A New Label-Free Technique for Analysing Evaporation Induced Self-Assembly of Viral Nanoparticles Based on Enhanced Dark-Field Optical Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteriophage Propagation and Purification
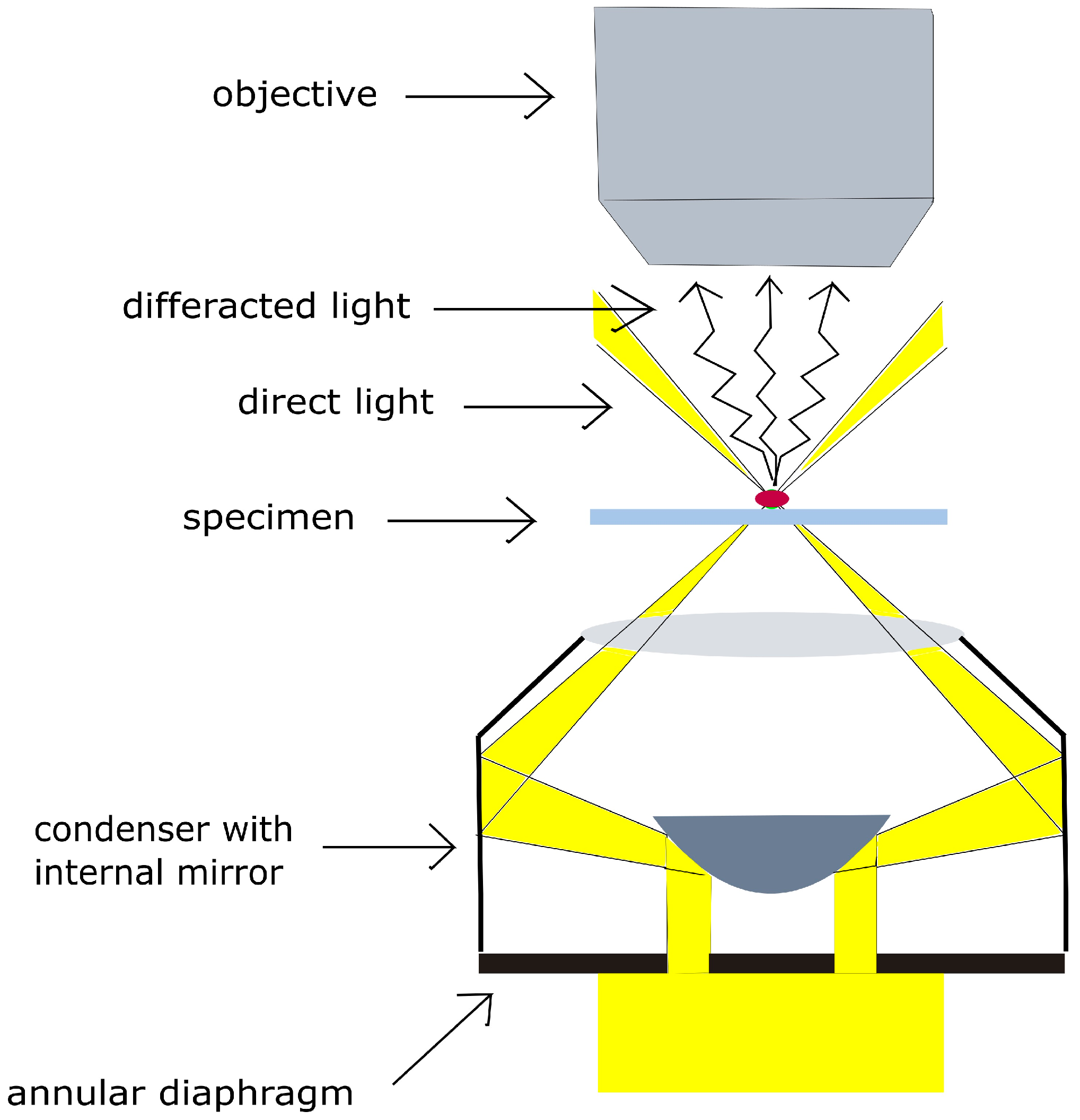
2.2. Experimental Setup: Enhanced Dark-Field Microscopy and Monitoring of Evaporation Process
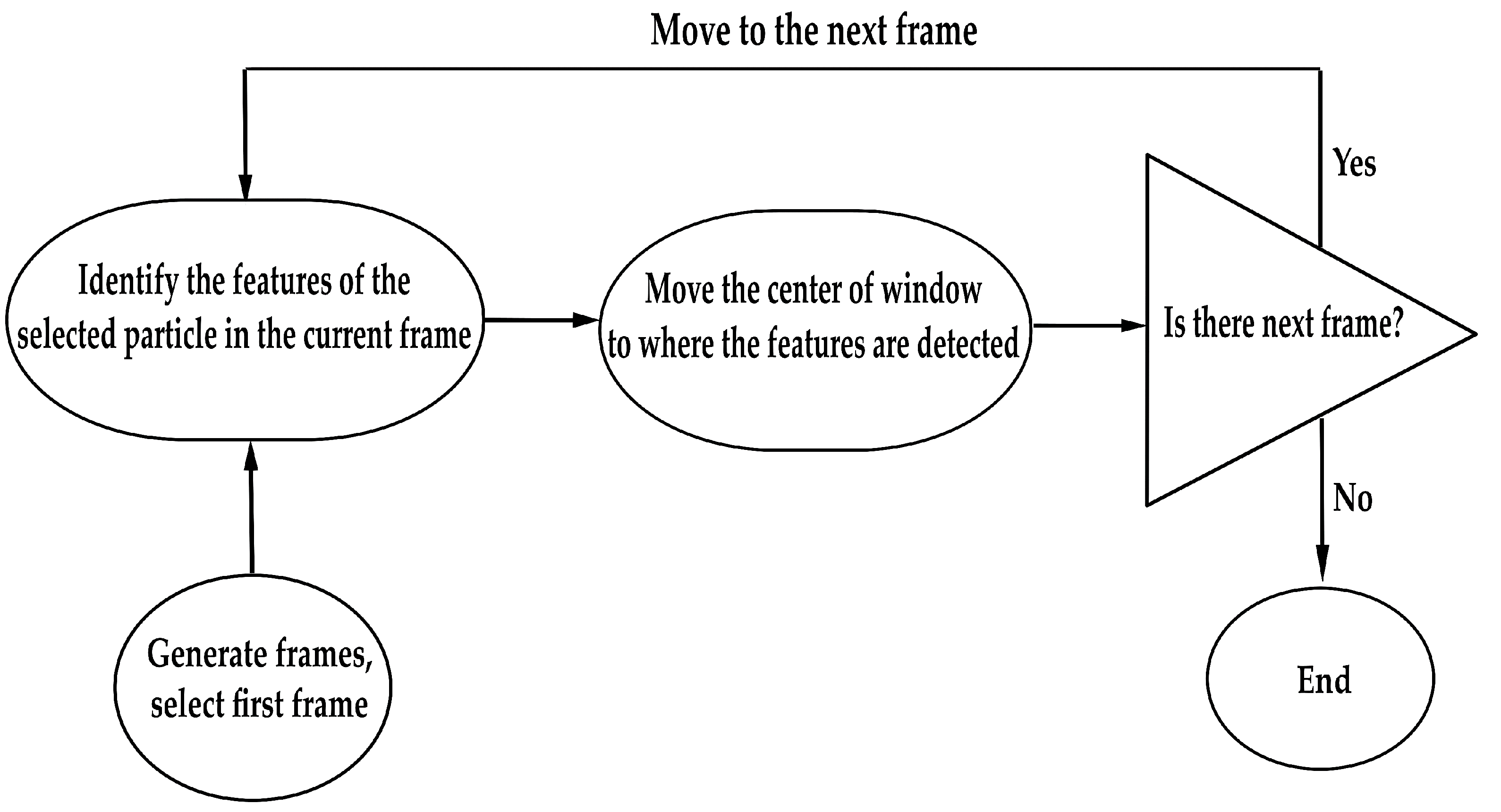
2.3. Particle Tracking and Image Analysis
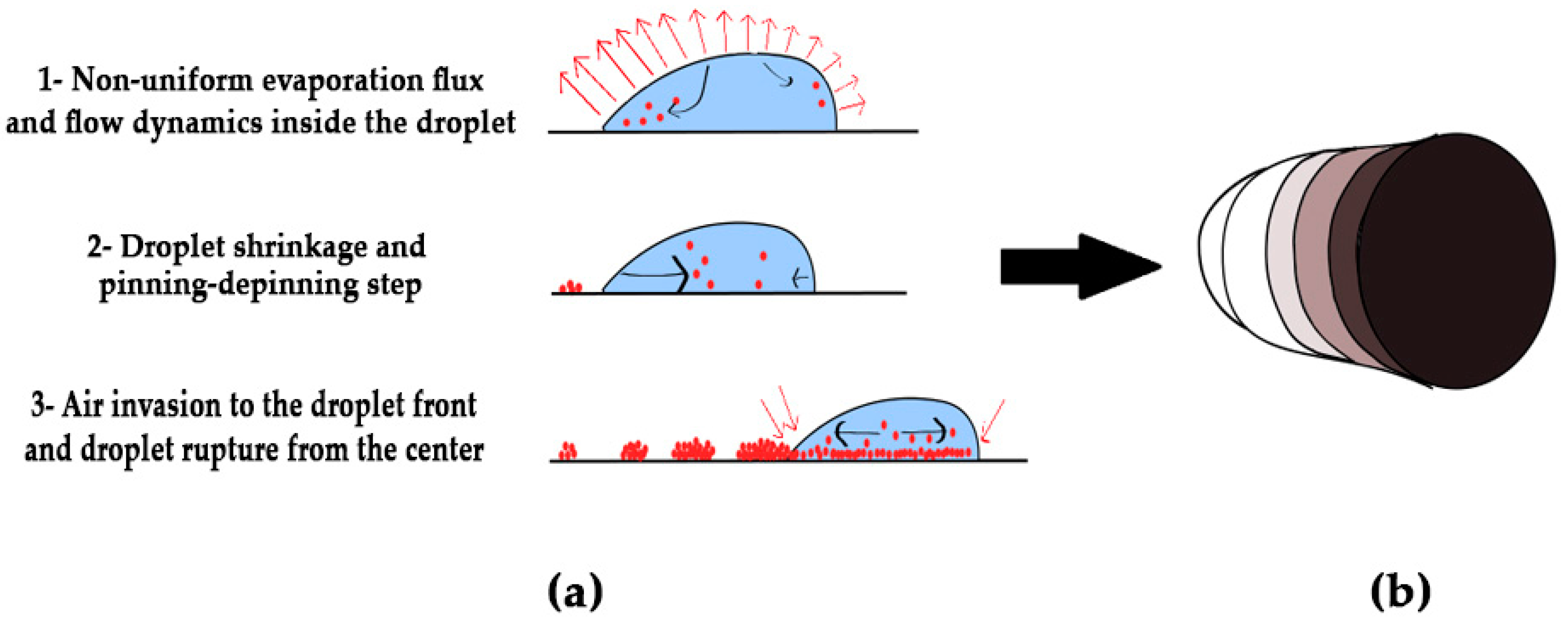
3. Results and Discussion
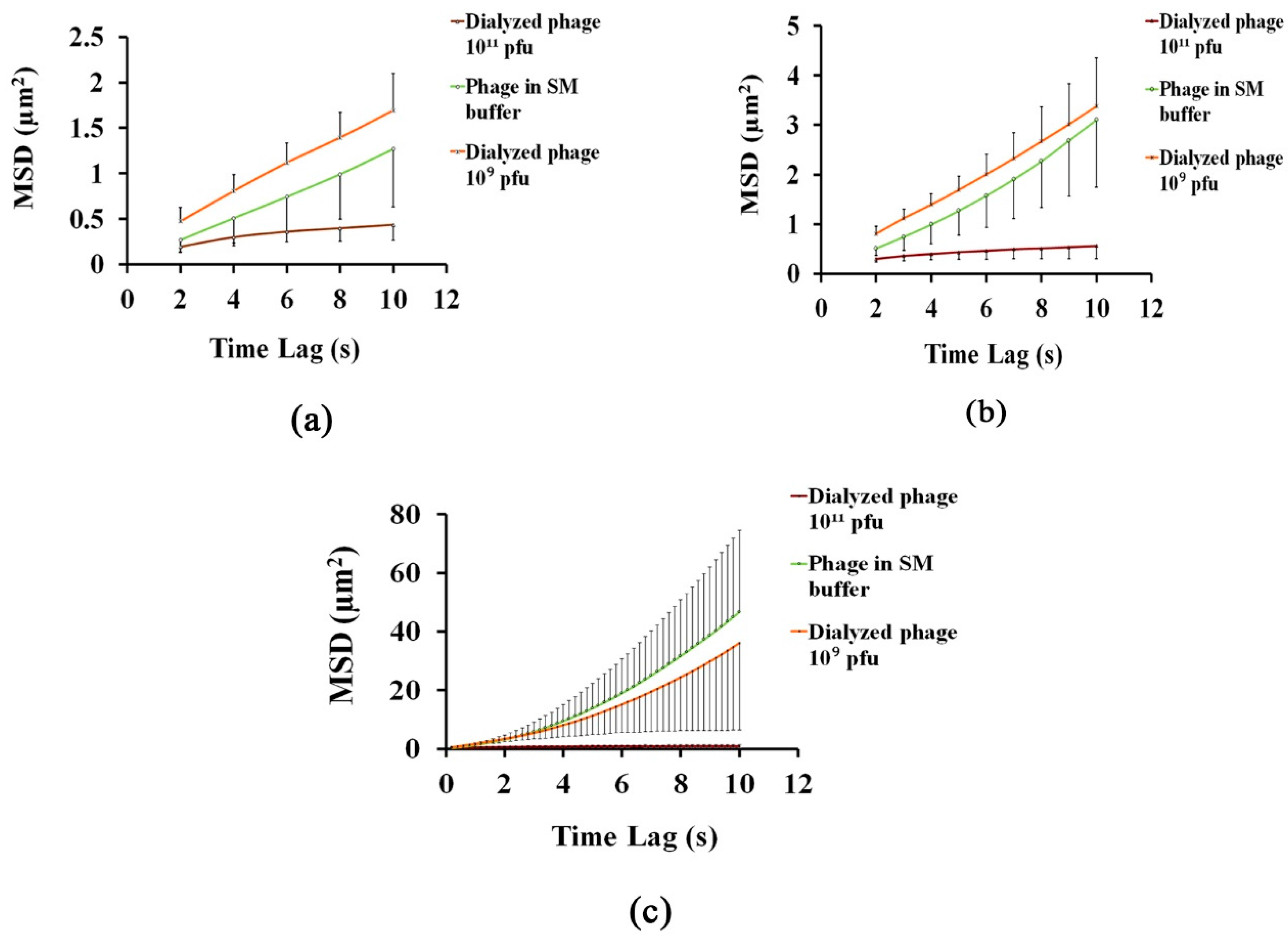
3.1. Analysing the Bacteriophage Motion and Its Influence on Phage Assembly onto the Glass Coverslip
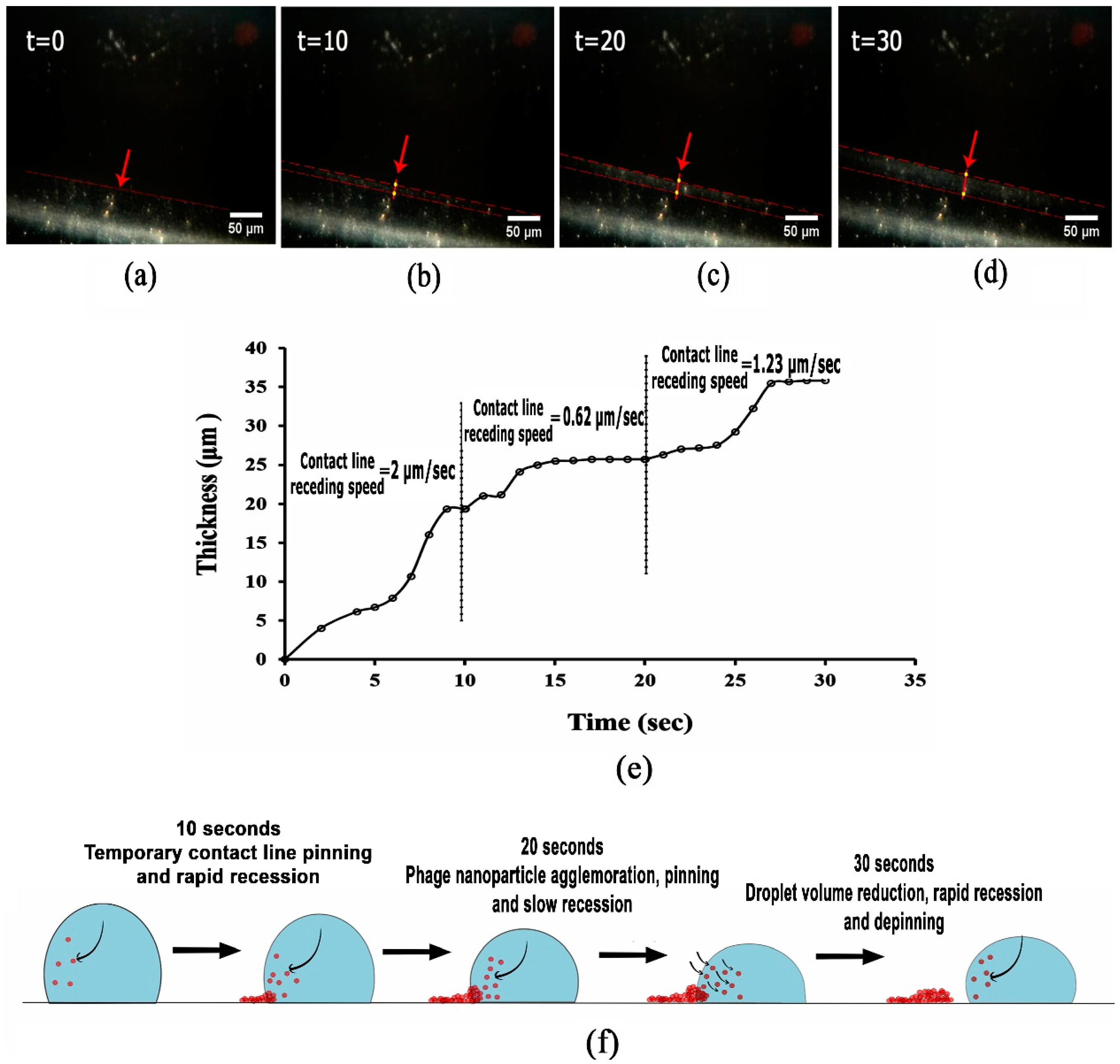
3.2. Analysing Fluid Flow through Monitoring Drying Process and Final Dried Patterns (Macroscopic Observations)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moghimian, P.; Srot, V.; Rothenstein, D.; Facey, S.J.; Harnau, L.; Hauer, B.; Bill, J.; van Aken, P.A. Adsorption and self-assembly of M13 phage into directionally organized structures on C and SiO2 films. Langmuir 2014, 30, 11428–11432. [Google Scholar] [CrossRef] [PubMed]
- Yoo, P.J.; Nam, K.T.; Qi, J.; Lee, S.-K.; Park, J.; Belcher, A.M.; Hammond, P.T. Spontaneous assembly of viruses on multilayered polymer surfaces. Nat. Mater. 2006, 5, 234. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.A.; Balizan, E.; Lin, Y.; Wang, Q. Assembly of Virus Particles and Virus-like Particles as Templates for Biomedical Applications. In Nanomaterials for Biomedicine; American Chemical Society: Washington, DC, USA, 2012; pp. 21–56. [Google Scholar]
- Lin, Y.; Su, Z.; Niu, Z.; Li, S.; Kaur, G.; Lee, L.A.; Wang, Q. Layer-by-layer assembly of viral capsid for cell adhesion. Acta Biomater. 2008, 4, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, Y.; Li, Z.; Wu, A.; Wei, G. Bottom-Up Synthesis and Sensor Applications of Biomimetic Nanostructures. Materials 2016, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lim, J.S.; Harris, M.T. Synthesis and application of virus-based hybrid nanomaterials. Biotechnol. Bioeng. 2012, 109, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Rink, V.; Muller-Renno, C.; Ziegler, C.; Braun, M.; Boonrod, K.; Krczal, G. Electrostatic conditions define the 2D self-assembly of tomato bushy stunt viruses on solid surfaces. Biointerphases 2017, 12, 04E402. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ryan, K.M. Centimetre scale assembly of vertically aligned and close packed semiconductor nanorods from solution. Chem. Commun. 2009, 42, 6421–6423. [Google Scholar] [CrossRef] [PubMed]
- Zanella, M.; Gomes, R.; Povia, M.; Giannini, C.; Zhang, Y.; Riskin, A.; Van Bael, M.; Hens, Z.; Manna, L. Self-Assembled Multilayers of Vertically Aligned Semiconductor Nanorods on Device-Scale Areas. Adv. Mater. 2011, 3, 2205–2209. [Google Scholar] [CrossRef] [PubMed]
- Bigioni, T.P.; Lin, X.-M.; Nguyen, T.T.; Corwin, E.I.; Witten, T.A.; Jaeger, H.M. Kinetically driven self assembly of highly ordered nanoparticle monolayers. Nat. Mater. 2006, 5, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Widmer-Cooper, A.; Toney, M.F.; Geissler, P.L.; Alivisatos, A.P. Device-Scale Perpendicular Alignment of Colloidal Nanorods. Nano Lett. 2010, 10, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Hao, J. Ordered patterns and structures via interfacial self-assembly: Superlattices, honeycomb structures and coffee rings. Chem. Soc. Rev. 2011, 40, 5457–5471. [Google Scholar] [CrossRef] [PubMed]
- Brezesinski, T.; Groenewolt, M.; Gibaud, A.; Pinna, N.; Antonietti, M.; Smarsly, B. Evaporation-Induced Self-Assembly (EISA) at Its Limit: Ultrathin, Crystalline Patterns by Templating of Micellar Monolayers. Adv. Mater. 2006, 18, 2260–2263. [Google Scholar] [CrossRef]
- Josten, E.; Wetterskog, E.; Glavic, A.; Boesecke, P.; Feoktystov, A.; Brauweiler-Reuters, E.; Rücker, U.; Salazar-Alvarez, G.; Brückel, T.; Bergström, L. Superlattice growth and rearrangement during evaporation-induced nanoparticle self-assembly. Sci. Rep. 2017, 7, 2802. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Wood, B.M.; Belcher, A.M. Chiral Smectic C Structures of Virus-Based Films. Langmuir 2003, 19, 1592–1598. [Google Scholar] [CrossRef]
- Wu, L.; Zang, J.; Lee, L.A.; Niu, Z.; Horvatha, G.C.; Braxtona, V.; Wibowo, A.C.; Bruckman, M.A.; Ghoshroy, S.; zur Loye, H.-C.; et al. Electrospinning fabrication, structural and mechanical characterization of rod-like virus-based composite nanofibers. J. Mater. Chem. 2011, 21, 8550–8557. [Google Scholar] [CrossRef]
- Joksimovic, R.; Watanabe, S.; Riemer, S.; Gradzielski, M.; Yoshikawa, K. Self-organized patterning through the dynamic segregation of DNA and silica nanoparticles. Sci. Rep. 2014, 4, 3660. [Google Scholar] [CrossRef] [PubMed]
- Kunstmann-Olsen, C.; Belic, D.; Brust, M. Monitoring pattern formation in drying and wetting dispersions of gold nanoparticles by ESEM. Faraday Discuss. 2015, 181, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Su, Z.; Xiao, G.; Balizan, E.; Kaur, G.; Niu, Z.; Wang, Q. Self-assembly of virus particles on flat surfaces via controlled evaporation. Langmuir 2011, 27, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Crivoi, A.; Duan, F. Three-dimensional patterns from the thin-film drying of amino acid solutions. Sci. Rep. 2015, 5, 10926. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.S.; Miller, S.E. Modern uses of electron microscopy for detection of viruses. Clin. Microbiol. Rev. 2009, 22, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Isenbugel, K.; Gehrke, Y.; Ritter, H. Evaporation-driven self-assembly of colloidal silica dispersion: New insights on janus particles. Macromol. Rapid Commun. 2012, 33, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Goehring, L.; Clegg, W.J.; Routh, A.F. Plasticity and fracture in drying colloidal films. Phys. Rev. Lett. 2013, 110, 024301. [Google Scholar] [CrossRef] [PubMed]
- Negishi, M.; Ichikawa, M.; Nakajima, M.; Kojima, M.; Fukuda, T.; Yoshikawa, K. Phase behavior of crowded like-charged mixed polyelectrolytes in a cell-sized sphere. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2011, 83, 061921. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, B.; Petersen, E.; Seo, Y.-S.; Samuilov, V.A.; Chen, Y.; Sokolov, J.C.; Shew, C.-Y.; Rafailovich, M.H. Drying of DNA Droplets. Langmuir 2006, 22, 6308–6312. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, S.; Zhang, L.; Zhu, Y.; Chang, H.C. Coupling between precipitation and contact-line dynamics: Multiring stains and stick-slip motion. Phys. Rev. Lett. 2008, 100, 044503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Maheshwari, S.; Chang, H.-C.; Zhu, Y. Evaporative Self-Assembly from Complex DNA-Colloid Suspensions. Langmuir 2008, 24, 3911–3917. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, L.; Esteban-Ferrer, D.; Cuervo, A.; Carrascosa, J.L.; Gomila, G. Label-free identification of single dielectric nanoparticles and viruses with ultraweak polarization forces. Nat. Mater. 2012, 11, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, B.; Zhuang, X. Virus trafficking—Learning from single-virus tracking. Nat. Rev. Microbiol. 2007, 5, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Lakadamyali, M.; Rust, M.J.; Babcock, H.P.; Zhuang, X. Visualizing infection of individual influenza viruses. Proc. Natl. Acad. Sci. USA 2003, 100, 9280–9285. [Google Scholar] [CrossRef] [PubMed]
- Ruthardt, N.; Lamb, D.C.; Brauchle, C. Single-particle tracking as a quantitative microscopy-based approach to unravel cell entry mechanisms of viruses and pharmaceutical nanoparticles. Mol. Ther. 2011, 19, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-L.; Spindler, S.; Ehrig, J.; Sandoghdar, V. Tracking Single Particles on Supported Lipid Membranes: Multimobility Diffusion and Nanoscopic Confinement. J. Phys. Chem. B 2014, 118, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Scherr, S.M.; Daaboul, G.G.; Trueb, J.; Sevenler, D.; Fawcett, H.; Goldberg, B.; Connor, J.H.; Ünlü, M.S. Real-Time Capture and Visualization of Individual Viruses in Complex Media. ACS Nano 2016, 10, 2827–2833. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shan, X.; Patel, U.; Huang, X.; Lu, J.; Li, J.; Tao, N. Label-free imaging, detection, and mass measurement of single viruses by surface plasmon resonance. Proc. Natl. Acad. Sci. USA 2010, 107, 16028–16032. [Google Scholar] [CrossRef] [PubMed]
- Faez, S.; Lahini, Y.; Weidlich, S.; Garmann, R.F.; Wondraczek, K.; Zeisberger, M.; Schmidt, M.A.; Orrit, M.; Manoharan, V.N. Fast, Label-Free Tracking of Single Viruses and Weakly Scattering Nanoparticles in a Nanofluidic Optical Fiber. ACS Nano 2015, 9, 12349–12357. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Deutsch, B.; Ignatovich, F.; Dykes, C.; Novotny, L. Nano-optofluidic Detection of Single Viruses and Nanoparticles. ACS Nano 2010, 4, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Ignatovich, F.; Novotny, L. Real-time optical detection of single human and bacterial viruses based on dark-field interferometry. Biosens. Bioelectron. 2012, 31, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Kukura, P.; Ewers, H.; Muller, C.; Renn, A.; Helenius, A.; Sandoghdar, V. High-speed nanoscopic tracking of the position and orientation of a single virus. Nat. Methods 2009, 6, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.D.; Hurley, K.R.; Feng, Z.V.; Haynes, C.L. Dark Field Transmission Electron Microscopy as a Tool for Identifying Inorganic Nanoparticles in Biological Matrices. Anal. Chem. 2015, 87, 4356–4362. [Google Scholar] [CrossRef] [PubMed]
- Theoret, T.; Wilkinson, K.J. Evaluation of enhanced darkfield microscopy and hyperspectral analysis to analyse the fate of silver nanoparticles in wastewaters. Anal. Methods 2017, 9, 3920–3928. [Google Scholar] [CrossRef]
- Li, H.; Luo, H.; Zhang, Z.; Li, Y.; Xiong, B.; Qiao, C.; Cao, X.; Wang, T.; He, Y.; Jing, G. Direct observation of nanoparticle multiple-ring pattern formation during droplet evaporation with dark-field microscopy. Phys. Chem. Chem. Phys. 2016, 18, 13018–13025. [Google Scholar] [CrossRef] [PubMed]
- Enoki, S.; Iino, R.; Morone, N.; Kaihatsu, K.; Sakakihara, S.; Kato, N.; Noji, H. Label-Free Single-Particle Imaging of the Influenza Virus by Objective-Type Total Internal Reflection Dark-Field Microscopy. PLoS ONE 2012, 7, e49208. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.-Y.; Zheng, L.-L.; Gao, P.-F.; Yang, X.-X.; Li, C.-M.; Li, Y.F.; Huang, C.Z. Real-Time Light Scattering Tracking of Gold Nanoparticles-bioconjugated Respiratory Syncytial Virus Infecting HEp-2 Cells. Sci. Rep. 2014, 4, 4529. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Guo, P.; El-Gohary, Y.; Prasadan, K.; Shiota, C.; Xiao, X.; Wiersch, J.; Paredes, J.; Tulachan, S.; Gittes, G.K. Rapid and simplified purification of recombinant adeno-associated virus. J. Virol. Methods 2012, 183, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, S. Mean-Shift Video Tracking. MATLAB Central File Exchange. Available online: https://cn.mathworks.com/matlabcentral/fileexchange/35520-mean-shift-video-tracking (accessed on 8 March 2012).
- Moon, J.-S.; Kim, W.-G.; Kim, C.; Park, G.-T.; Heo, J.; Yoo, S.Y.; Oh, J.-W. M13 Bacteriophage-Based Self-Assembly Structures and Their Functional Capabilities. Mini-Rev. Org. Chem. 2015, 12, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Chung, W.J.; McFarland, S.; Lee, S.W. Assembly of bacteriophage into functional materials. Chem. Rec. 2013, 13, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Bodiguel, H.; Leng, J. Imaging the drying of a colloidal suspension. Soft Matter 2010, 6, 5451–5460. [Google Scholar] [CrossRef]
- Fan, F.; Stebe, K.J. Assembly of Colloidal Particles by Evaporation on Surfaces with Patterned Hydrophobicity. Langmuir 2004, 20, 3062–3067. [Google Scholar] [CrossRef] [PubMed]
- Boulogne, F.; Giorgiutti-Dauphine, F.; Pauchard, L. Surface patterns in drying films of silica colloidal dispersions. Soft Matter 2015, 11, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Polson, A. Diffusion constants of the E. coli bacteriophages. Proc. Soc. Exp. Biol. Med. 1948, 67, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Polson, A.; Shepard, C.C. On the diffusion rates of bacteriophages. Biochim. Biophys. Acta 1949, 3, 137–145. [Google Scholar] [CrossRef]
- Cookson, J.T.; North, W.J. Adsorption of viruses on activated carbon. Equilibriums and kinetics of the attachment of Escherichia coli bacteriophage T4 on activated carbon. Environ. Sci. Technol. 1967, 1, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ho, C.-M.; Wong, T.-S. Minimal Size of Coffee Ring Structure. J. Phys. Chem. B 2010, 114, 5269–5274. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.J. Particle Convection in an Evaporating Colloidal Droplet. Langmuir 2002, 18, 60–67. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Analysis of the Microfluid Flow in an Evaporating Sessile Droplet. Langmuir 2005, 21, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Nadir Kaplan, C.; Wu, N.; Mandre, S.; Aizenberg, J.; Mahadevan, L. Dynamics of evaporative colloidal patterning. Phys. Fluids 2015, 27, 092105. [Google Scholar] [CrossRef]
- Kaplan, C.N.; Mahadevan, L. Evaporation-driven ring and film deposition from colloidal droplets. J. Fluid Mech. 2015, 781. [Google Scholar] [CrossRef]
- Rio, E.; Daerr, A.; Lequeux, F.; Limat, L. Moving Contact Lines of a Colloidal Suspension in the Presence of Drying. Langmuir 2006, 22, 3186–3191. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Balizan, E.; Lee, L.A.; Niu, Z.; Wang, Q. Self-Assembly of Rodlike Bio-nanoparticles in Capillary Tubes. Angew. Chem. Int. Ed. 2010, 49, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Moffat, J.R.; Sefiane, K.; Shanahan, M.E.R. Effect of TiO2 Nanoparticles on Contact Line Stick-Slip Behavior of Volatile Drops. J. Phys. Chem. B 2009, 113, 8860–8866. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, P.; Yan, H.J. Pinning-Depinning Mechanism of the Contact Line during Evaporation on Chemically Patterned Surfaces: A Lattice Boltzmann Study. Langmuir 2016, 32, 9389–9396. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.A.; Fairhurst, D.J. Classifying dynamic contact line modes in drying drops. Soft Matter 2015, 11, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Muller-Plathe, F.; Leroy, F. Pinning of the Contact Line during Evaporation on Heterogeneous Surfaces: Slowdown or Temporary Immobilization? Insights from a Nanoscale Study. Langmuir 2015, 31, 7544–7552. [Google Scholar] [CrossRef] [PubMed]
- Orejon, D.; Sefiane, K.; Shanahan, M.E.R. Stick-Slip of Evaporating Droplets: Substrate Hydrophobicity and Nanoparticle Concentration. Langmuir 2011, 27, 12834–12843. [Google Scholar] [CrossRef] [PubMed]
- Tarasevich, Y.Y.; Vodolazskaya, I.V.; Isakova, O.P. Desiccating colloidal sessile drop: Dynamics of shape and concentration. Colloid Polym. Sci. 2011, 289, 1015–1023. [Google Scholar] [CrossRef]
- Sáenz, P.J.; Wray, A.W.; Che, Z.; Matar, O.K.; Valluri, P.; Kim, J.; Sefiane, K. Dynamics and universal scaling law in geometrically-controlled sessile drop evaporation. Nat. Commun. 2017, 8, 14783. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, T.; Kaneko, D.; Doi, M. Dynamical Visualization of “Coffee Stain Phenomenon” in Droplets of Polymer Solution via Fluorescent Microscopy. Langmuir 2008, 24, 12369–12374. [Google Scholar] [CrossRef] [PubMed]
- Trantum, J.R.; Eagleton, Z.E.; Patil, C.A.; Tucker-Schwartz, J.M.; Baglia, M.L.; Skala, M.C.; Haselton, F.R. Cross-Sectional Tracking of Particle Motion in Evaporating Drops: Flow Fields and Interfacial Accumulation. Langmuir 2013, 29, 6221–6231. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Q.; Li, M.; Song, Y. Rate-dependent interface capture beyond the coffee-ring effect. Sci. Rep. 2016, 6, 24628. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooij, H.M.; van de Kerkhof, G.T.; Sprakel, J. A mechanistic view of drying suspension droplets. Soft Matter 2016, 12, 2858–2867. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooij, H.M.; de Kool, M.; van der Gucht, J.; Sprakel, J. Coalescence, Cracking, and Crack Healing in Drying Dispersion Droplets. Langmuir 2015, 31, 4419–4428. [Google Scholar] [CrossRef] [PubMed]
- Marín, Á.G.; Gelderblom, H.; Lohse, D.; Snoeijer, J.H. Order-to-Disorder Transition in Ring-Shaped Colloidal Stains. Phys. Rev. Lett. 2011, 107, 085502. [Google Scholar] [CrossRef] [PubMed]
- Eales, A.D.; Routh, A.F.; Dartnell, N.; Simon, G. Evaporation of pinned droplets containing polymer—An examination of the important groups controlling final shape. AIChE J. 2015, 61, 1759–1767. [Google Scholar] [CrossRef]


| T4 Phage Suspensions | Diffusion Coefficient (cm2/s) |
|---|---|
| T4 phage suspension in water at 23 °C (108 to 109 pfu/mL) [52] | 4 × 10−8–8 × 10−8 |
| T4 phage suspension in SM buffer (109 pfu/mL) | 4.8 × 10−8 |
| Concentrated T4 phage suspension in water (1011 pfu/mL) | 5.6 × 10−10 |
| Diluted T4 phage suspension in water (109 pfu/mL) | 3.6 × 10−8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaeli, I.; Hosseinidoust, Z.; Zolfagharnasab, H.; Jorge Monteiro, F. A New Label-Free Technique for Analysing Evaporation Induced Self-Assembly of Viral Nanoparticles Based on Enhanced Dark-Field Optical Imaging. Nanomaterials 2018, 8, 1. https://doi.org/10.3390/nano8010001
Ghaeli I, Hosseinidoust Z, Zolfagharnasab H, Jorge Monteiro F. A New Label-Free Technique for Analysing Evaporation Induced Self-Assembly of Viral Nanoparticles Based on Enhanced Dark-Field Optical Imaging. Nanomaterials. 2018; 8(1):1. https://doi.org/10.3390/nano8010001
Chicago/Turabian StyleGhaeli, Ima, Zeinab Hosseinidoust, Hooshiar Zolfagharnasab, and Fernando Jorge Monteiro. 2018. "A New Label-Free Technique for Analysing Evaporation Induced Self-Assembly of Viral Nanoparticles Based on Enhanced Dark-Field Optical Imaging" Nanomaterials 8, no. 1: 1. https://doi.org/10.3390/nano8010001






