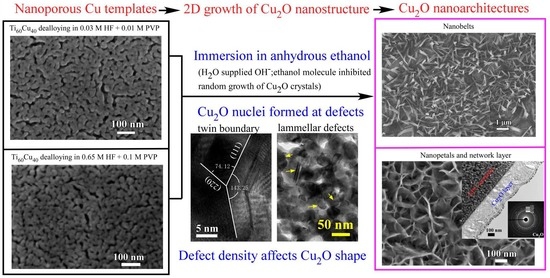
Ethanol-Mediated 2D Growth of Cu2O Nanoarchitectures on Nanoporous Cu Templates in Anhydrous Ethanol
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
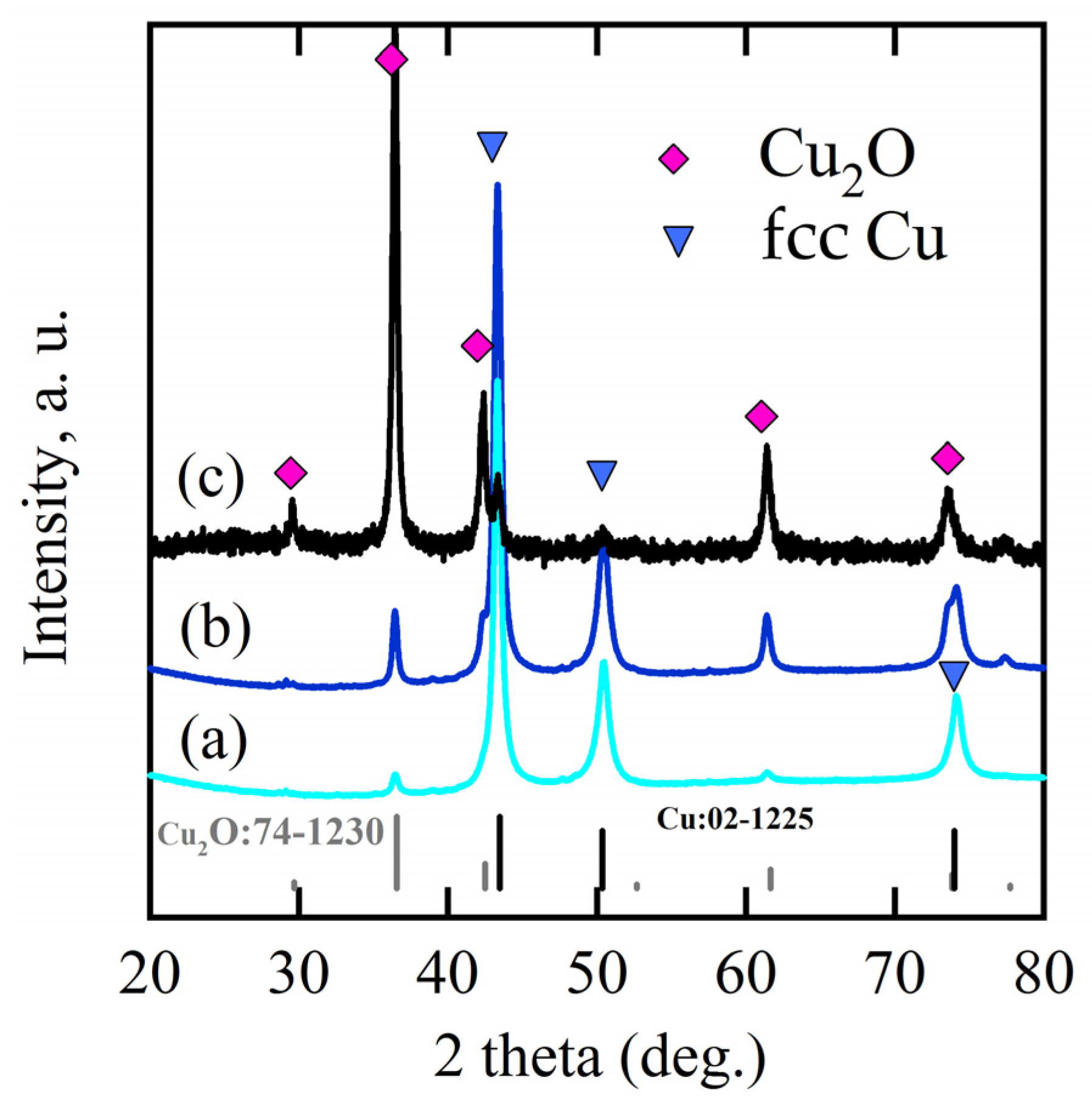
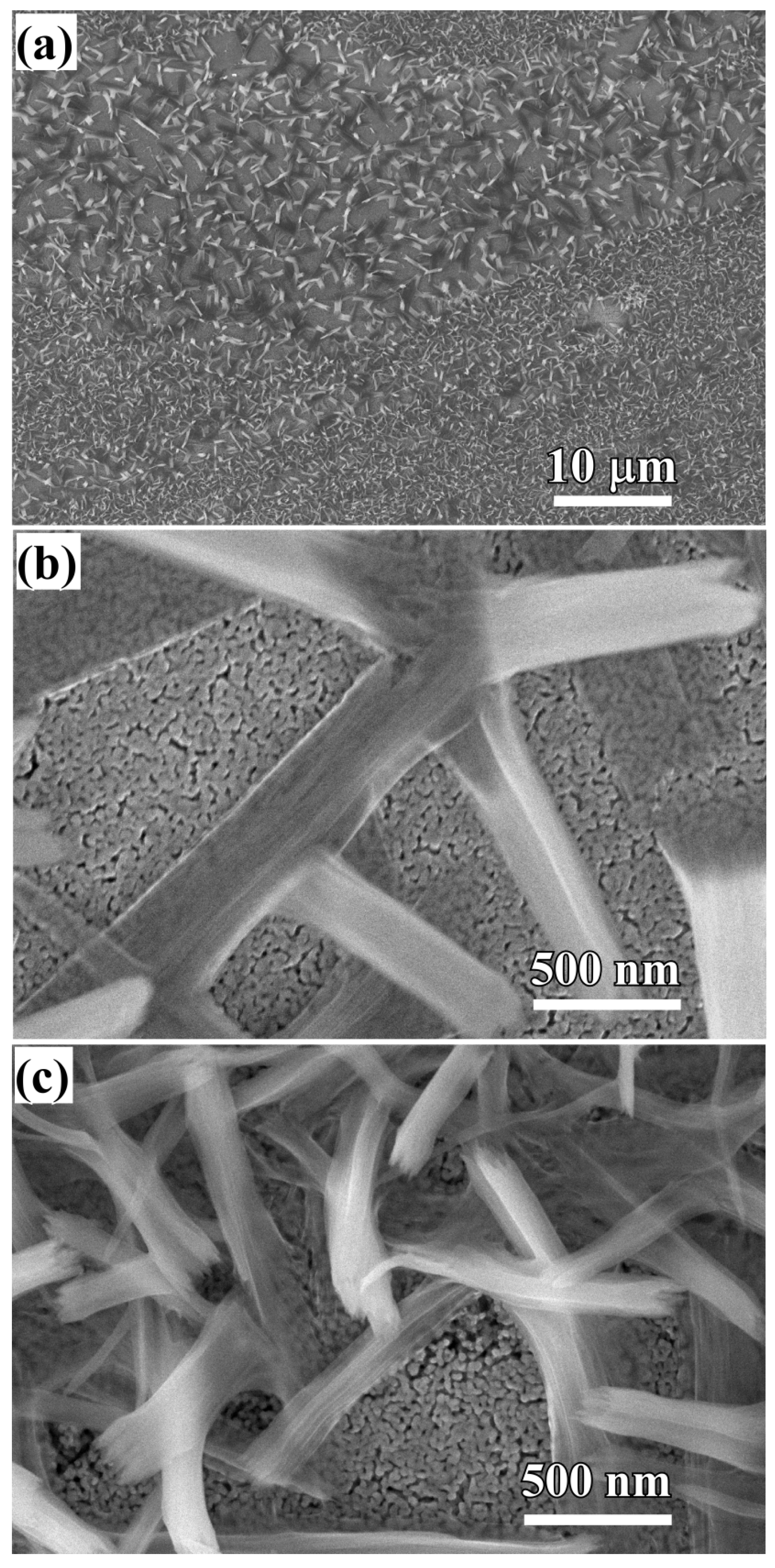
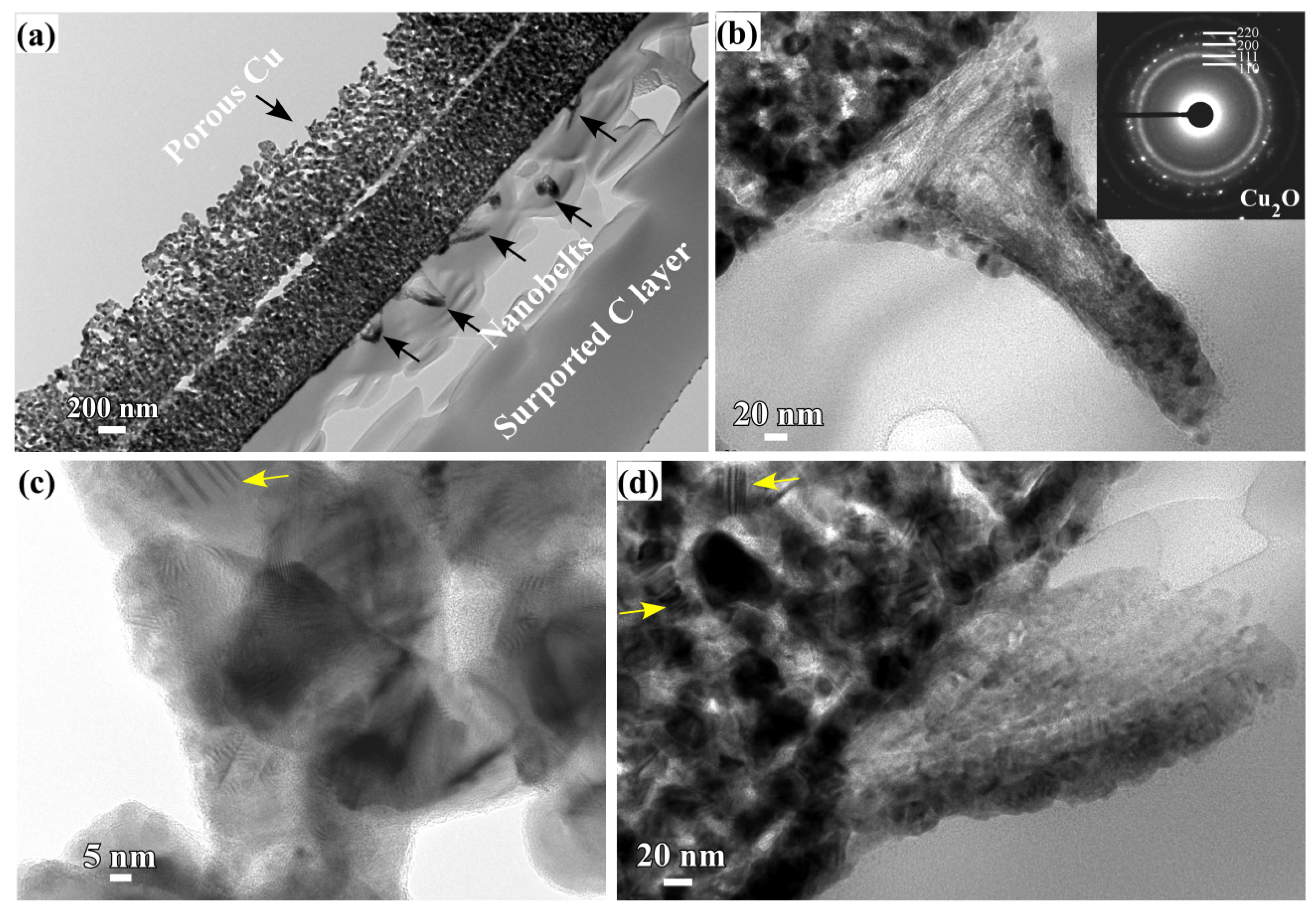
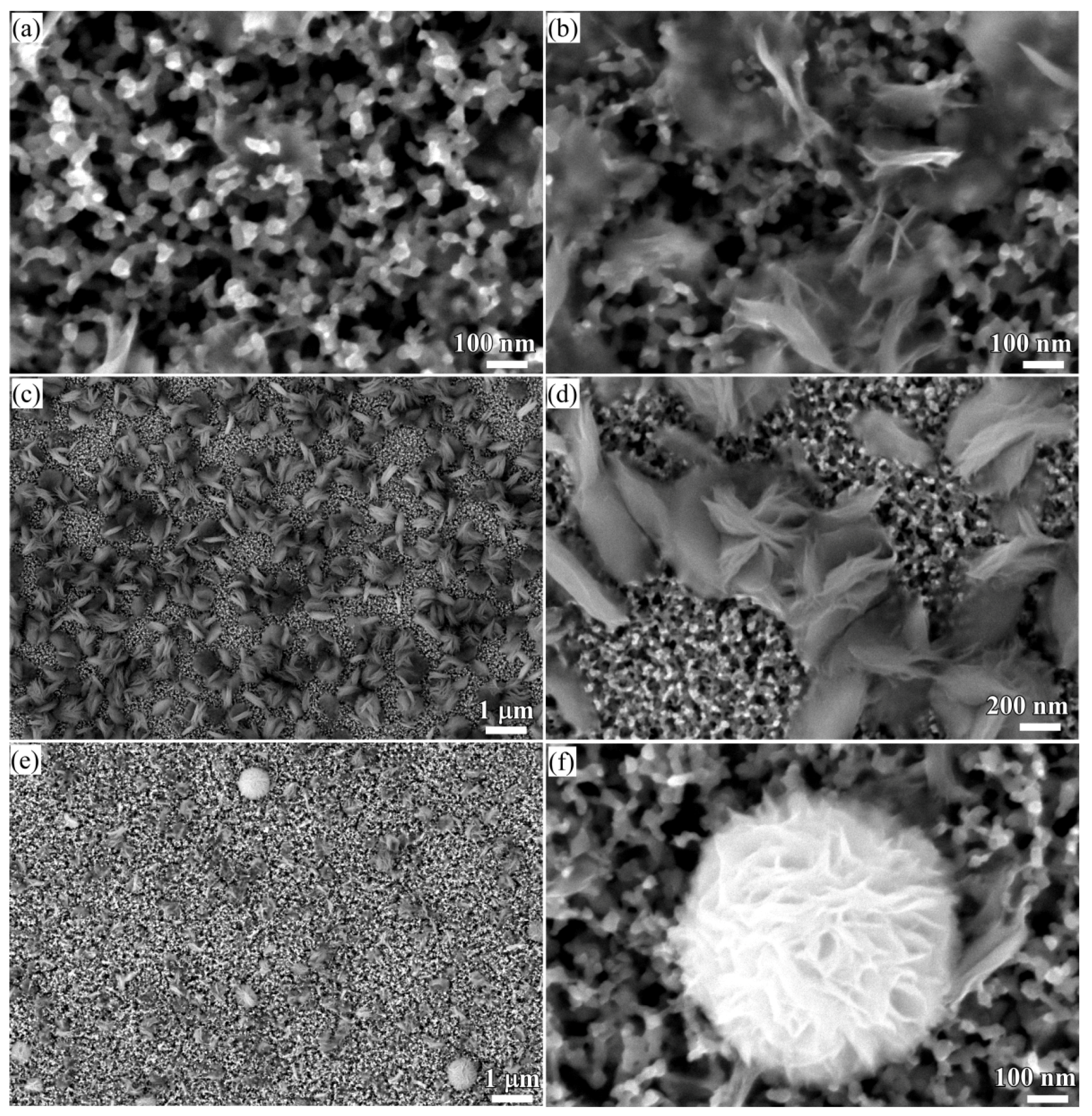
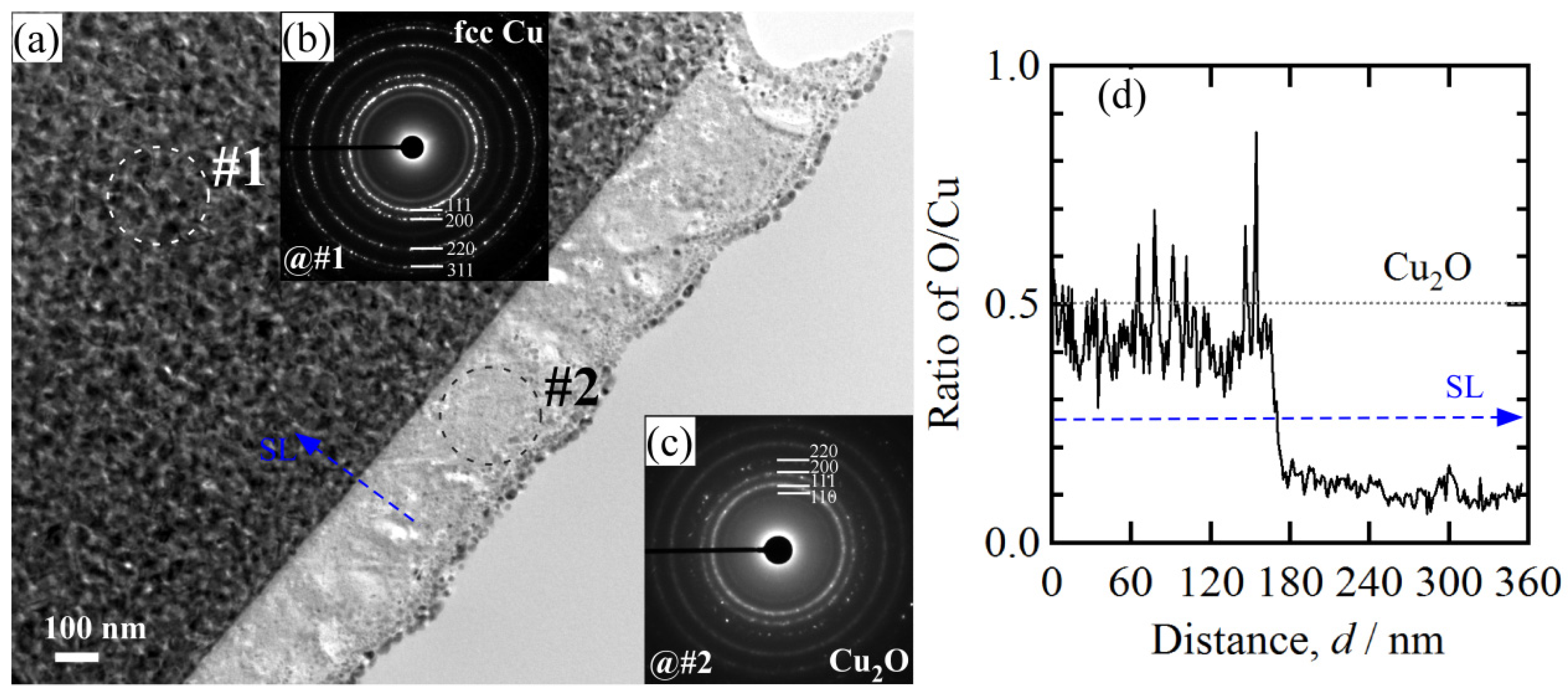
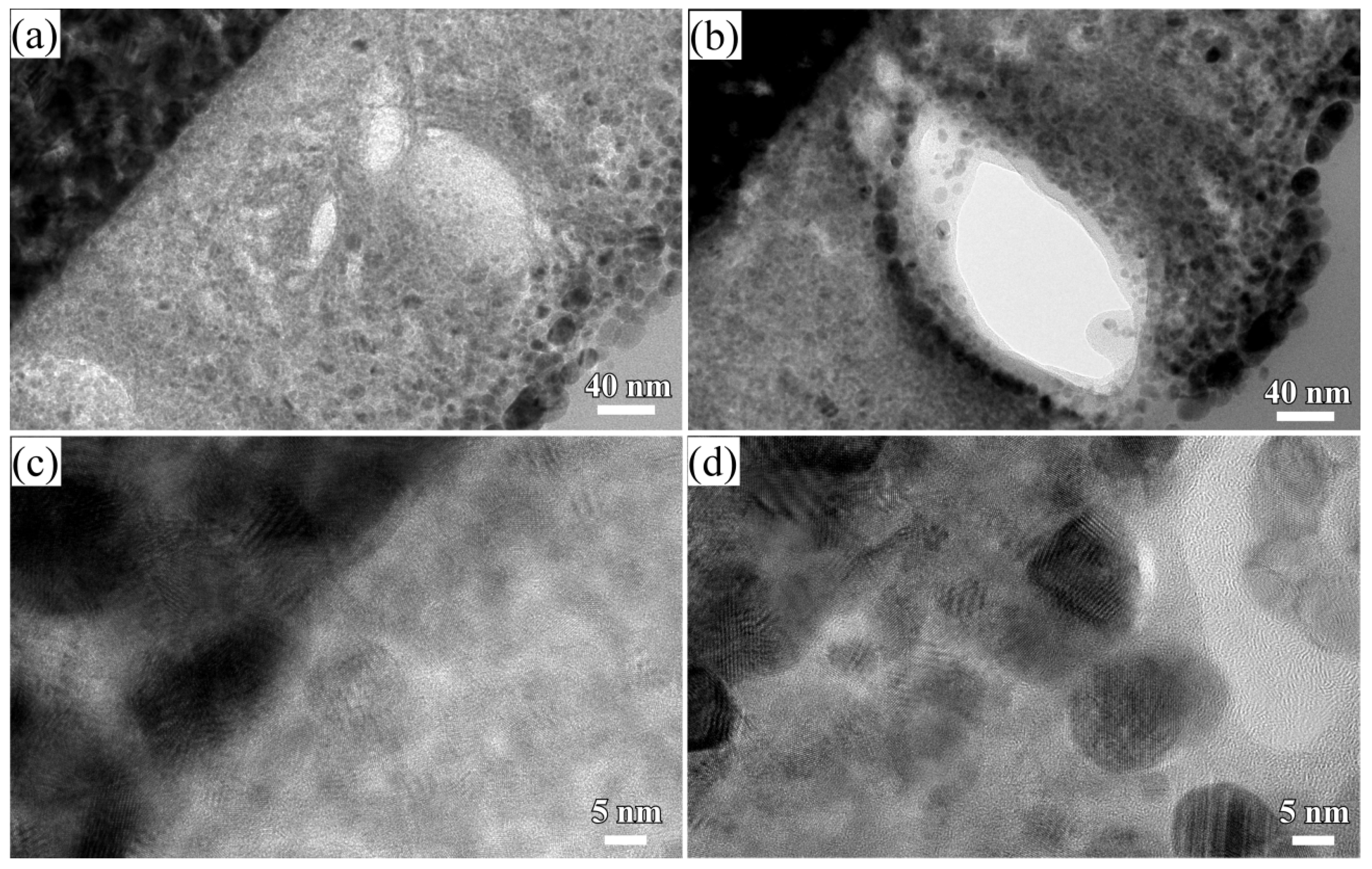
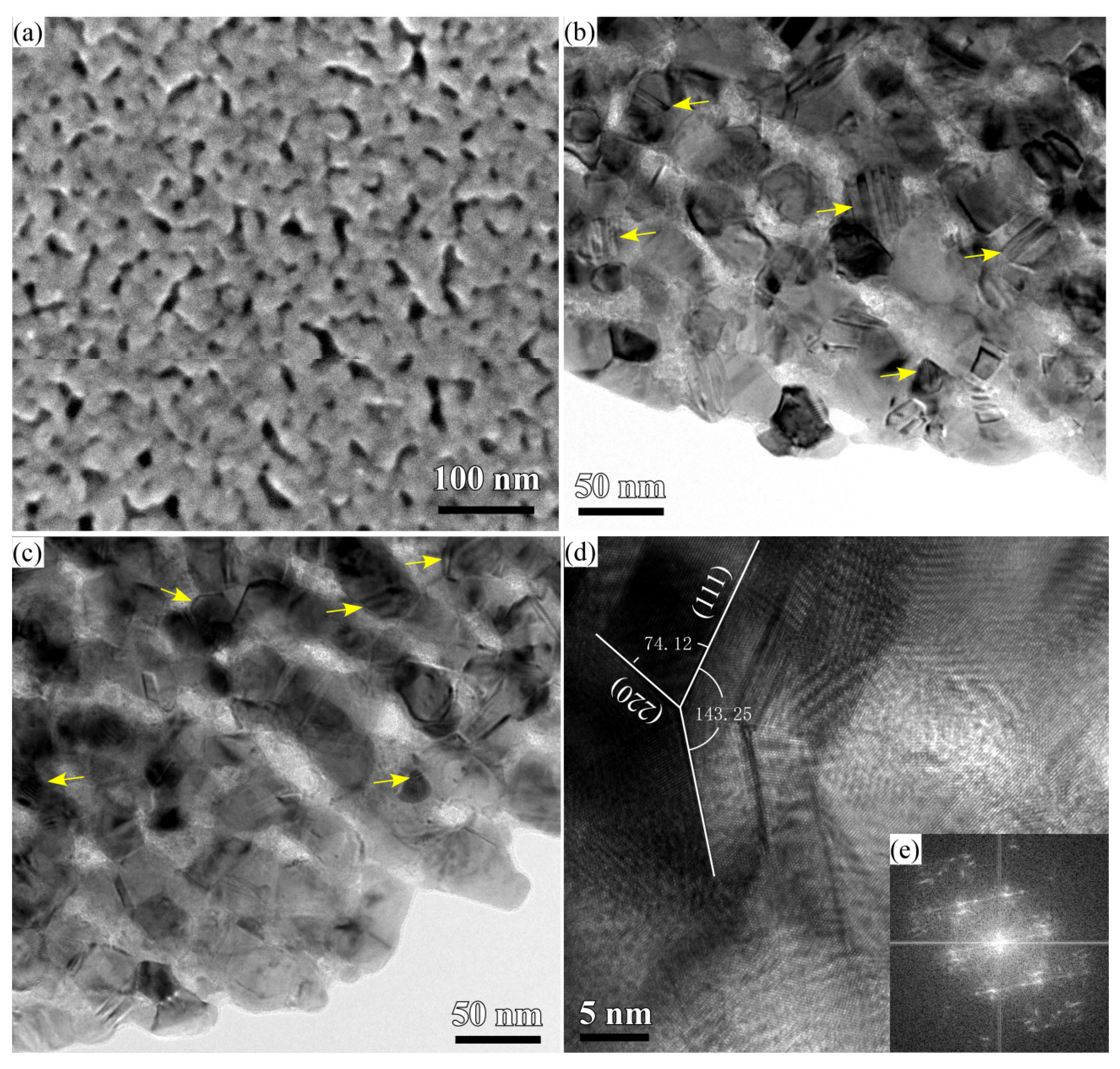
3.1. 2D Growth of Cu2O Nanobelts in Anhydrous Ethanol on NPC Templates from Ti60Cu40 Ribbons Dealloying in 0.03 M HF and 0.01 M PVP Solution
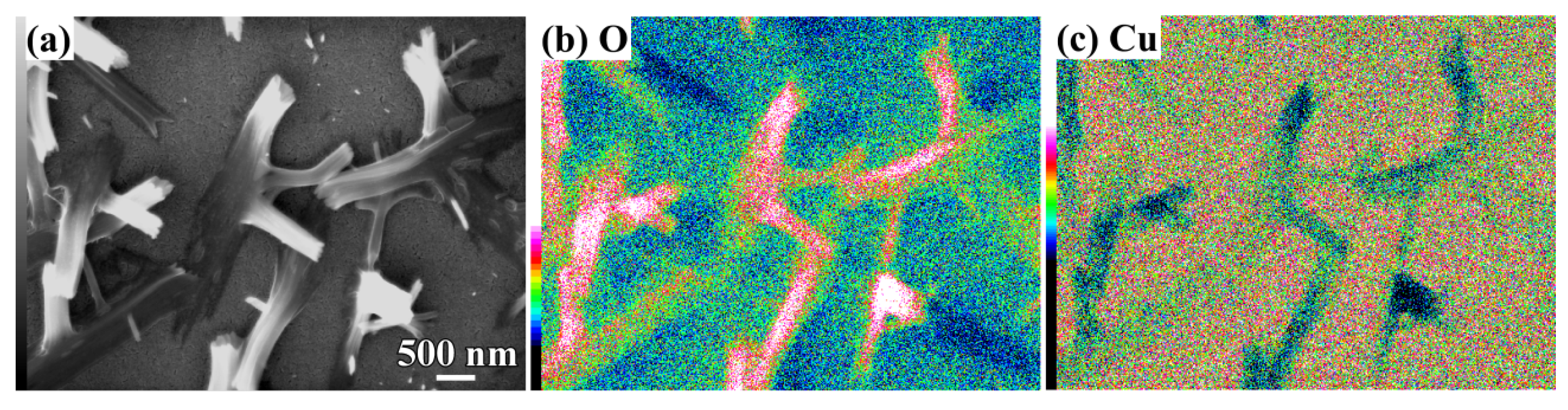
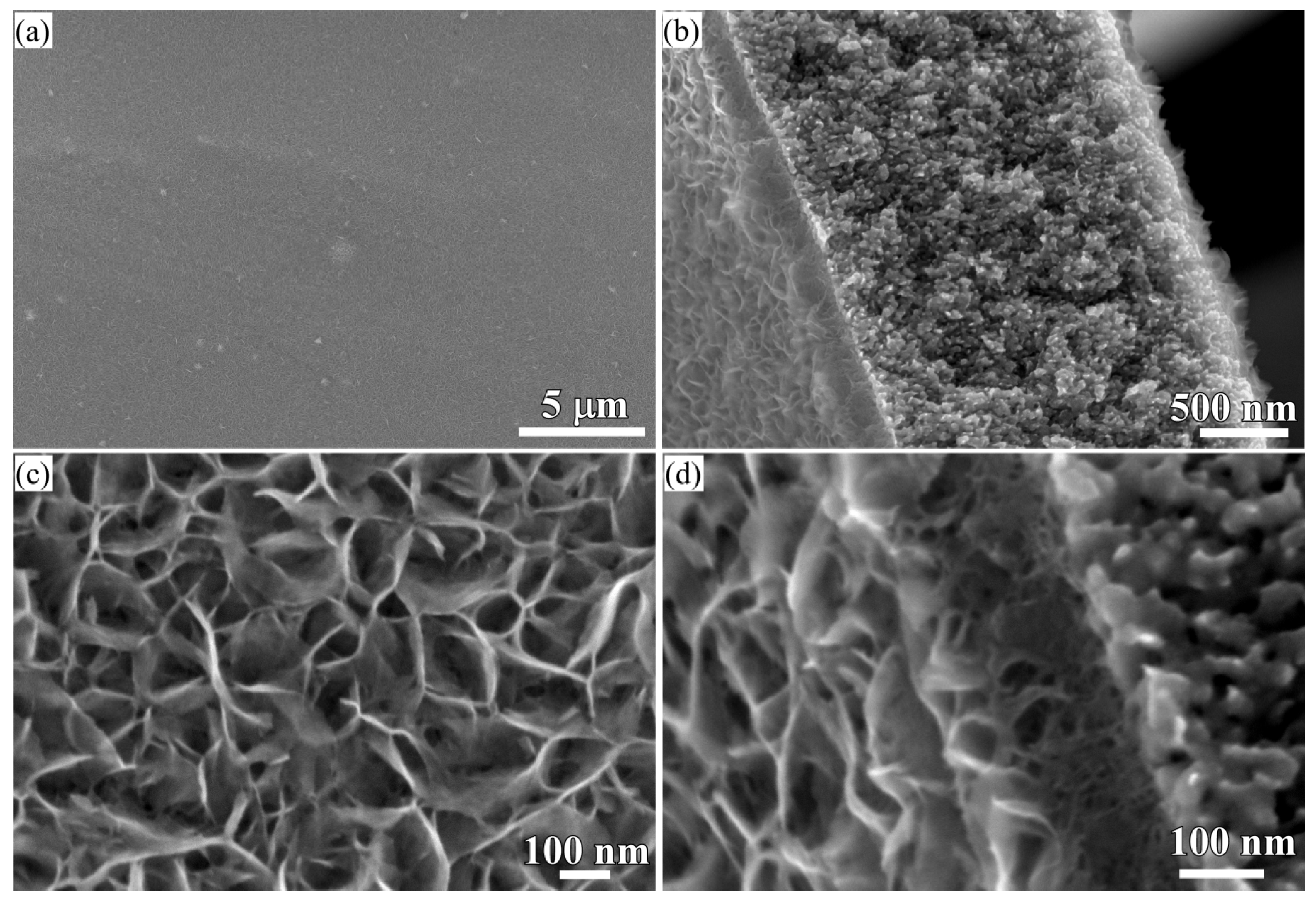
3.2. 2D Growth of Cu2O Nanopetal Networks in Anhydrous Ethanol on NPC Templates from Ti60Cu40 Ribbons Dealloying in 0.65 M HF and 0.1 M PVP Solution
3.3. Synergistic Effect of Initial Microstructure of NPC Templates and Stabilizing Agent of Ethanol Molecule on the Shapes of Cu2O Nanostructure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, M.H.; Lim, B.; Lee, E.P.; Xia, Y.N. Polyol synthesis of Cu2O nanoparticles: Use of chloride to promote the formation of a cubic morphology. J. Mater. Chem. 2008, 18, 4069–4073. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Shi, E.W.; Zheng, Y.Q.; Li, W.J.; Xiao, B.; Zhuang, J.Y. Growth of hex-pod-like Cu2O whisker under hydrothermal conditions. J. Cryst. Growth 2003, 249, 294–300. [Google Scholar] [CrossRef]
- Xu, H.L.; Wang, W.Z.; Zhu, W. Shape evolution and size-controllable synthesis of Cu2O octahedra and their morphology-dependent photocatalytic properties. J. Phys. Chem. B 2006, 110, 13829–13834. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.S.; Tu, Y.C.; Ren, Q.F.; Dai, X.J.; Xing, L.L.; Li, J.L. Surfactant-free fabrication of Cu2O nanosheets from Cu colloids and their tunable optical properties. J. Solid State Chem. 2009, 182, 182–186. [Google Scholar] [CrossRef]
- Cao, M.H.; Hu, C.W.; Wang, Y.H.; Guo, Y.H.; Guo, C.X.; Wang, E.B. A controllable synthetic route to Cu, Cu2O, and CuO nanotubes and nanorods. Chem. Commun. 2003, 1884–1885. [Google Scholar] [CrossRef]
- Luo, Y.S.; Li, S.Q.; Ren, Q.F.; Liu, J.P.; Xing, L.L.; Wang, Y.; Yu, Y.; Jia, Z.J.; Li, J.L. Facile synthesis of flowerlike Cu2O nanoarchitectures by a solution phase route. Cryst. Growth Des. 2007, 7, 87–92. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Yang, L.C.; Wu, Y.P. Fabrication of a graphene–cuprous oxide composite. J. Solid State Chem. 2009, 182, 2486–2490. [Google Scholar] [CrossRef]
- Wang, D.B.; Mo, M.S.; Yu, D.B.; Xu, L.Q.; Li, F.Q.; Qian, Y.T. Large-scale growth and shape evolution of Cu2O cubes. Cryst. Growth Des. 2003, 3, 717–720. [Google Scholar] [CrossRef]
- Tan, Y.W.; Xue, X.Y.; Peng, Q.; Zhao, H.; Wang, T.H.; Li, Y.D. Controllable fabrication and electrical performance of single crystalline Cu2O nanowires with high aspect ratios. Nano Lett. 2007, 7, 3723–3728. [Google Scholar] [CrossRef]
- Kuo, C.H.; Chen, C.H.; Huang, M.H. Seed-Mediated synthesis of monodispersed Cu2O nanocubes with five different size ranges from 40 to 420 nm. Adv. Funct. Mater. 2007, 17, 3773–3780. [Google Scholar] [CrossRef]
- Gou, L.F.; Murphy, C.J. Solution-phase synthesis of Cu2O nanocubes. Nano Lett. 2003, 3, 231–234. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.G.; Mayers, B.; Xia, Y.N. Shape-controlled synthesis of metal nanostructures: The case of silver. Chem. Eur. J. 2005, 11, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.J.; Xia, Y.N. Shape-controlled synthesis of metal nanostructures: The case of palladium. Adv. Mater. 2007, 19, 3385–3391. [Google Scholar] [CrossRef]
- Sun, Y.G.; Xia, Y.N. Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in aqueous medium. J. Am. Chem. Soc. 2004, 126, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Zhu, Q.S.; Zhang, Y.; Wang, Y.; Zhao, L.; Yu, B. One-pot synthesis and hierarchical assembly of hollow Cu2O microspheres with nanocrystals-composed porous multishell and their gas-sensing properties. Adv. Funct. Mater. 2007, 17, 2766–2771. [Google Scholar] [CrossRef]
- Xu, J.S.; Xue, D.F. Five branching growth patterns in the cubic crystal system: A direct observation of cuprous oxide microcrystals. Acta Mater. 2007, 55, 2397–2406. [Google Scholar] [CrossRef]
- Chang, Y.; Zeng, H.C. Manipulative synthesis of multipod frameworks for self-organization and self-amplification of Cu2O microcrystals. Cryst. Growth Des. 2004, 4, 273–278. [Google Scholar] [CrossRef]
- Xiong, Y.J.; Li, Z.Q.; Zhang, R.; Xie, Y.; Yang, J.; Wu, C.Z. From complex chains to 1D metal oxides: A novel strategy to Cu2O nanowires. J. Phys. Chem. B 2003, 107, 3697–3702. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.; Xu, F.; Liu, X.H.; Xu, D. Shape-controlled synthesis of submicro-sized cuprous oxide octahedra. Inorg. Chem. Commun. 2003, 6, 1390–1392. [Google Scholar] [CrossRef]
- Zhang, H.W.; Zhang, X.; Li, H.Y.; Qu, Z.K.; Fan, S.; Ji, M.Y. Hierarchical growth of Cu2O double tower-tip-like nanostructures in water/oil microemulsion. Cryst. Growth Des. 2007, 7, 820–824. [Google Scholar] [CrossRef]
- Siegfried, M.J.; Choi, K.S. Electrochemical crystallization of cuprous oxide with systematic shape evolution. Adv. Mater. 2004, 16, 1743–1746. [Google Scholar] [CrossRef]
- Siegfried, M.J.; Choi, K.S. Directing the architecture of cuprous oxide crystals during electrochemical growth. Angew. Chem. Int. Ed. 2005, 44, 3282–3287. [Google Scholar] [CrossRef]
- Siegfried, M.J.; Choi, K.S. Elucidation of an overpotential-limited branching phenomenon observed during the electrocrystallization of cuprous oxide. Angew. Chem. Int. Ed. 2008, 47, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, Y.; Cai, Q.; Sun, Q.Y.; Li, H.D.; Chen, X.H.; Wang, X.P.; Yan, Y.J.; Vireling, E.G. Patterning of nanostructured cuprous oxide by surfactant-assisted electrochemical deposition. Cryst. Growth Des. 2008, 8, 2652–2659. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, Y.; Qi, Z.; Zhang, W.H.; Qin, J.Y.; Frenzel, J. Generalized fabrication of nanoporous metals (Au, Pd, Pt, Ag, and Cu) through chemical dealloying. J. Phys. Chem. C 2009, 113, 12629–12636. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan, Z.H.; Qin, F.X.; Wada, T.; Yamaura, S.; Xie, G.Q.; Sugawara, Y.; Muto, I.; Makino, A.; Hara, N. Nanoporous palladium fabricated from an amorphous Pd42.5Cu30Ni7.5P20 precursor and its ethanol electro-oxidation performance. Electrochim. Acta 2013, 108, 512–519. [Google Scholar] [CrossRef]
- Yu, J.S.; Ding, Y.; Xu, C.X.; Inoue, A.; Sakurai, T.; Chen, M.W. Nanoporous metals by dealloying multicomponent metallic glasses. Chem. Mater. 2008, 20, 4548–4550. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Sugawara, Y.; Muto, I.; Hara, N. Fabrication of nanoporous copper by dealloying amorphous binary Ti-Cu alloys in hydrofluoric acid solutions. Intermetallics 2012, 9, 14–20. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Hara, N. Refinement of nanoporous copper: A summary of micro-alloying of Au-group and Pt-group elements. Mater. Trans. 2014, 55, 796–800. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Sugawara, Y.; Muto, I.; Hara, N. Bimodal nanoporous nickel prepared by dealloying Ni38Mn62 alloys. Intermetallics 2012, 31, 157–164. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Sugawara, Y.; Muto, I.; Hara, N. Elaboration of nanoporous copper by modifying surface diffusivity by the minor addition of gold. Microporous Mesoporous Mater. 2013, 165, 257–264. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Yamaura, S.; Xie, G.Q.; Makino, A.; Hara, N. Refinement of nanoporous copper by dealloying MgCuY amorphous alloys in sulfuric acids containing polyvinylpyrrolidone. J. Electrochem. Soc. 2014, 161, C120–C125. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Hara, N. Polyvinylpyrrolidone macromolecules function as a diffusion barrier during dealloying. Mater. Chem. Phys. 2014, 146, 277–282. [Google Scholar] [CrossRef]
- Kou, T.Y.; Jin, C.H.; Zhang, C.; Sun, J.Z.; Zhang, Z.H. Nanoporous core–shell Cu@Cu2O nanocomposites with superior photocatalytic properties towards the degradation of methyl orange. RSC Adv. 2012, 2, 12636–12643. [Google Scholar] [CrossRef]
- Liu, D.Q.; Yang, Z.B.; Wang, P.; Li, F.; Wang, D.S.; He, D.Y. Preparation of 3D nanoporous copper-supported cuprous oxide for high-performance lithium ion battery anodes. Nanoscale 2013, 5, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.B.; Zhang, S.C.; Li, N.; Zheng, J.W.; Xing, Y.L. A facile one-pot route to fabricate nanoporous copper with controlled hierarchical pore size distributions through chemical dealloying of Al–Cu alloy in an alkaline solution. Microporous Mesoporous Mater. 2011, 138, 1–7. [Google Scholar] [CrossRef]
- Chanquía, C.M.; Sapag, K.; Rodríguez-Castellόn, E.; Herrero, E.R.; Eimer, G.A. Nature and location of copper nanospecies in mesoporous molecular sieves. J. Phys. Chem. C 2010, 114, 1481–1490. [Google Scholar] [CrossRef]
- Li, D.; McCann, J.; Matthew, T.; Xia, Y.N. Photocatalytic deposition of gold nanoparticles on electrospun nanofibers of titania. Chem. Phys. Lett. 2004, 394, 387–391. [Google Scholar] [CrossRef]
- Leff, D.V.; Ohara, P.C.; Heath, J.R.; Gelbart, W.M. Thermodynamic control of gold nanocrystal size: Experiment and theory. J. Phys. Chem. 1995, 99, 7036–7041. [Google Scholar] [CrossRef]
- Wiley, B.J.; Xiong, Y.J.; Li, Z.Y.; Yin, Y.D.; Xia, Y.N. Right bipyramids of silver: A new shape derived from single twinned seeds. Nano Lett. 2006, 6, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Bowker, M.; Madix, R.J. XPS, UPS and thermal desorption studies of alcohol adsorption on Cu(110): II. Higher alcohols. Surf. Sci. 1982, 116, 549–572. [Google Scholar] [CrossRef]
- Ko, E.S.; Choi, J.; Okamoto, K.; Tak, Y.S.; Lee, J.Y. Cu2O nanowires in an alumina template: Electrochemical conditions for the synthesis and photoluminescence characteristics. ChemPhysChem 2006, 7, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Ko, E.S.; Kang, J.W.; Tak, Y.S.; Lee, J.Y. Influence of solution pH on the electrochemical fabrication of functional metal oxides using a nanoporous alumina template. J. Ind. Eng. Chem. 2007, 13, 305–309. [Google Scholar]
- Parida, S.; Kramer, D.; Volkert, C.A.; Rosner, H.; Erlebacher, J.; Weissmüller, A. Volume change during the formation of nanoporous gold by dealloying. Phys. Rev. Lett. 2006, 97, 35504. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.T.; Wang, J.X.; Xu, G.Y. Fast Synthesis of Cu2O Hollow Microspheres and Their Application in DNA Biosensor of Hepatitis B Virus. Cryst. Growth Des. 2009, 9, 633–638. [Google Scholar] [CrossRef]
- Luo, J.S.; Steier, L.; Son, M.K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O nanowire photocathodes for efficient and durable solar water splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic hydrogen production: A rift into the future energy supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Zhang, J.T.; Liu, J.F.; Peng, Q.; Wang, X.; Li, Y.D. Nearly monodisperse Cu2O and CuO nanospheres: Preparation and applications for sensitive gas sensors. Chem. Mater. 2006, 18, 867–871. [Google Scholar] [CrossRef]
- Zhang, C.; Tu, J.; Huang, X.; Yuan, Y.; Chen, X.; Mao, F. Preparation and electrochemical performances of cubic shape Cu2O as anode material for lithium ion batteries. J. Alloy. Compd. 2007, 441, 52–56. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dan, Z.; Lu, J.; Li, F.; Qin, F.; Chang, H. Ethanol-Mediated 2D Growth of Cu2O Nanoarchitectures on Nanoporous Cu Templates in Anhydrous Ethanol. Nanomaterials 2018, 8, 18. https://doi.org/10.3390/nano8010018
Dan Z, Lu J, Li F, Qin F, Chang H. Ethanol-Mediated 2D Growth of Cu2O Nanoarchitectures on Nanoporous Cu Templates in Anhydrous Ethanol. Nanomaterials. 2018; 8(1):18. https://doi.org/10.3390/nano8010018
Chicago/Turabian StyleDan, Zhenhua, Jiafei Lu, Feng Li, Fengxiang Qin, and Hui Chang. 2018. "Ethanol-Mediated 2D Growth of Cu2O Nanoarchitectures on Nanoporous Cu Templates in Anhydrous Ethanol" Nanomaterials 8, no. 1: 18. https://doi.org/10.3390/nano8010018