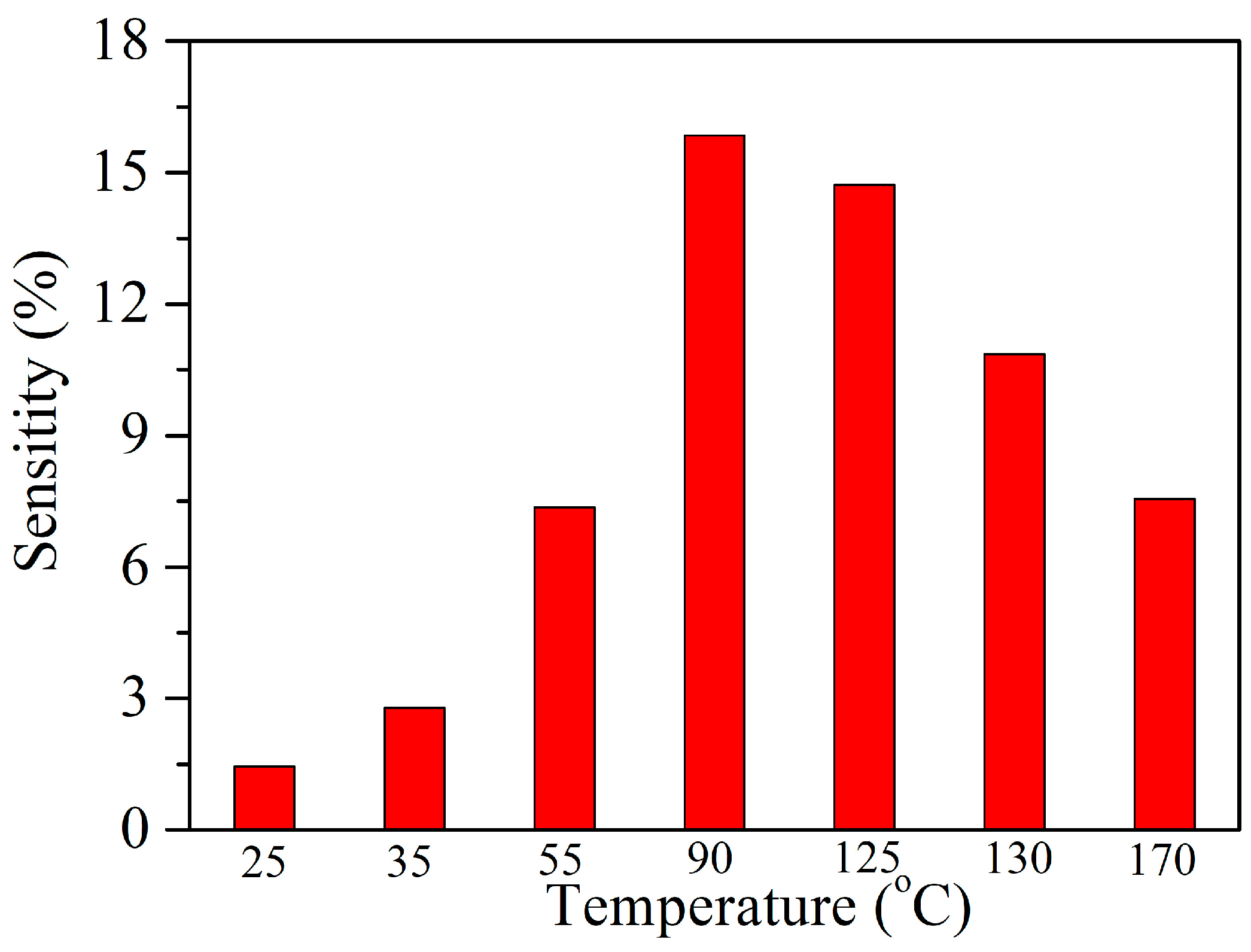
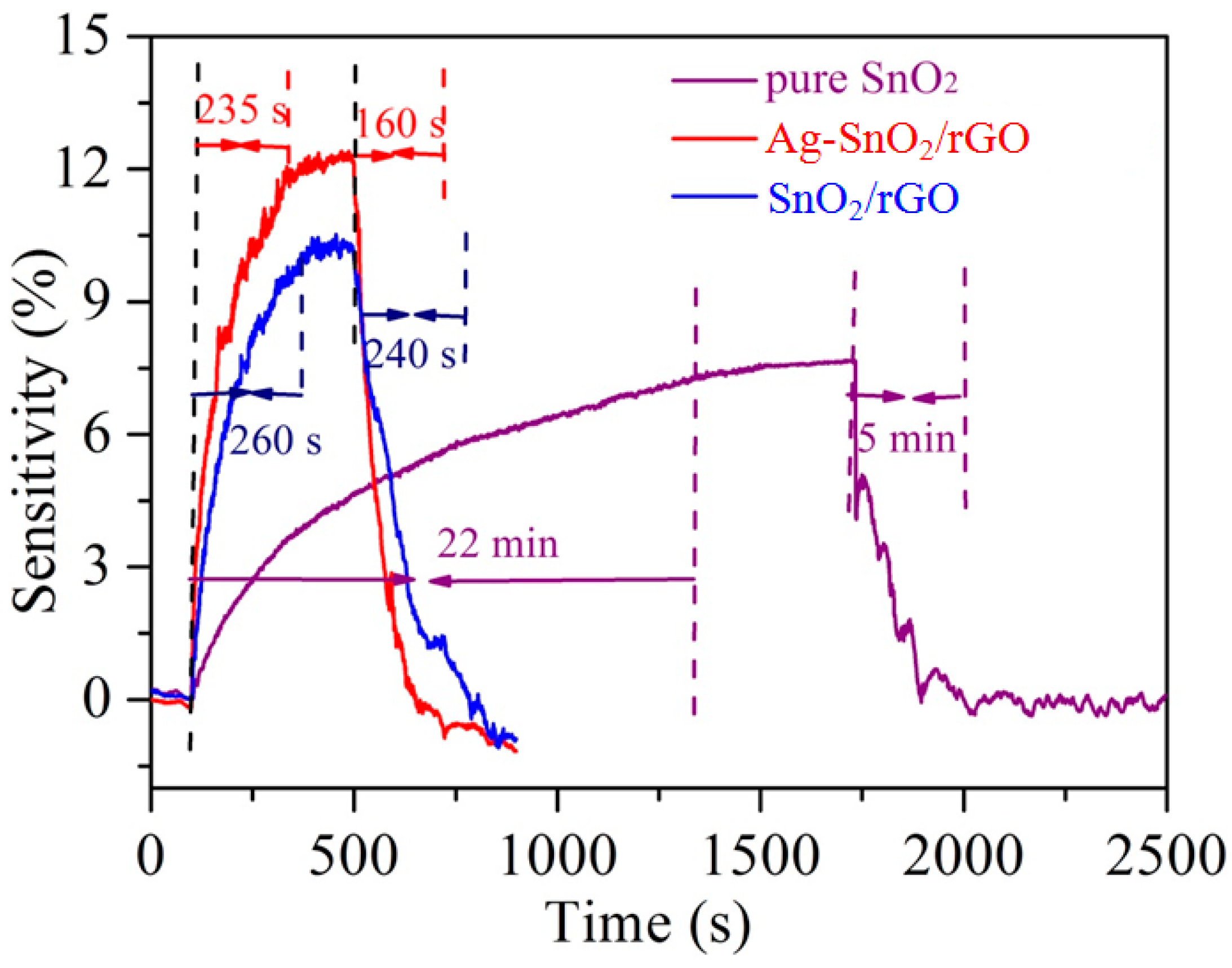
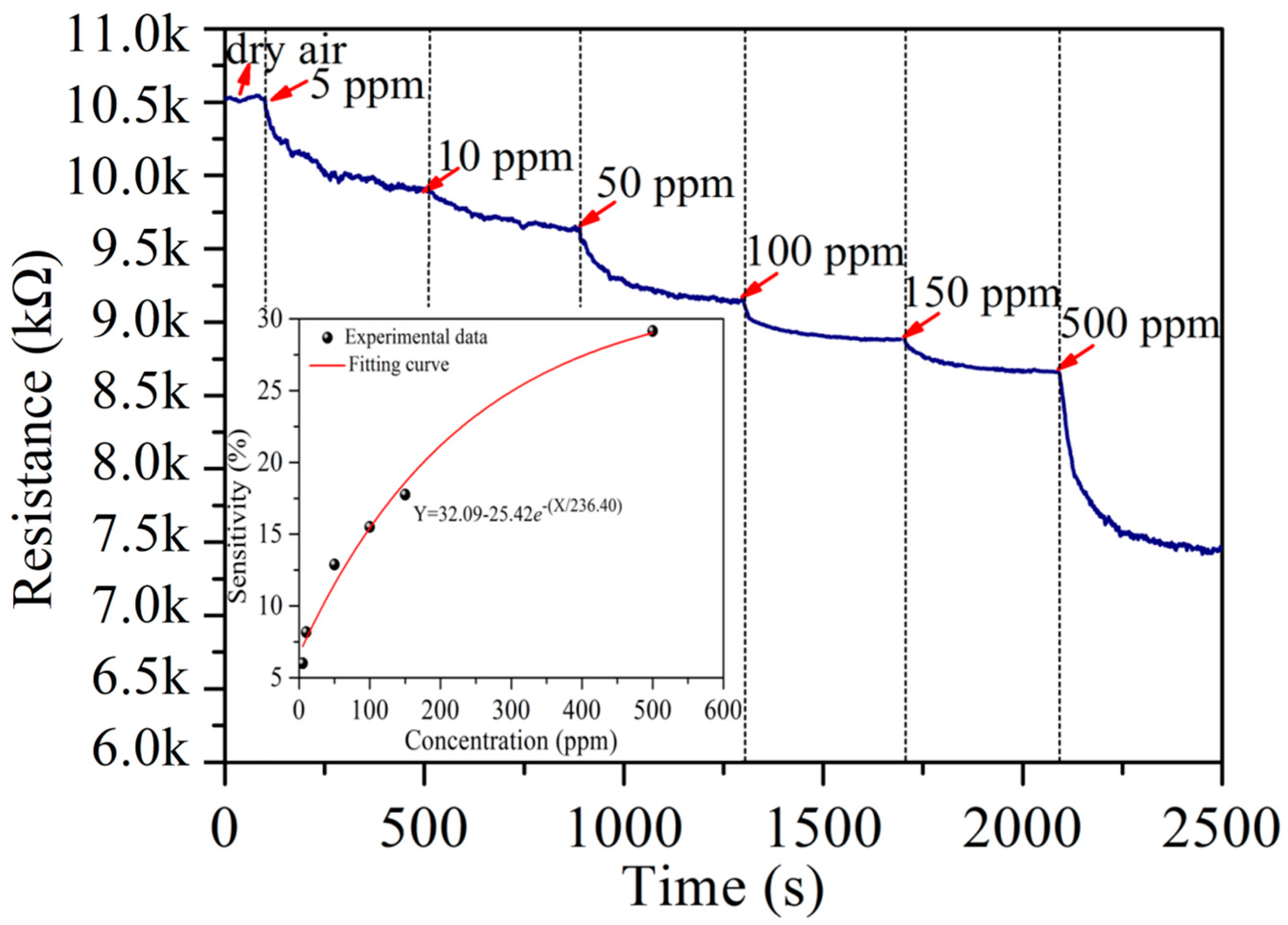
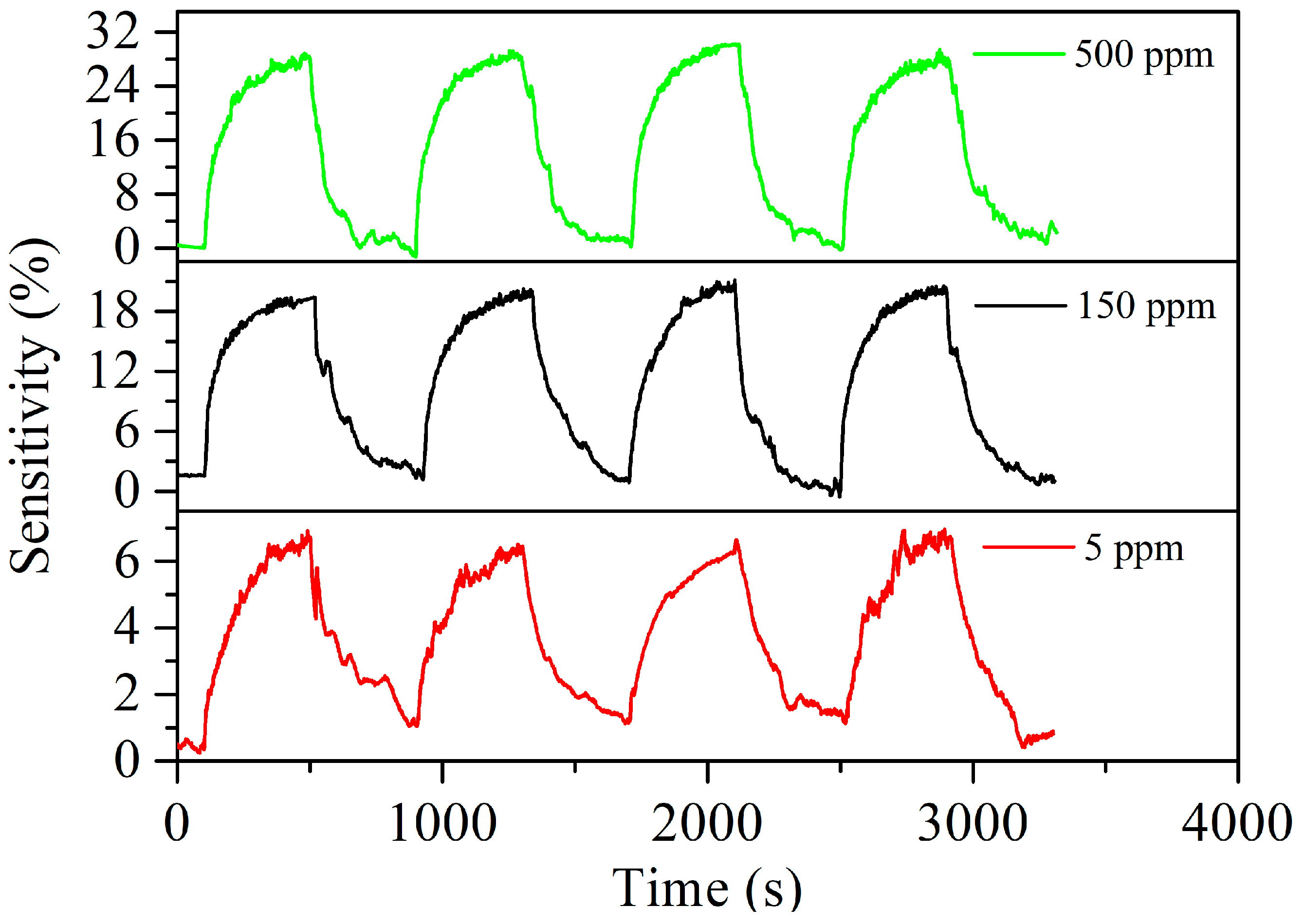
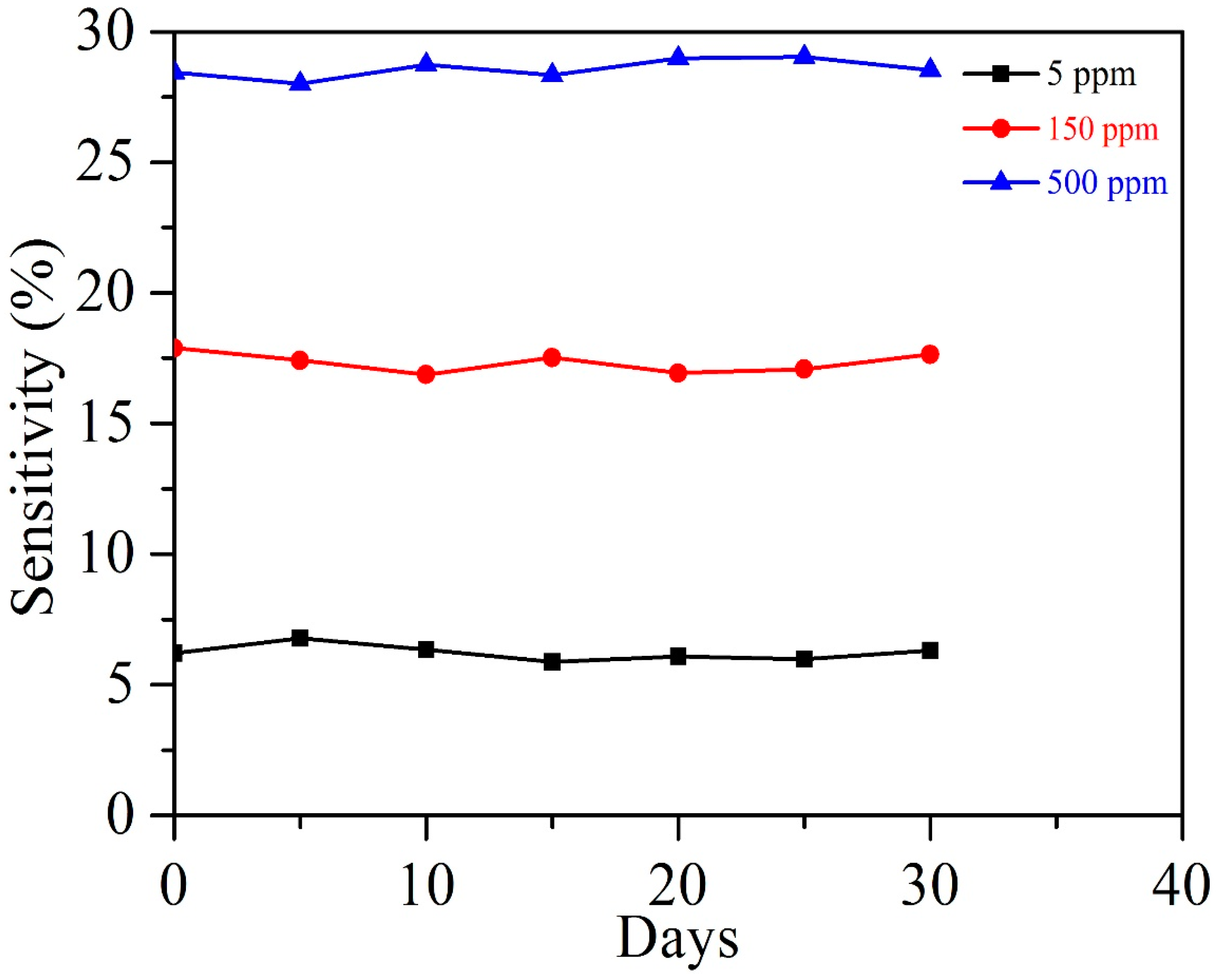
3.2. Acetylene Sensing Properties
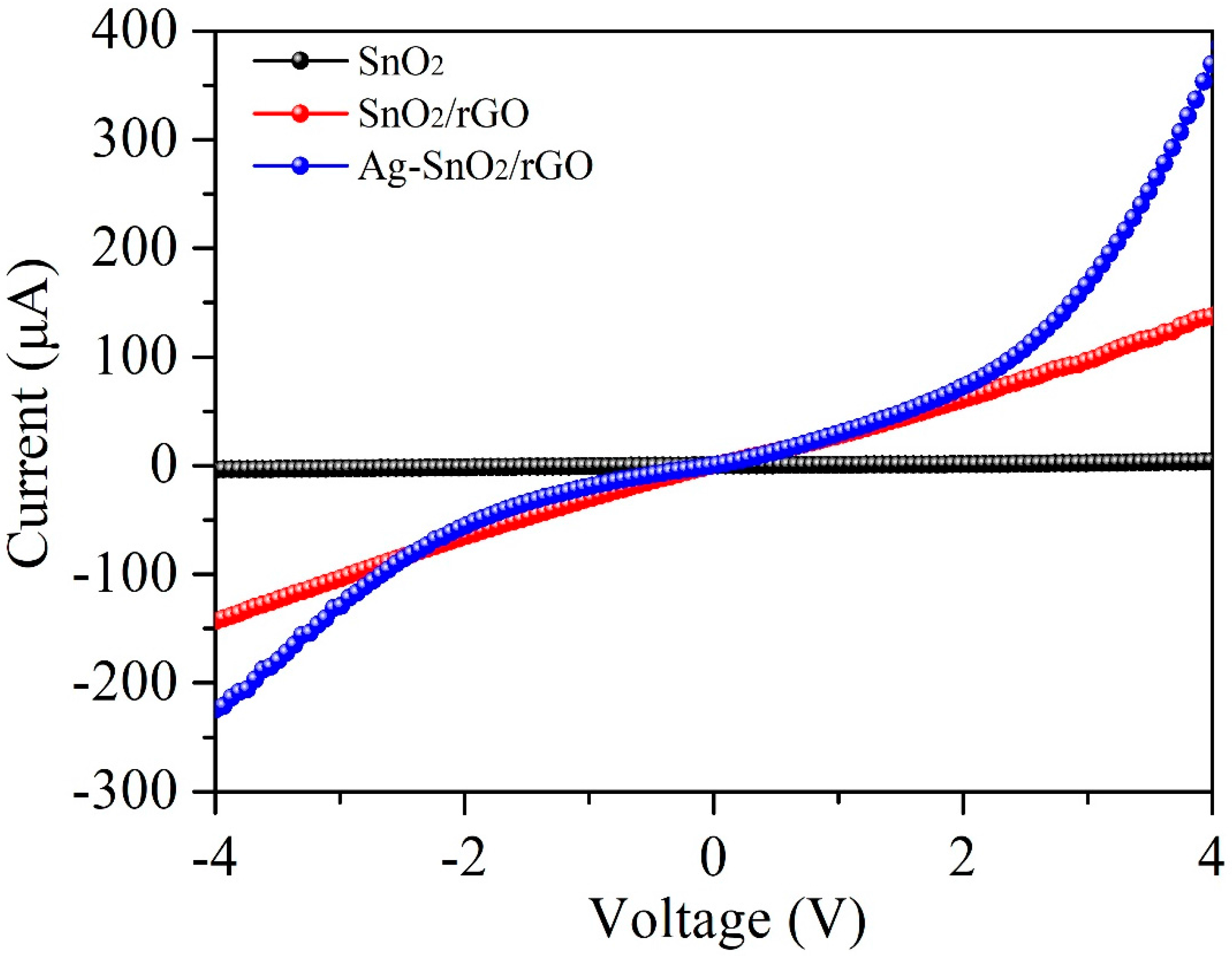
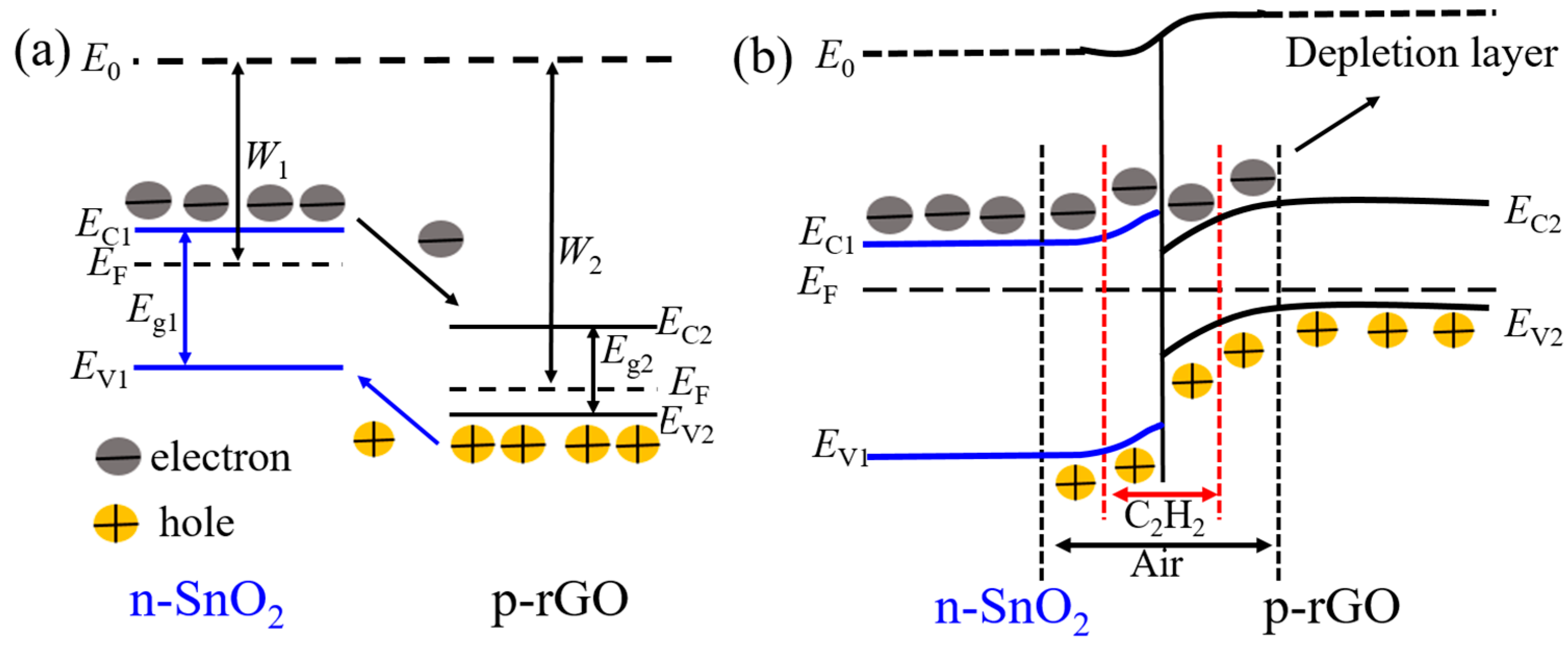
3.3. Acetylene-Sensing Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Antonsson, A.-B.; Christensson, B.; Berge, J.; Sjögren, B. Fatal carbon monoxide intoxication after acetylene gas welding of pipes. Ann. Occup. Hyg. 2013, 57, 662–666. [Google Scholar] [PubMed]
- Steinmetz, J.; Lee, H.-J.; Kwon, S.; Lee, D.-S.; Goze-Bac, C.; Abou-Hamad, E.; Kim, H.; Park, Y.W. Routes to the synthesis of carbon nanotube-polyacetylene composites by Ziegler-Natta polymerization of acetylene inside carbon nanotubes. Curr. Appl. Phys. 2007, 7, 39–41. [Google Scholar] [CrossRef]
- Saroha, R.; Panwar, A.K. Effect of in situ pyrolysis of acetylene (C2H2) gas as a carbon source on the electrochemical performance of LiFePO4 for rechargeable lithium-ion batteries. J. Phys. D Appl. Phys. 2017, 50, 255501. [Google Scholar] [CrossRef]
- Bastos, D.C.; Dos Santos, A.E.F.; Simao, R.A. Acetylene coating on cornstarch plastics produced by cold plasma technology. Starch-Stärke 2014, 66, 267–273. [Google Scholar] [CrossRef]
- Jin, L.; Chen, W.; Zhang, H.; Xiao, G.; Yu, C.; Zhou, Q. Characterization of reduced greaphene oxide (rGO)-loaded SnO2 nanocomposite and applications in C2H2 gas detection. Appl. Sci. 2017, 7, 19. [Google Scholar] [CrossRef]
- Wan, F.; Zhou, Q.; Zou, J.; Gu, Z.; Chen, W.; Wang, C. Using a sensitive optical system to analyze gases dissolved in samples extracted from transformer oil. IEEE Electr. Insul. Mag. 2014, 30, 15–22. [Google Scholar] [CrossRef]
- Chen, W.; Liu, B.; Zhou, H.; Wang, Y.; Wang, C. Diode laser-based photoacoustic spectroscopy detection of acetylene gas and its quantitative analysis. Eur. Trans. Electr. Power 2012, 22, 226–234. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, A.P.; Ma, G.; Wang, B.; Kim, B.; Im, J.; He, S.; Chung, Y. Fiber-optic acetylene gas sensor based on microstructured optical fiber bragg gratings. IEEE Photonic Technol. Lett. 2011, 23, 1588–1590. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, H.; Hong, C.; Xu, L.; Chen, W. Fabrication and enhanced acetylene sensing properties of PdO-decorated SnO2 composites chemical sensor. Sens. Lett. 2016, 14, 1144–1149. [Google Scholar] [CrossRef]
- Li, C.L.; Su, Y.; Lv, X.Y.; Xia, H.L.; Wang, Y.J. Electrochemical acetylene sensor based on Au/MWCNTs. Sens. Actuators B Chem. 2010, 149, 427–431. [Google Scholar] [CrossRef]
- Zhou, Q.; Cao, M.; Li, W.; Tang, C.; Zhu, S. Research on acetylene sensing properties and mechanism of SnO2 based chemical gas sensor decorated with Sm2O3. J. Nanosci. Nanotechnol. 2015, 2015, 714072. [Google Scholar]
- Uddin, A.S.M.I.; Yaqoob, U.; Phan, D.-T.; Chung, G.-S. A novel flexible acetylene gas sensor based on PI/PTFE-supported Ag-loaded vertical ZnO nanorods array. Sens. Actuators B Chem. 2016, 222, 536–543. [Google Scholar] [CrossRef]
- Lee, K.-W.; Phan, D.-T.; Chung, G.-S. Fabrication of low-temperature acetylene gas sensor based on Ag nanoparticles loaded hierarchical ZnO nanostructures. Electron. Lett. 2015, 51, 572–574. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Phan, D.-T.; Chung, G.-S. Low temperature acetylene gas sensor based on Ag nanoparticles-loaded ZnO-reduced graphene oxide hybrid. Sens. Actuators B Chem. 2015, 207, 362–369. [Google Scholar] [CrossRef]
- Lin, Y.; Li, C.; Wei, W.; Li, Y.; Wen, S.; Sun, D.; Chen, Y.; Ruan, S. A new type of acetylene gas sensor based on a hollow heterostructure. RSC Adv. 2015, 5, 61521–61527. [Google Scholar] [CrossRef]
- Akash, K.; Sun-Woo, C.; Hyoun, W.K.; Sang, S.K. Highly sensitive and selective H2 sensing by ZnO nanofibers and the underlying sensing mechanism. J. Hazard. Mater. 2015, 286, 229–235. [Google Scholar]
- Rawal, I. Facial synthesis of hexagonal metal oxide nanoparticles for low temperature ammonia gas sensing applications. RSC Adv. 2015, 5, 4135–4142. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Shim, Y.S.; Kim, D.H.; Jeong, H.Y.; Kim, Y.H.; Nahm, S.H.; Kang, C.Y.; Kim, J.S.; Lee, W.; Jang, H.W. Utilization of both-side metal decoration in close-packed SnO2 nanodome arrays for ultrasensitive gas sensing. Sens. Actuators B Chem. 2015, 213, 314–321. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Kaneti, Y.V.; Jiang, X.C.; Yu, A.B. Hydrothermal synthesis of sodium vanadium oxide nanorods for gas sensing application. Sens. Actuators B Chem. 2014, 202, 803–809. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Zheng, J.; Li, L.; Zhu, Z. Hydrothermal synthesis of hierarchical nanoparticle-decorated ZnO microdisks and the structure-enhanced acetylene sensing properties at high temperatures. Sens. Actuators B Chem. 2014, 158, 144–150. [Google Scholar] [CrossRef]
- Tamaekong, N.; Liewhiran, C.; Wisitsoraat, A.; Phanichphant, S. Acetylene sensor based on Pt/ZnO thick films as prepared by flame spray pyrolysis. Sens. Actuators B Chem. 2011, 152, 155–161. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Q.; Gao, T.; Su, X.; Wan, F. Pd-doped SnO2-based sensor detecting characteristic fault hydrocarbon gases in transformer oil. J. Nanomater. 2013, 2013, 127345. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Lee, K.-W.; Chung, G.-S. Acetylene gas sensing properties of an Ag-loaded hierarchical ZnO nanostructure-decorated reduced graphene oxide hybrid. Sens. Actuators B Chem. 2015, 216, 33–40. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Xia, B. Quantitative detection of formaldehyde and ammonia gas via metal oxide-modified graphene-based sensor array combining with neural network model. Sens. Actuators B Chem. 2017, 240, 55–65. [Google Scholar] [CrossRef]
- Alzari, V.; Sanna, V.; Biccai, S.; Caruso, T.; Politano, A.; Scaramuzza, N.; Sechi, M.; Nuvoli, D.; Sanna, R.; Mariani, A. Tailoring the physical properties of nanocomposite films by the insertion of graphene and other nanoparticles. Compos. Part B Eng. 2014, 60, 29–35. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Politano, A.; Drioli, E. The advent of graphene and other two-dimensional materials in membrane science and technology. Curr. Opin. Chem. Eng. 2017, 16, 78–85. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Chung, G.-S. Synthesis of highly dispersed ZnO nanoparticles on graphene surface and their acetylene sensing properties. Sens. Actuators B Chem. 2014, 205, 338–344. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, N.; Xia, B. Facile fabrication of ZnO nanocrystalline-modified graphene hybrid nanocomposite toward methane gas sensing application. J. Mater. Sci.-Mater. Electron. 2015, 26, 5937–5945. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Liu, J.; Cao, Y. Carbon monoxide gas sensing at room temperature using copper oxide-decorated graphene hybrid nanocomposite prepared by layer-by-layer self-assembly. Sens. Actuators B Chem. 2017, 247, 875–882. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Jiang, C.; Li, P.; Sun, Y. High-performance sulfur dioxide sensing properties of layer-by-layer self-assembled titania-modified graphene hybrid nanocomposite. Sens. Actuators B Chem. 2017, 245, 560–567. [Google Scholar] [CrossRef]
- Bai, S.L.; Liu, J.C.; Guo, J.; Luo, R.X.; Li, D.Q.; Song, Y.J.; Liu, C.C.; Chen, A.F. Facile preparation of SnO2/NiO composites and enhancement of sensing performance to NO2. Sens. Actuators B Chem. 2017, 249, 22–29. [Google Scholar]
- Ding, C.; Ma, Y.; Lai, X.; Yang, Q.; Xue, P.; Hu, F.; Geng, W. Mesoporous Ag/In2O3 composite derived from indium organic framework as high performance formaldehyde sensor. J. Solid State Chem. 2017, 251, 170–175. [Google Scholar]
- Zhang, D.; Liu, J.; Xia, B. Nitrogen dioxide-sensing properties at room temperature of metal oxide-modified graphene composite via one-step hydrothermal method. J. Electron. Mater. 2016, 45, 4324–4330. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Chang, H.; Liu, A.; Xia, B. Characterization of a hybrid composite of SnO2 nanocrystal-decorated reduced graphene oxide for ppm-level ethanol gas sensing application. RSC Adv. 2015, 5, 18666–18672. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, H.; Li, P.; Liu, R.; Xue, Q. Fabrication and characterization of an ultra-sensitive humidity sensor based on metal oxide/graphene hybrid nanocomposite. Sens. Actuators B Chem. 2016, 225, 233–240. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, M.; Liu, F.; Jia, J.; Li, X.; Cao, L. C2H2 gas sensor based on Ni-doped ZnO electrospun nanofibers. Ceram. Int. 2013, 39, 2883–2887. [Google Scholar] [CrossRef]
- Liewhiran, C.; Tamaekong, N.; Wisitsoraat, A.; Phanichphant, S. Highly selective environmental sensors based on flame-spray-made SnO2 nanoparticles. Sens. Actuators B Chem. 2012, 163, 51–60. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Zheng, X.; Fan, H.; Liu, L.; Wang, R.; Zeng, Y. Electrical response of Sm2O3-doped SnO2 to C2H2 and effect of humidity interference. Sens. Actuators B Chem. 2008, 134, 36–42. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.; Jiang, C.; Zhang, Y. Room temperature hydrogen gas sensor based on palladium decorated tin oxide/molybdenum disulfide ternary hybrid via hydrothermal route. Sens. Actuators B 2017, 242, 15–24. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Yu, D.; Li, L. Seed/catalyst free growth and self-powered photoresponse of vertically aligned ZnO nanorods on reduced graphene oxide nanosheets. Cryst. Growth Des. 2016, 16, 4831–4838. [Google Scholar] [CrossRef]
- Di Bartolomeo, A. Graphene schottky diodes: An experimental review of the rectifying graphene/semiconductor heterojunction. Phys. Rep. 2016, 606, 1–58. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, H.; Lee, S.; Chin, I.J.; Seong, T.Y.; Lee, W.I.; Lee, C. Enhanced ethanol sensing properties of TiO2 nanotube sensors. Sens. Actuators B Chem. 2012, 173, 441–446. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.; Jiang, C.; Yao, Y.; Wang, D.; Zhang, Y. Room-temperature highly sensitive CO gas sensor based on Ag-loaded zinc oxide/molybdenum disulfide ternary nanocomposite and its sensing properties. Sens. Actuators B Chem. 2017, 253, 1120–1128. [Google Scholar] [CrossRef]


| Sensing Material | Fabrication Method | Work Temperature | Response | Reference |
|---|---|---|---|---|
| Ag–SnO2/rGO | Layer-by-layer self-assembly | 90 °C | 15.8 @100 ppm | This paper |
| Ag–ZnO nanorods | Hydrothermal-radio frequency (RF) magnetron sputtering | 200 °C | 27.2 @1000 ppm | [12] |
| Ag-hierarchical ZnO | Hydrothermal method | 200 °C | 1.92 @1000 ppm | [13] |
| NiO/SnO2 | Hydrothermal method | 206 °C | 13.8 @100 ppm | [15] |
| PdO-SnO2 | Hydrothermal method | 350 °C | 7.22 @100 ppm | [23] |
| Ni-ZnO | Electrospinning method | 250 °C | 17 @2000 ppm | [36] |
| SnO2 | Spin-coating method | 300 °C | 6.3 @10000 ppm | [37] |
| Sm2O3/SnO2 | Sol-gel method | 180 °C | 63.8 @1000 ppm | [38] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Zhang, D.; Yin, N.; Yao, Y.; Shaymurat, T.; Zhou, X. Acetylene Gas-Sensing Properties of Layer-by-Layer Self-Assembled Ag-Decorated Tin Dioxide/Graphene Nanocomposite Film. Nanomaterials 2017, 7, 278. https://doi.org/10.3390/nano7090278
Jiang C, Zhang D, Yin N, Yao Y, Shaymurat T, Zhou X. Acetylene Gas-Sensing Properties of Layer-by-Layer Self-Assembled Ag-Decorated Tin Dioxide/Graphene Nanocomposite Film. Nanomaterials. 2017; 7(9):278. https://doi.org/10.3390/nano7090278
Chicago/Turabian StyleJiang, Chuanxing, Dongzhi Zhang, Nailiang Yin, Yao Yao, Talgar Shaymurat, and Xiaoyan Zhou. 2017. "Acetylene Gas-Sensing Properties of Layer-by-Layer Self-Assembled Ag-Decorated Tin Dioxide/Graphene Nanocomposite Film" Nanomaterials 7, no. 9: 278. https://doi.org/10.3390/nano7090278





