Improvement in Electrode Performance of Novel SWCNT Loaded Three-Dimensional Porous RVC Composite Electrodes by Electrochemical Deposition Method
Abstract
:1. Introduction
2. Materials, Methods and Experimental Section
2.1. Chemicals and Materials
2.2. Functionalization of Carbon Nanotubes
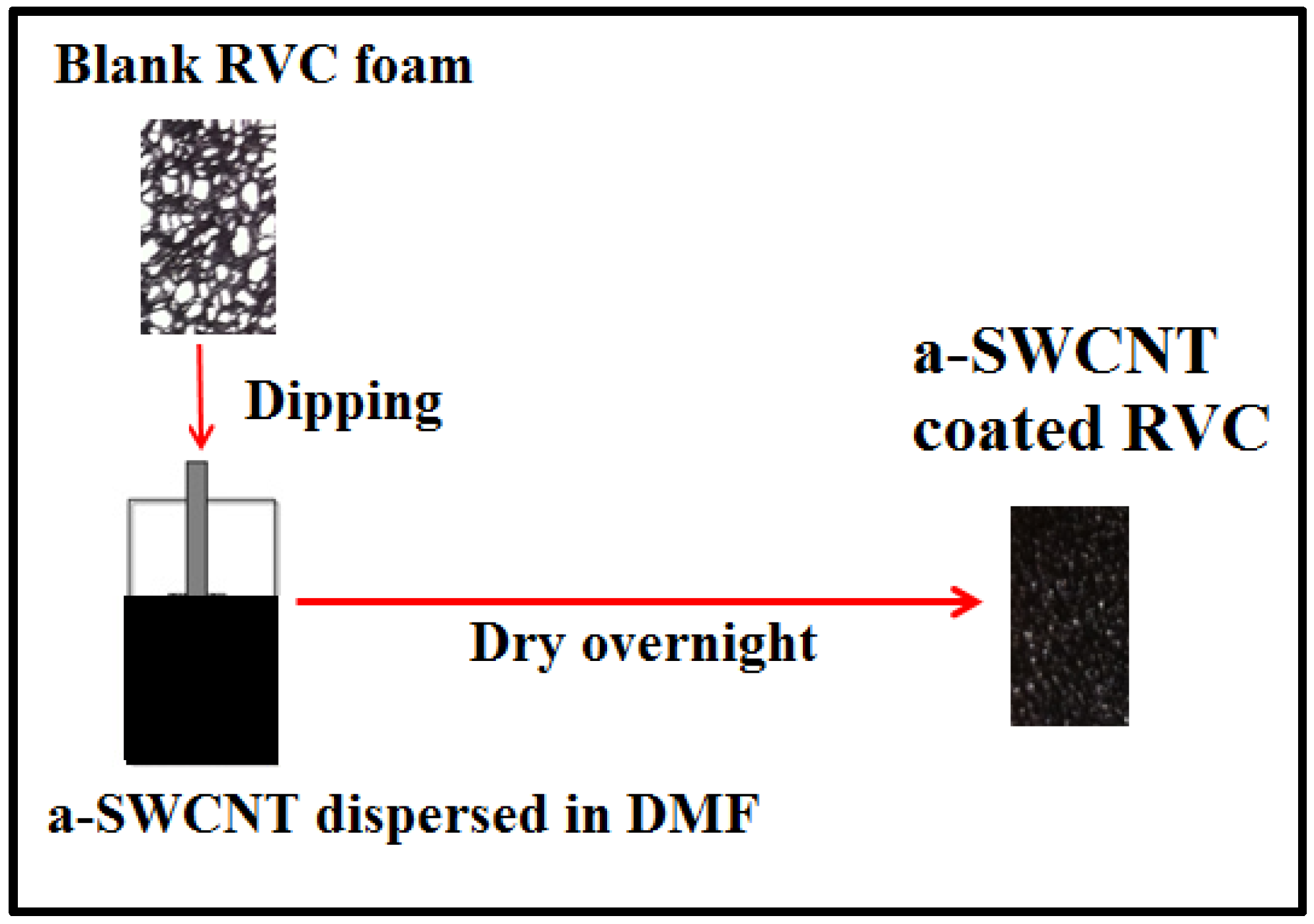
2.3. Dispersion of a-SWCNT
2.4. Pre-Treatment of the RVC Electrode
2.5. a-SWCNT Dip Coated RVC Electrode
2.6. Electrochemical Characterization
2.7 Physical Characterization
3. Results and Discussion
3.1. Characterization of SWCNT after Functionalization by Raman Spectroscopy
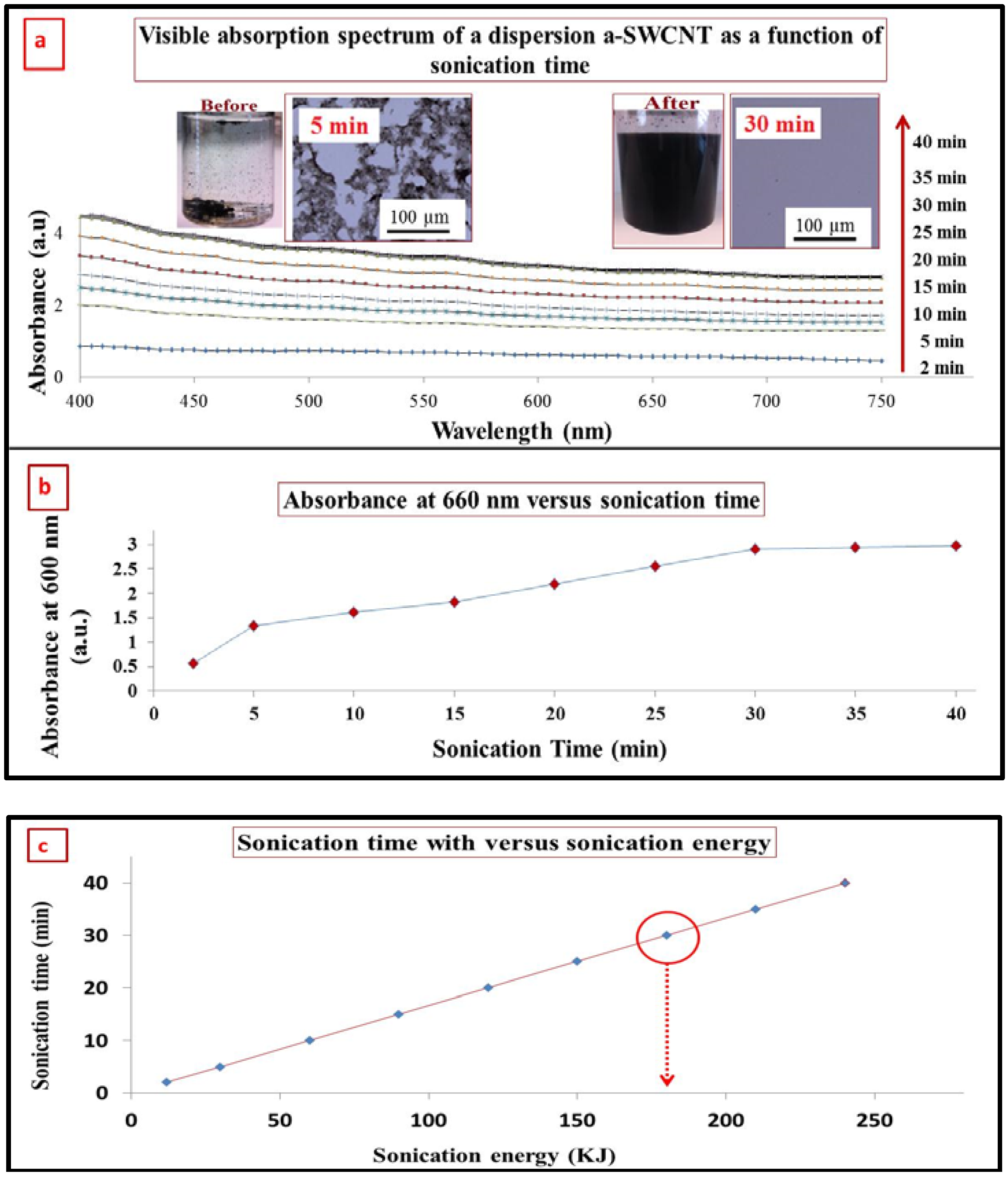
3.2. Characterization of SWCNT after Functionalization by Visible Spectrophotometry
3.3. Dispersion of a-SWCNT in DMF
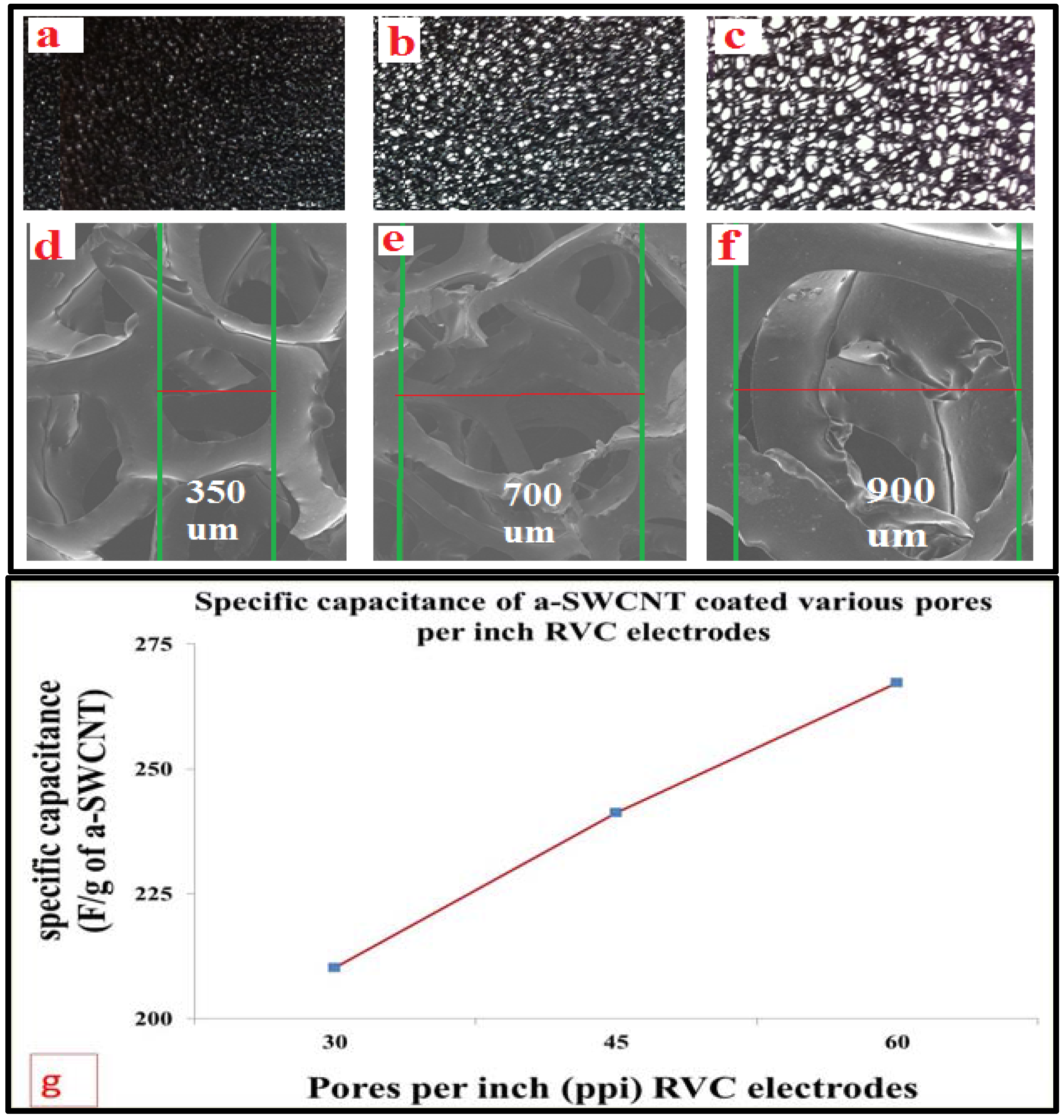
3.4. Optimization of RVC Electrodes Coated with a-SWCNT
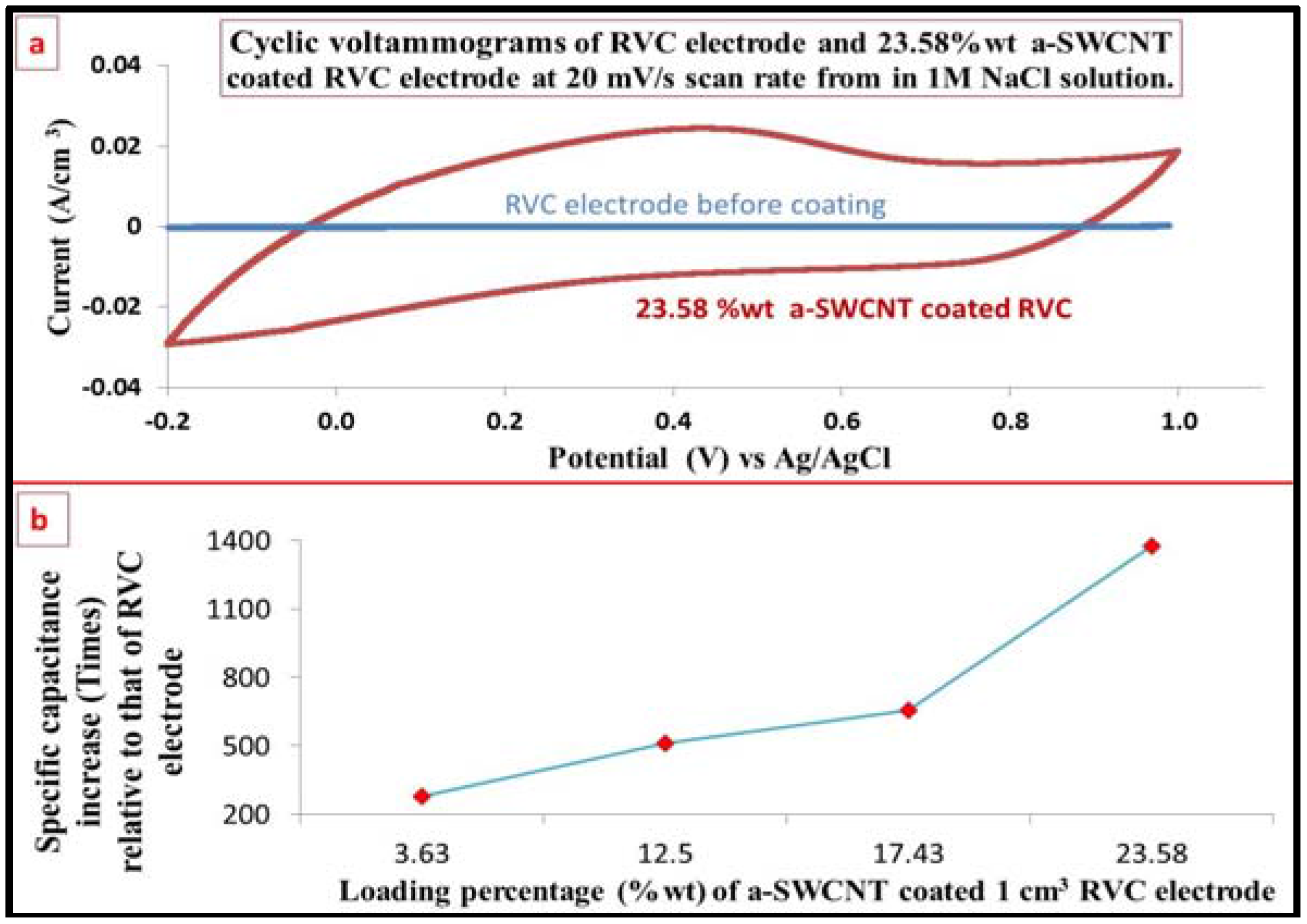
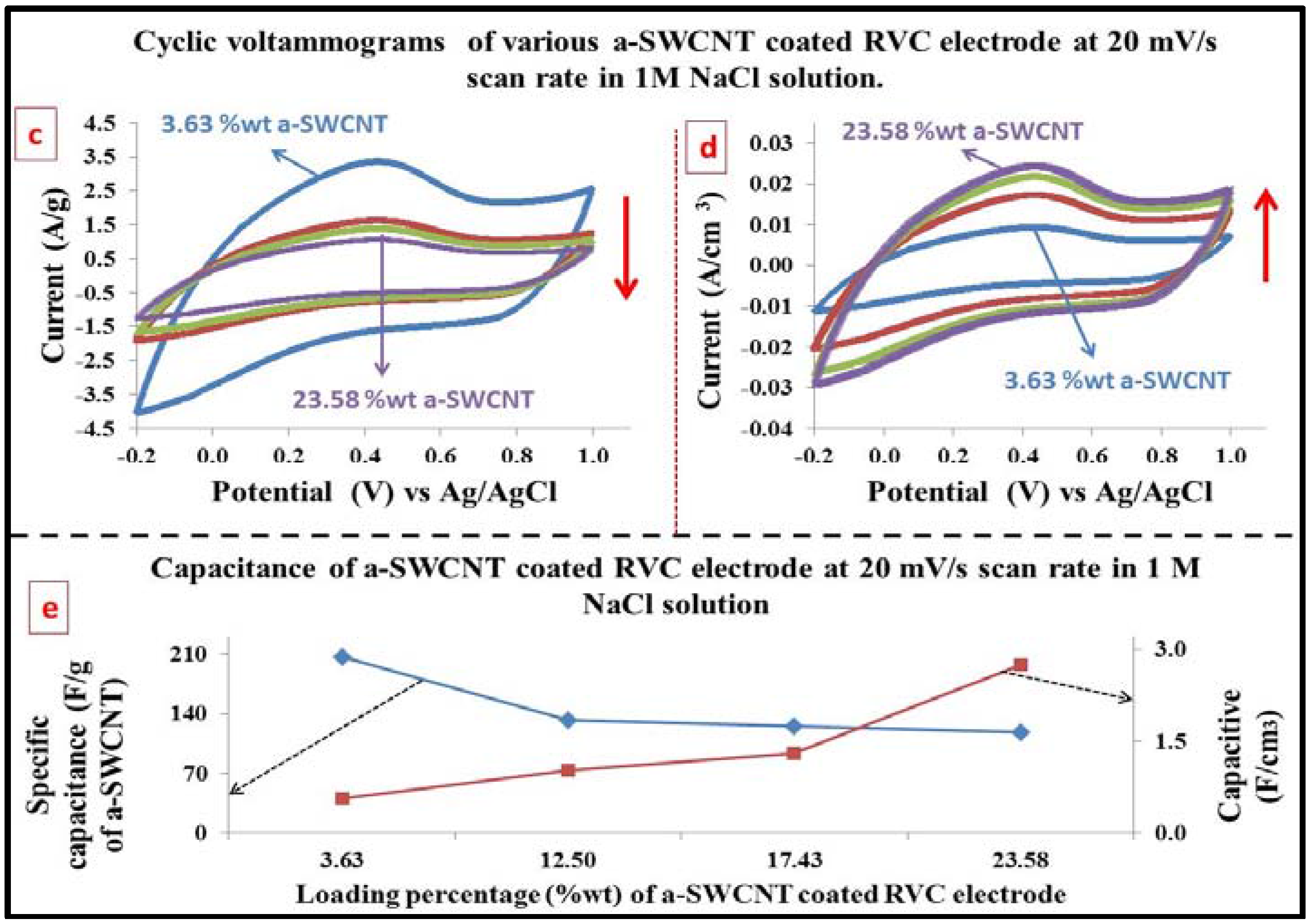
3.5. Optimization of the Loading Level of a-SWCNT on the RVC Electrode
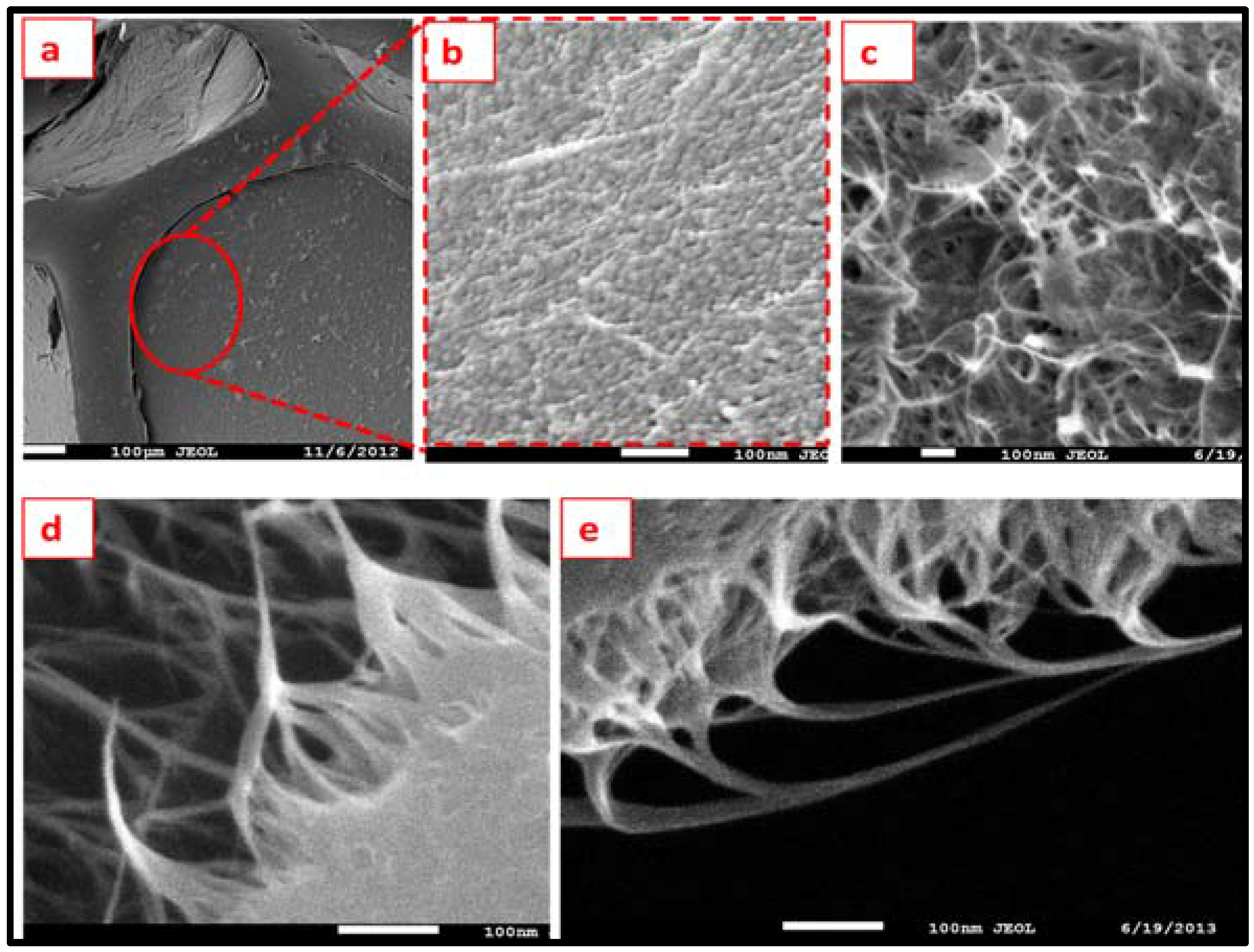
3.6. Scanning Electron Microscopy of a-SWCNT-Coated RVC Electrodes
3.7. Electrochemical Behavior Evaluation Using Cyclic Voltammetry
3.8. The Capacitance of RVC Electrode before and after Loading with a-SWCNT
3.9. Effect of Increasing the Scan Rate on the Electrode Capacitance
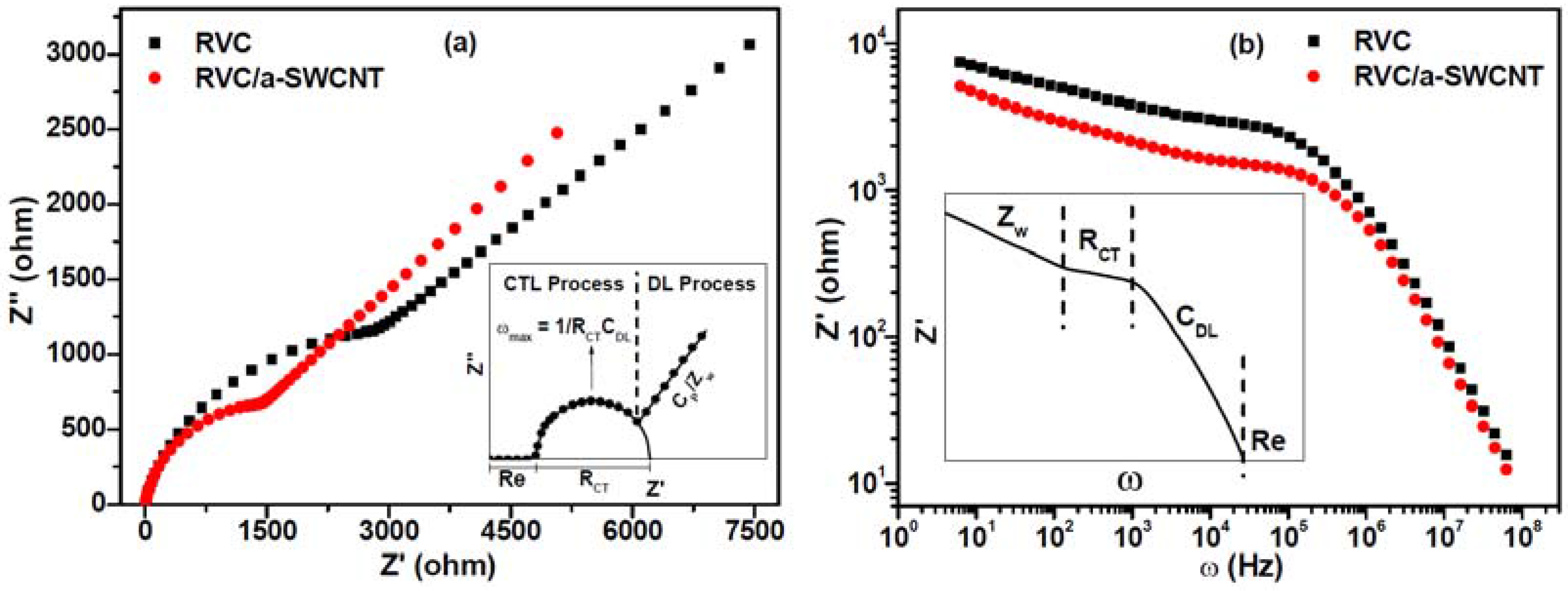
3.10. Electrochemical Impedance Spectroscopy
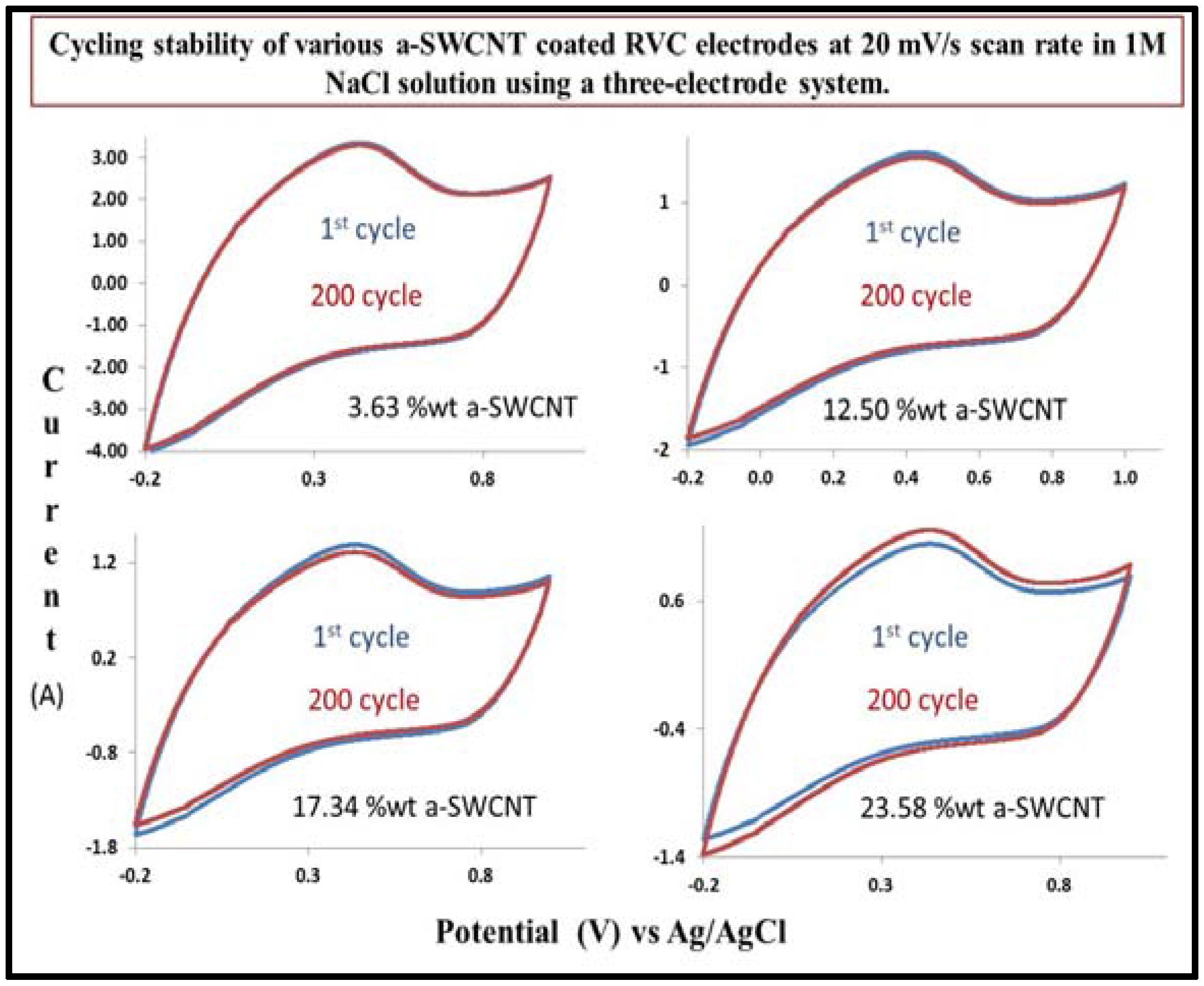
3.11. Cycling Stability of a-SWCNT/RVC Electrodes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pekala, R.W.; Farmer, J.C.; Alviso, C.T.; Tran, T.D.; Mayer, S.T.; Miller, J.M.; Dunn, B. Carbon aerogels for electrochemical applications. J. Non Cryst. Solids 1998, 225, 74–80. [Google Scholar] [CrossRef]
- Oren, Y. Capacitive deionization (CDI) for desalination and water treatment past, present and future (a review). Desalination 2008, 228, 10–29. [Google Scholar] [CrossRef]
- Conway, B.E.; Ayranci, E.; Al-Maznai, H. Use of quasi-3-dimensional porous electrodes for adsorption and electrocatalytic removal of impurities from waste-waters. Electrochim. Acta 2001, 47, 705–718. [Google Scholar] [CrossRef]
- Gabelich, C.J.; Tran, T.D.; Suffet, I.H. Electrosorption of inorganic salts from aqueous solution using carbon aerogels. Environ. Sci. Technol. 2002, 36, 3010–3019. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-J.; Lee, J.-H.; Ahn, H.-J.; Jeong, Y.; Kim, Y.-J.; Chi, C.-S. Nanoporous activated carbon cloth for capacitive deionization of aqueous solution. Thin Solid Films 2006, 515, 220–225. [Google Scholar] [CrossRef]
- Ryoo, M.; Kim, J.; Seo, G. Role of titania incorporated on activated carbon cloth for capacitive deionization of NaCl solution. J. Colloid Interface Sci. 2003, 264, 414–419. [Google Scholar] [CrossRef]
- Wang, X.Z.; Li, M.G.; Chen, Y.W.; Cheng, R.M.; Huang, S.M.; Pan, L.K.; Sun, Z. Electrosorption of ions from aqueous solutions with carbon nanotubes and nanofibers composite film electrodes. Appl. Phys. Lett. 2006, 89, 53127. [Google Scholar] [CrossRef]
- Pan, L.; Wang, X.; Gao, Y.; Zhang, Y.; Chen, Y.; Sun, Z. Electrosorption of anions with carbon nanotube and nanofibre composite film electrodes. Desalination 2009, 244, 139–143. [Google Scholar] [CrossRef]
- Kim, B.C.; Ko, J.M.; Wallace, G.G. A novel capacitor material based on Nafion-doped polypyrrole. J. Power Sources 2008, 177, 665–668. [Google Scholar] [CrossRef]
- Meyyappan, M. Carbon Nanotubes: Science and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon Nanotubes-the Route toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Myung, S. Nanotube electronics: A flexible approach to mobility. Nat. Nanotechnol. 2007, 2, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Itoh, E.; Suzuki, I.; Miyairi, K. Field emission from carbon-nanotube-dispersed conducting polymer thin film and its application to photovoltaic devices. Jpn. J. Appl. Phys. 2005, 44, 636–640. [Google Scholar] [CrossRef]
- Li, L.; Li, F.; Xiao, Y.; Aigin, Z. The effect of carbonyl, carboxyl and hydroxyl groups on the capacitance of carbon nanotubes. New Carbon Mater. 2012, 26, 224–228. [Google Scholar] [CrossRef]
- Kaplan, R.B. Open Cell Tantalum Structures for Cancellous Bone Implants and Cell and Tissue Receptors. U.S. Patent 52,828,611,994, 14 June 2000. [Google Scholar]
- Jenkins, G.M.; Grigson, C.J. The fabrication of artifacts out of glassy carbon and carbon-fiber-reinforced carbon for biomedical applications. J. Biomed. Mater. Res. 1979, 13, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.L., Jr.; Walker, P.L., Jr. Carbon molecular sieve supports for metal catalysts-II. Selective hydrogenation of hydrocarbons over platinum supported on polyfurfuryl alcohol carbon. Carbon 1972, 10, 87–92. [Google Scholar] [CrossRef]
- Lestrade, C.; Guyomar, P.Y.; Astruc, M. Electrochemical removal of dilute heavy metals with carbon felt porous electrodes. Environ. Technol. Lett. 1981, 2, 409–418. [Google Scholar] [CrossRef]
- Tan, W.X.; Hasnat, M.A.; Ramalan, N.H.M.; Soh, W.M.; Mohamed, N. Influence of flow rates on the electrogenerative Co2+ recovery at a reticulated vitreous carbon cathode. Chem. Eng. J. 2012, 189–190, 182–187. [Google Scholar] [CrossRef]
- Saleh, M.M.; Weidlich, C.; Mangold, K.M.; Jattner, K. Absorption/desorption of calcium ions on polypyrrole-loaded reticulated vitreous carbon. J. Appl. Electrochem. 2006, 36, 179–186. [Google Scholar] [CrossRef]
- Czerwiski, A.; Rogulski, Z.; Obrecbowski, S.; Siwek, H.; Paleska, I.; Chotkowski, M.; Łukaszewski, M.A. RVC as new carbon material for batteries. J. Appl. Electrochem. 2009, 39, 559–567. [Google Scholar] [CrossRef]
- Czerwiski, A.; Obrecbowski, S.; Rogulski, Z. New high-energy lead-acid battery with reticulated vitreous carbon as a carrier and current collector. J. Power Sources 2012, 198, 378–382. [Google Scholar] [CrossRef]
- El-Deab, M.S.; Saleh, M.M. Electrocatalytic production of hydrogen on reticulated vitreous carbon. Int. J. Hydrog. Energy 2003, 28, 1199–1206. [Google Scholar] [CrossRef]
- Chakhovskoi, A.G.; Hunt, C.E.; Forsberg, G.; Nilsson, T.; Persson, P. Reticulated vitreous carbon field emission cathodes for light source applications. J. Vac. Sci. Technol. B 2003, 21, 571–575. [Google Scholar] [CrossRef]
- Semiat, R. Desalination: Present and Future. Water Int. 2000, 25, 54–65. [Google Scholar] [CrossRef]
- Ukaszewski, M.; Zurowski, A.; Czerwiski, A. Hydrogen in thin Pd-based layers deposited on reticulated vitreous carbon—A new system for electrochemical capacitors. J. Power Sources 2008, 185, 1598–1604. [Google Scholar] [CrossRef]
- Tangirala, R.; Low, C.T.J.; De-Lean, P.; Campbell, S.; Walsh, F. Copper deposition at segmented, reticulated vitreous carbon cathode in Hull cell. Trans. Inst. Met. Finish. 2010, 88, 84–92. [Google Scholar] [CrossRef]
- Ausman, K.D.; Piner, R.; Lourie, O.; Ruoff, R.S.; Korobov, M. Organic Solvent Dispersions of Single-Walled Carbon Nanotubes: Toward Solutions of Pristine Nanotubes. J. Phys. Chem. B 2000, 104, 8911–8915. [Google Scholar] [CrossRef]
- Shedge, H.Y. Specific and Non-Specific Binding of Proteins and Nucleic Acids on Chemically Modified Reticulated Vitreous Carbon Electrodes. Master’s Thesis, Clemson University, Clemson, SC, USA, 2009. [Google Scholar]
- Roberts, M.; Huang, A.F.; Johns, P.; Owen, J. Dip–spin coating of reticulated vitreous carbon with composite materials to act as an electrode for 3D microstructured lithium ion batteries. J. Power Sources 2013, 224, 250–259. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Mussi, V.; Biale, C.; Visentin, S.; Barbero, N.; Rocchia, M.; Valbusa, U. Raman analysis and mapping for the determination of COOH groups on oxidized single walled carbon nanotubes. Carbon 2010, 48, 3391–3398. [Google Scholar] [CrossRef]
- Dresselhaus, M.S. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Yuca, N.; Karatepe, N.; Yakuphanoglu, F. Thermal and electrical properties of carbon nanotubes purified by acid digestion. World Acad. Sci. Eng. Technol. 2011, 79, 611–616. [Google Scholar]
- Kim, Y.; Lee, D.; Oh, Y.; Choi, J.; Baik, S. The effects of acid treatment methods on the diameter dependent length separation of single walled carbon nanotubes. Synth. Met. 2006, 156, 999–1003. [Google Scholar] [CrossRef]
- Saini, R.K.; Chiang, I.W.; Peng, H.; Smalley, R.E.; Billups, W.E.; Hauge, R.H.; Margrave, J.L. Covalent Sidewall Functionalization of Single Wall Carbon Nanotubes. J. Am. Chem. Soc. 2003, 125, 3617–3621. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, A.; Tu, Y.; Foo, G.; Yeo, C.; Chan, M.; Jiang, R.; Chen, Y. How carboxylic groups improve the performance of single-walled carbon nanotube electrochemical capacitors? Energy Environ. Sci. 2011, 4, 4220–4229. [Google Scholar] [CrossRef]
- Sfeir, M.; Beetz, T.; Wang, F.; Huang, L.; Huang, H.; Huang, M.; Hone, J.; Obrien, S.; Misewich, J.; Heinz, T.; et al. Optical Spectroscopy of Individual Single-Walled Carbon Nanotubes of Defined Chiral Structure. Science 2006, 312, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.; Hamon, M.A.; Hu, H.; Zhao, B.; Bhowmik, P.; Sen, R.; Itkis, M.E.; Haddon, R.C. Chemistry of Single-Walled Carbon Nanotubes. Acc. Chem. Res. 2002, 35, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Attal, S.; Thiruvengadathan, R.; Regev, O. Determination of the concentration of single-walled carbon nanotubes in aqueous dispersions using UV—Visible absorption spectroscopy. Anal. Chem. 2006, 78, 8098–8104. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, N.; Regev, O.; Loos, J.; Meuldijk, J.; Koning, C.E. Time-dependent study of the exfoliation process of carbon nanotubes in aqueous dispersions by using UV−Visible spectroscopy. Anal. Chem. 2005, 77, 5135–5139. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Jha, P.; Chouksey, A.; Raman, R.; Islam, S.S.; Islam, T.; Choudhary, P.K. Spectroscopic Investigation of Modified Single Wall Carbon Nanotube (SWCNT). J. Mod. Phys. 2011, 2, 538–543. [Google Scholar] [CrossRef]
- Benedict, B.; Pehrsson, P.E.; Zhao, W. Optically sensing additional sonication effects on dispersed HiPco nanotubes in aerated water. J. Phys. Chem. B 2005, 109, 7778–7780. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Aldalbahi, A.; in het Panhuis, M. Electrical and mechanical characteristics of buckypapers and evaporative cast films prepared using single and multi-walled carbon nanotubes and the biopolymer carrageenan. Carbon 2012, 50, 1197–1208. [Google Scholar] [CrossRef]
- Friedrich, J.M. Reticulated vitreous carbon as an electrode material. J. Electroanal. Chem. Interfacial Electrochem. 2004, 561, 203–217. [Google Scholar] [CrossRef]
- Tondi, G.; Fierro, V.; Pizzi, A.; Celzard, A. Tannin-based carbon foams. Carbon 2009, 47, 1480–1492. [Google Scholar] [CrossRef]
- Chen, J.H.; Li, W.Z.; Wang, D.Z.; Yang, S.X.; Wen, J.G.; Ren, Z.F. Electrochemical characterization of carbon nanotubes as electrode in electrochemical double-layer capacitors. Carbon 2002, 40, 1193–1197. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Tu, Y.; Li, Y.; Coulter, J.Y.; Zheng, L.; Zhao, Y.; Jia, Q.; Peterson, D.E.; Zhu, Y. Strong Carbon-Nanotube Fibers Spun from Long Carbon-Nanotube Arrays. Small 2007, 3, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Holesinger, T.G.; Arendt, P.N.; Huang, J.; Kirven, P.D.; Clapp, T.G.; DePaula, R.F.; Liao, X.; Zhao, Y.; et al. Ultrastrong, Stiff, and Lightweight Carbon-Nanotube Fibers. Adv. Mater. 2007, 19, 4198–4201. [Google Scholar] [CrossRef]
- Naoi, K.; Simon, P. New Materials and New Configurations for Advanced Electrochemical Capacitors. J. Electrochem. Soc. 2008, 17, 34–37. [Google Scholar]
- Aldalbahi, A.; Rahaman, M.; Almoiqli, M. A Strategy to Enhance the Electrode Performance of Novel Three-Dimensional PEDOT/RVC Composites by Electrochemical Deposition Method. Polymers 2017, 9, 157. [Google Scholar] [CrossRef]
- Bard, A. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Bard, A.J., Faulkner, L.R., Eds.; John Wiley: New York, NY, USA, 2001. [Google Scholar]
- Nian, Y.R.; Teng, H.S. Nitric acid modification of activated carbon electrodes for improvement of electrochemical capacitance. J. Electrochem. Soc. 2002, 149, A1008–A1014. [Google Scholar] [CrossRef]
- Wang, D.; Li, F.; Liu, M.; Cheng, H. Improved capacitance of SBA-15 templated mesoporous carbons after modification with nitric acid oxidation. New Carbon Mater. 2007, 22, 307–314. [Google Scholar] [CrossRef]
- Oda, H.; Yamashita, A.; Minoura, S.; Okamoto, M.; Morimoto, T. Modification of the oxygen-containing functional group on activated carbon fiber in electrodes of an electric double-layer capacitor. J. Power Sources 2006, 158, 1510–1516. [Google Scholar] [CrossRef]
- Teng, H.; Chang, Y.J.; Hsieh, C.T. Performance of electric double-layer capacitors using carbons prepared from phenol-formaldehyde resins by KOH etching. Carbon 2001, 39, 1981–1987. [Google Scholar] [CrossRef]
- Mitali, S.; Soma, D.; Monica, D. A Study of Effect of Electrolytes on the Capacitive Properties of Mustard Soot Containing Multiwalled Carbon Nanotubes. Res. J. Chem. Sci. 2011, 1, 109–113. [Google Scholar]
- Guo, Y.; Qi, J.; Jiang, Y.; Yang, S.; Wang, Z.; Xu, H. Performance of electrical double layer capacitors with porous carbons derived from rice husk. Mater. Chem. Phys. 2003, 80, 704–709. [Google Scholar] [CrossRef]
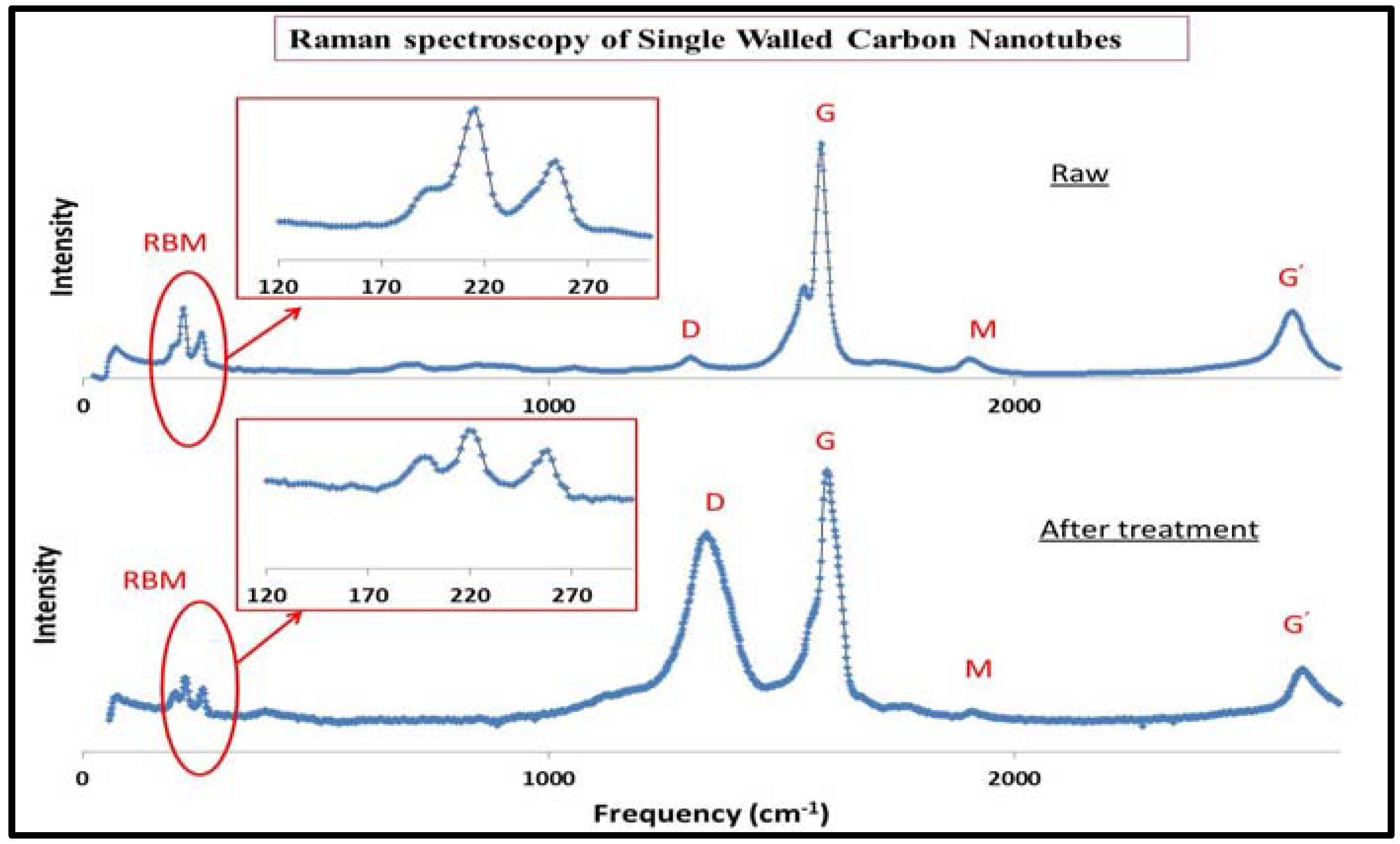



| (SWCNT) | (cm−1) | dt (nm) | ID/IG |
|---|---|---|---|
| Raw | 191 | 1.30 | 0.0615 |
| 213 | 1.16 | ||
| 254 | 0.98 | ||
| Treated | 196 | 1.27 | 0.7452 |
| 219 | 1.13 | ||
| 258 | 0.96 |
| Sample | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| RVC (mg) | 159 | 161 | 161 | 162 |
| After coating (mg) | 165 | 184 | 195 | 212 |
| Amount of a-SWCNT (mg) in sample | 6 | 23 | 34 | 50 |
| Amount of a-SWCNT in sample (% w/w) | 3.63 | 12.50 | 17.43 | 23.58 |
| Sample | 3.63 wt % a-SWCNT-Coated RVC | ||||||
| Scan rate (mV/s) | 5 | 10 | 20 | 50 | 100 | 200 | |
| Specific Capacitance | (F/g) | 267.24 | 239.58 | 207.40 | 132.69 | 84.78 | 51.15 |
| (F/cm2) | 0.09 | 0.08 | 0.07 | 0.03 | 0.03 | 0.02 | |
| (F/cm3) | 0.72 | 0.64 | 0.56 | 0.36 | 0.23 | 0.14 | |
| Sample | 12.50 wt % a-SWCNT-Coated RVC | ||||||
| Scan rate (mV/s) | 5 | 10 | 20 | 50 | 100 | 200 | |
| Specific Capacitance | (F/g) | 165.20 | 150.33 | 132.16 | 99.12 | 56.17 | 34.69 |
| (F/cm2) | 0.21 | 0.18 | 0.12 | 0.07 | 0.04 | 0.02 | |
| (F/cm3) | 1.76 | 1.46 | 1.02 | 0.55 | 0.31 | 0.17 | |
| Sample | 17.43 wt % a-SWCNT-Coated RVC | ||||||
| Scan rate (mV/s) | 5 | 10 | 20 | 50 | 100 | 200 | |
| Specific Capacitance | (F/g) | 153.79 | 138.94 | 125.20 | 100.77 | 61.07 | 41.23 |
| (F/cm2) | 0.29 | 0.23 | 0.16 | 0.08 | 0.04 | 0.02 | |
| (F/cm3) | 2.42 | 1.93 | 1.31 | 0.67 | 0.37 | 0.19 | |
| Sample | 23.58 wt % a-SWCNT-Coated RVC | ||||||
| Scan rate (mV/s) | 5 | 10 | 20 | 50 | 100 | 200 | |
| Specific Capacitance | (F/g) | 139.65 | 131.27 | 117.70 | 103.34 | 68.43 | 51.67 |
| (F/cm2) | 0.39 | 0.37 | 0.33 | 0.29 | 0.19 | 0.14 | |
| (F/cm3) | 3.23 | 3.04 | 2.75 | 2.39 | 1.58 | 1.20 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldalbahi, A.; Rahaman, M.; Almoigli, M.; Meriey, A.Y.; Alharbi, K.N. Improvement in Electrode Performance of Novel SWCNT Loaded Three-Dimensional Porous RVC Composite Electrodes by Electrochemical Deposition Method. Nanomaterials 2018, 8, 19. https://doi.org/10.3390/nano8010019
Aldalbahi A, Rahaman M, Almoigli M, Meriey AY, Alharbi KN. Improvement in Electrode Performance of Novel SWCNT Loaded Three-Dimensional Porous RVC Composite Electrodes by Electrochemical Deposition Method. Nanomaterials. 2018; 8(1):19. https://doi.org/10.3390/nano8010019
Chicago/Turabian StyleAldalbahi, Ali, Mostafizur Rahaman, Mohammed Almoigli, Al Yahya Meriey, and Khalid N. Alharbi. 2018. "Improvement in Electrode Performance of Novel SWCNT Loaded Three-Dimensional Porous RVC Composite Electrodes by Electrochemical Deposition Method" Nanomaterials 8, no. 1: 19. https://doi.org/10.3390/nano8010019





