UV-Vis-Induced Degradation of Phenol over Magnetic Photocatalysts Modified with Pt, Pd, Cu and Au Nanoparticles
Abstract
:1. Introduction
2. Results
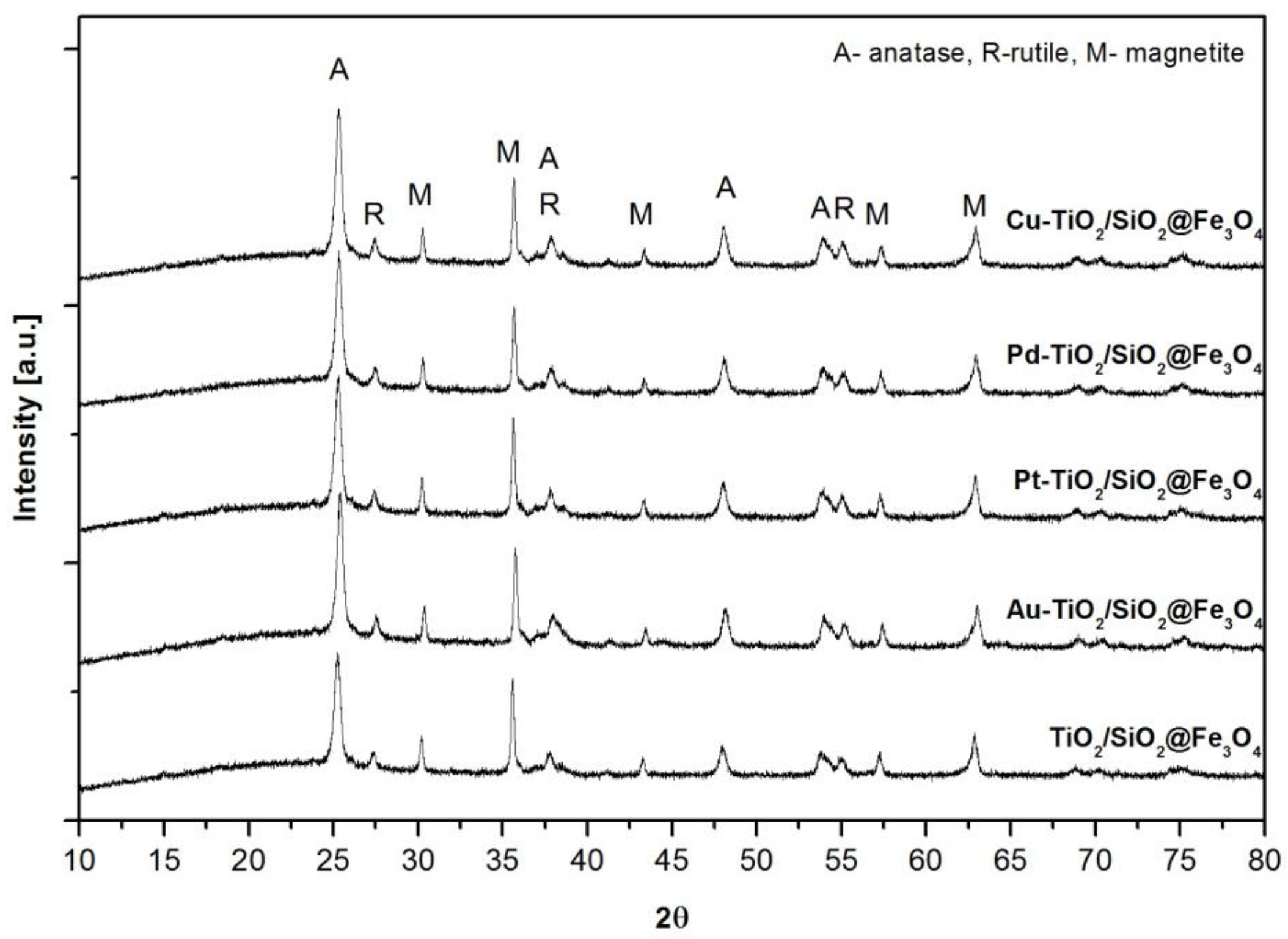
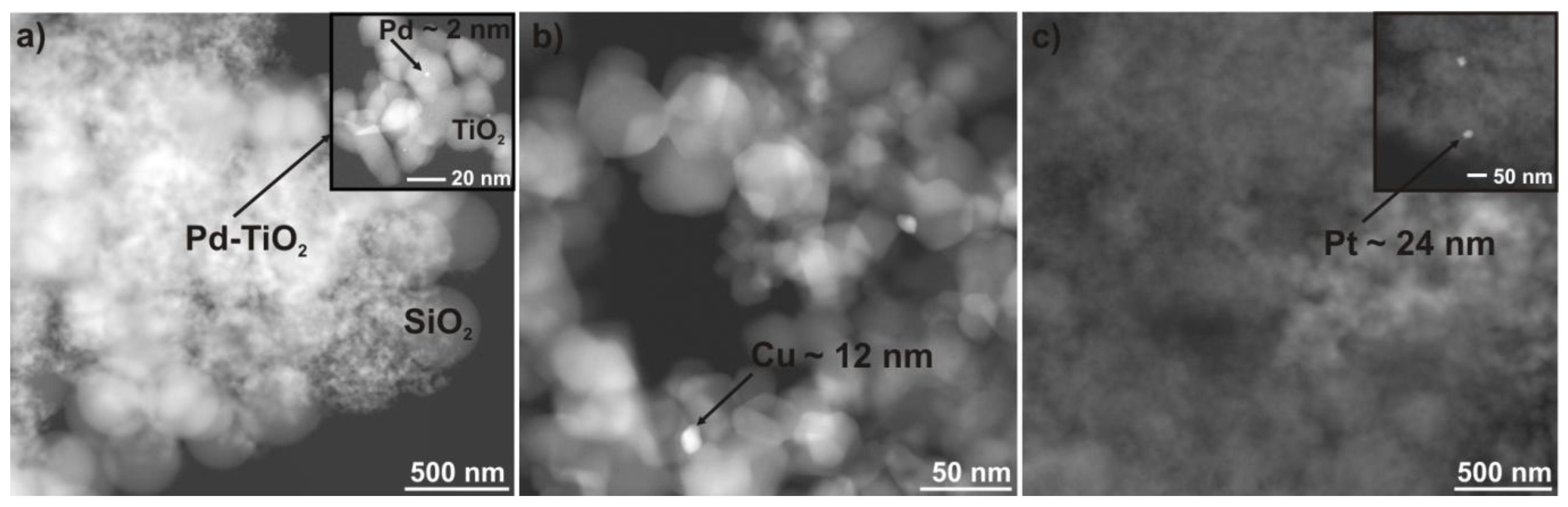
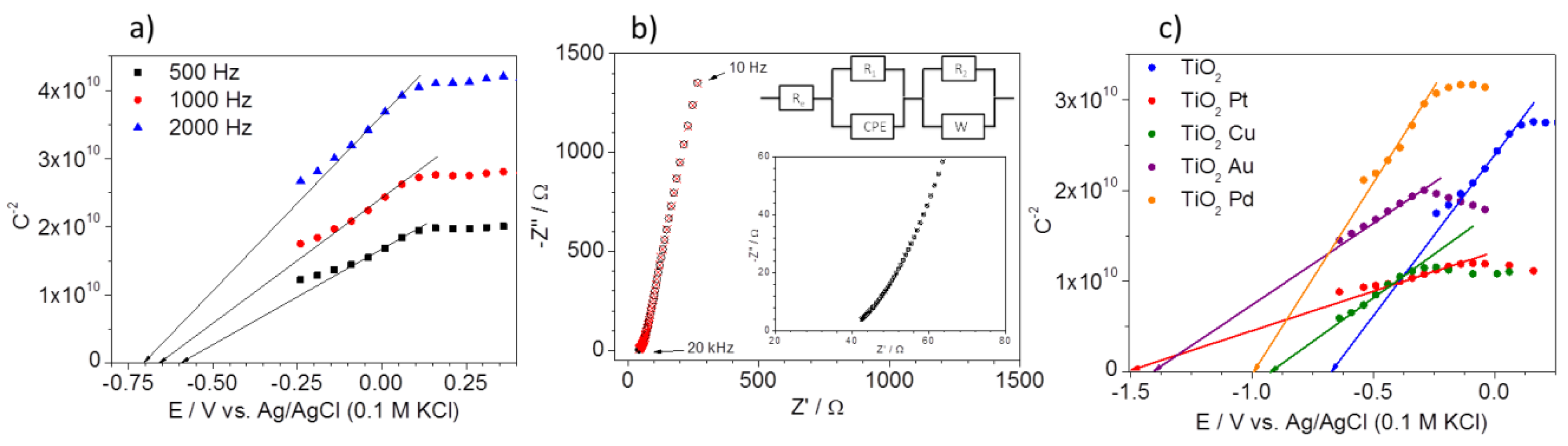
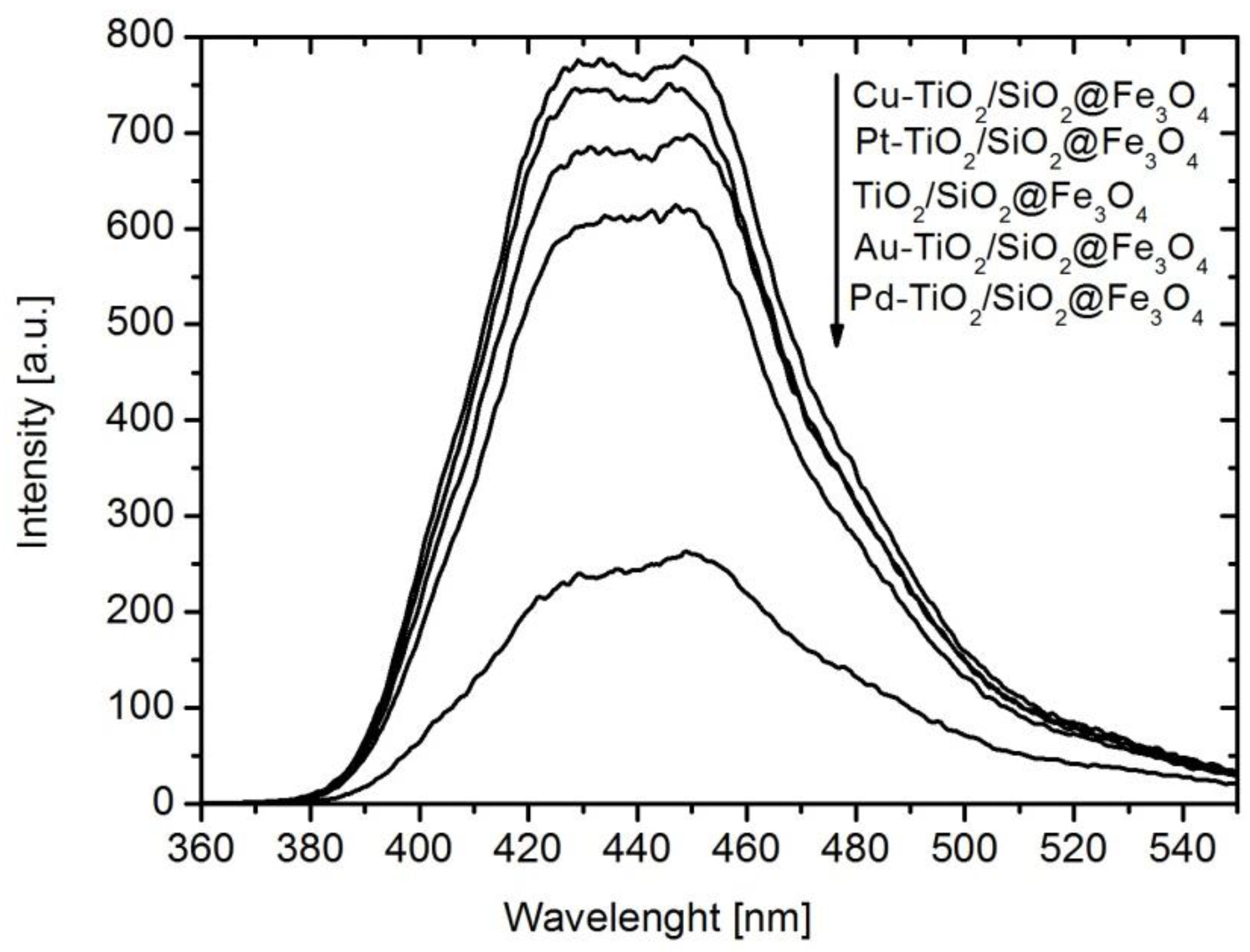
2.1. Characterization of Nanocomposites
2.2. Phenol Photocatalytic Degradation
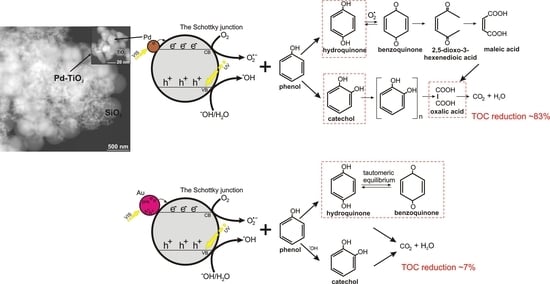
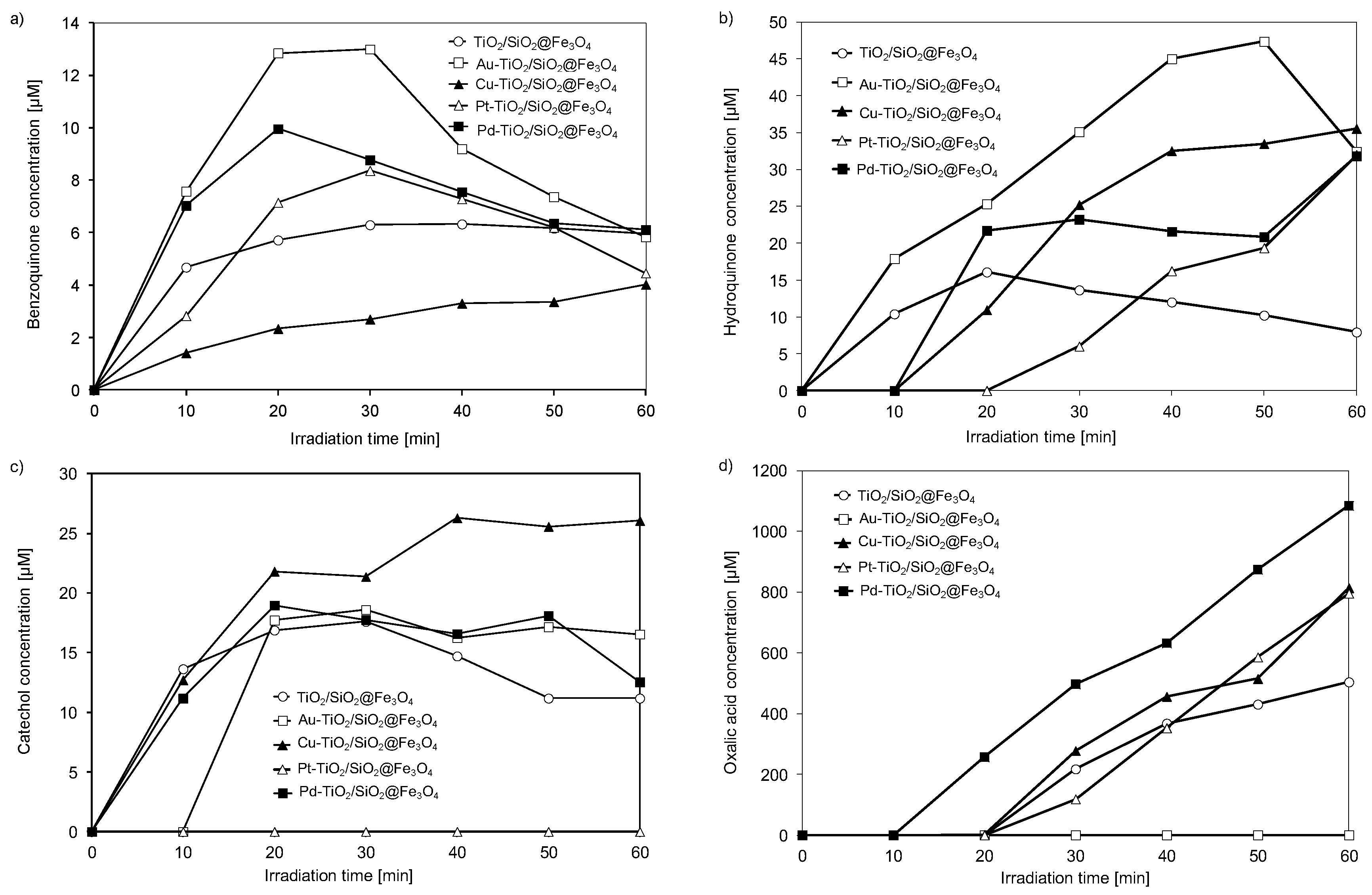
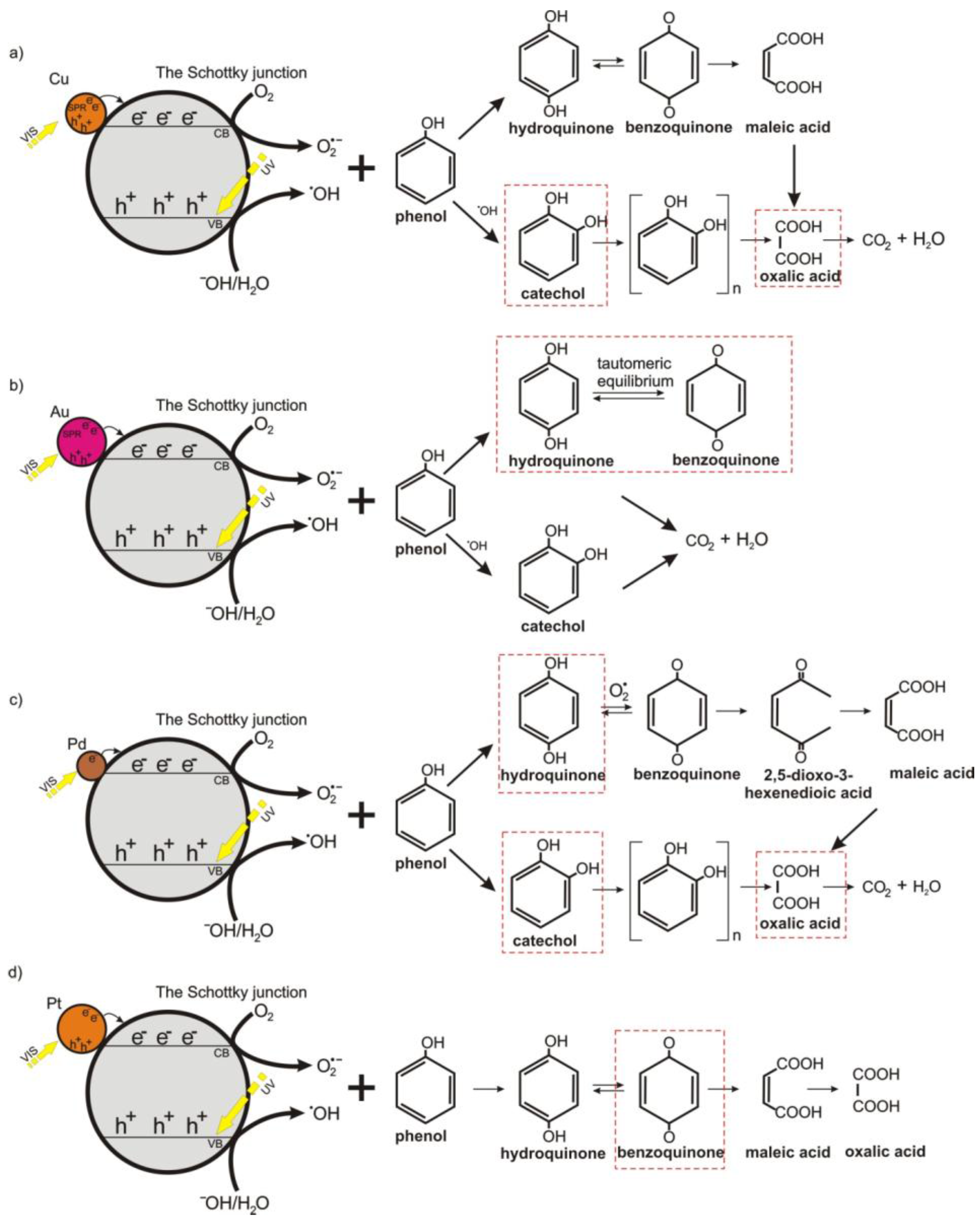
2.3. Identification of Degradation Intermediates
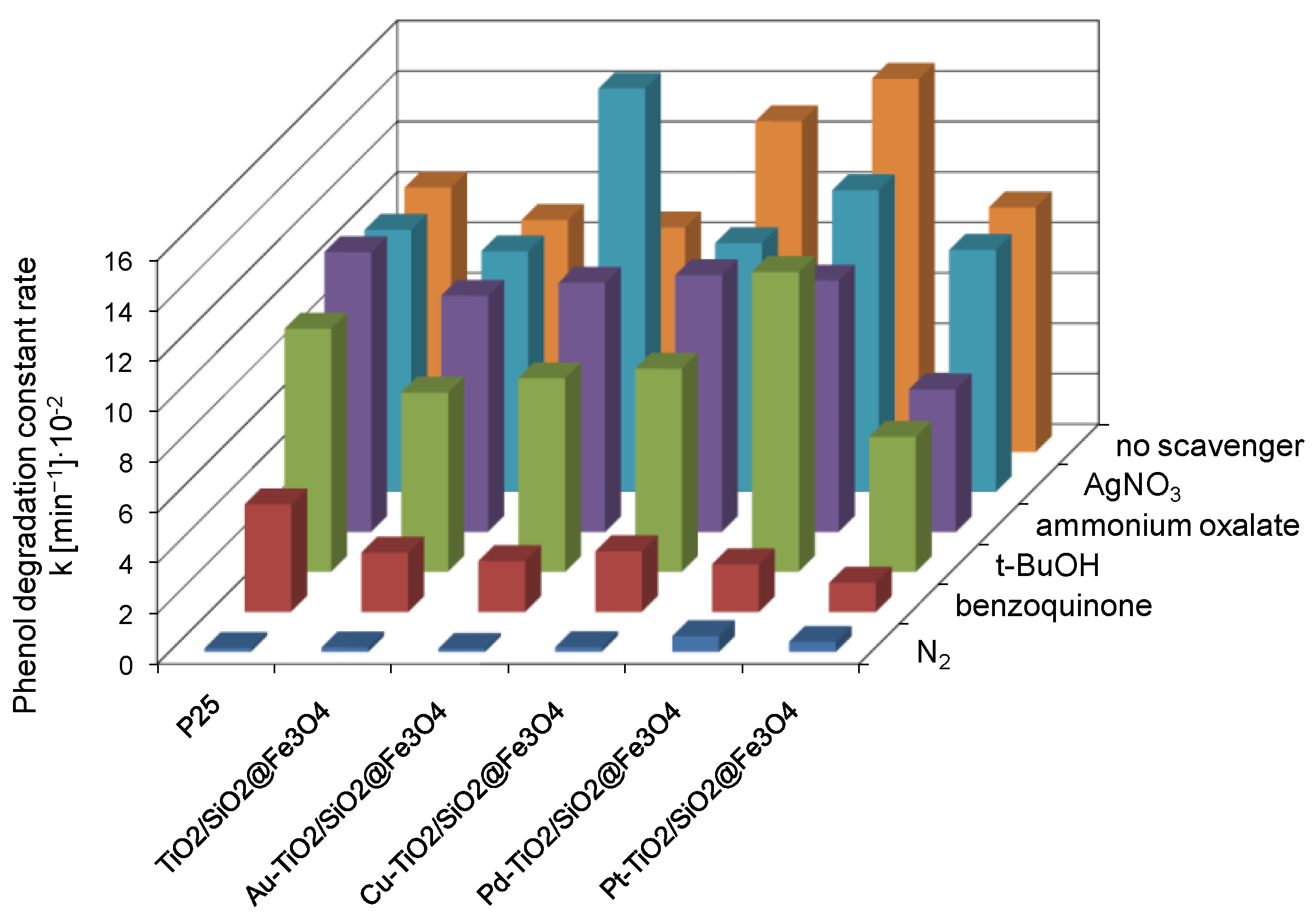
2.4. Verification of the Degradation Mechanism Using Scavengers and Under N2 Purging
2.4.1. Effect of N2 Purging
2.4.2. Effect of Benzoquinone
2.4.3. Effect of t-BuOH
2.4.4. Effect of Ammonium Oxalate
2.4.5. Effect of Silver Nitrate
3. Discussion and Proposed Mechanism
4. Materials and Methods
4.1. Materials and Instruments
4.2. Preparation of TiO2/SiO2@Fe3O4 and Me-TiO2/SiO2@Fe3O4 Photocatalysts
4.3. Measurements of Photocatalytic Activity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonic) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Wang, K.; Wei, Z.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum- loaded titania particles prepared from P25. Catal. Today 2017. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M. Magnetite hybrid photocatalysis: Advance environmental remediation. Rev. Inorg. Chem. 2016, 36, 135–151. [Google Scholar] [CrossRef]
- Beydoun, D.; Amal, R.; Low, G.K.-C.; McEvoy, S. Novel Photocatalyst:Titania-Coated Magnetite. Activity and Photodissolution. J. Phys. Chem. B 2000, 104, 4387–4396. [Google Scholar] [CrossRef]
- Beydoun, D.; Amal, R.; Low, G.; McEvoy, S. Occurrence and prevention of photodissolution at the phase junction of magnetite and titanium dioxide. J. Mol. Catal. A Chem. 2002, 180, 193–200. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M. Modified iron oxide nanomaterials: Functionalization and application. J. Magn. Magn. Mater. 2016, 416, 117–133. [Google Scholar] [CrossRef]
- Yu, X.; Liu, S.; Yu, J. Superparamagnetic γ-Fe2O3@SiO2@TiO2 composite microspheres with superior photocatalytic properties. Appl. Catal. B Environ. 2011, 104, 12–20. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Wu, W.; Tian, Q.; Cui, S.; Dai, Z.; Ren, F.; Xiao, X.; Jiang, C. 3D Flowerlike α-Fe2O3@TiO2 Core-Shell Nanostructures: General Synthesis and Enhanced Photocatalytic Performance. ACS Sustain. Chem. Eng. 2015, 3, 2975–2984. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Bielan, Z.; Dudziak, S.; Wolak, I.; Sobczak, Z.; Klimczuk, T.; Hupka, J. Design and Application of Magnetic Photocatalysts for Water Treatment. The Effect of Particle Charge on Surface Functionality. Catalysts 2017, 7. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, J.; Cha, S.; Choi, S.; Park, Y.C.; Liu, C. Magnetically separable Au-TiO2/nanocube ZnFe2O4 composite for chlortetracycline removal in wastewater under visible light. J. Ind. Eng. Chem. 2017, 47, 303–314. [Google Scholar] [CrossRef]
- Laohhasurayotin, K.; Pookboonmee, S.; Viboonratanasri, D. Preparation of magnetic photocatalyst nanoparticles—TiO2/SiO2/Mn–Zn ferrite—and its photocatalytic activity influenced by silica interlayer. Mater. Res. Bull. 2012, 47, 1500–1507. [Google Scholar] [CrossRef]
- Chen, C.; Jaihindh, D.; Hu, S.; Fu, Y. Magnetic recyclable photocatalysts of Ni-Cu-Zn ferrite@SiO2@TiO2@Ag and their photocatalytic activities. J. Photochem. Photobiol. A Chem. 2017, 334, 74–85. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D.; Murcia, J.J.; Hidalgo, M.C.; Ciambelli, P.; Navío, J.A. Photocatalytic removal of patent blue V dye on Au-TiO2 and Pt-TiO2 catalysts. Appl. Catal. B Environ. 2016, 188, 134–146. [Google Scholar] [CrossRef]
- Hamzezadeh-Nakhjavani, S.; Tavakoli, O.; Akhlaghi, S.P.; Salehi, Z.; Esmailnejad-Ahranjani, P.; Arpanaei, A. Efficient photocatalytic degradation of organic pollutants by magnetically recoverable nitrogen-doped TiO2 nanocomposite photocatalysts under visible light irradiation. Environ. Sci. Pollut. Res. 2015, 22, 18859–18873. [Google Scholar] [CrossRef] [PubMed]
- Long, N.Q.; Uyen, N.T.T.; Hoang, T.D.; Trung, D.B. Preparation, characterization and photocatalytic activity under visible light of magnetic N-dopped TiO2. Int. J. Renew. Energy Environ. Eng. 2015, 3, 2–5. [Google Scholar]
- Larumbe, S.; Monge, M.; Gomez-Polo, C. Magnetically separable photocatalyst Fe3 O4/SiO2/N-TiO2 hybrid nanostructure. IEEE Trans. Mag. 2014, 50, 6971–6975. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Fang, L.; Lo, I.M.C. Visible-light-driven N-TiO2@SiO2@Fe3O4 magnetic nanophotocatalysts: Synthesis, characterization, and photocatalytic degradation of PPCPs. J. Hazard. Mater. 2017, 1–9. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A. Progress, challenge, and perspective of bimetallic TiO2-based photocatalysts. J. Nanomater. 2015, 1–17. [Google Scholar] [CrossRef]
- Choi, K.; Park, S.; Joo, B.; Jung, J. Recyclable Ag-coated Fe3O4@TiO2 for efficient photocatalytic oxidation of chlorophenol. Surf. Coat. Technol. 2017, 320, 240–245. [Google Scholar] [CrossRef]
- Khojasteh, H.; Salavati-Niasari, M.; Mazhari, M.-P.; Hamadanian, M. Preparation and characterization of Fe3O4@SiO2@TiO2@Pd and Fe3O4@SiO2@TiO2@Pd-Ag nanocomposites and their utilization in enhanced degradation systems. RSC Adv. 2016, 6, 78043–78052. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Song, S.; Zhang, H. Fe3O4@SiO2@TiO@Pt Hierarchical Core-Shell Microspheres: Controlled Synthesis, Enhanced Degradation System, and Rapid Magnetic Separation to Recycle. Cryst. Growth Des. 2014, 14, 5506–5511. [Google Scholar] [CrossRef]
- Mohammadi-Aghdam, S.; Sarkhosh, B.; Tajoddin, N.N. Recyclable Fe3O4/SiO2/TiO2/Cu nanocomposites: Synthesis, characterization and investigation of the photocatalytic and magnetic property. J. Mater. Sci. Mater. Electron. 2017, 28, 9456–9463. [Google Scholar] [CrossRef]
- Chun-Te Lin, J.; Sopajaree, K.; Jitjanesuwan, T.; Lu, M.-C. Application of visible light on copper-doped titanium dioxide catalyzing degradation of chlorophenols. Sep. Purif. Technol. 2017, 191, 233–243. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, S.; Choi, W. Visible Light Active Platinum-Ion-Doped TiO2 Photocatalyst. J. Phys. Chem. B 2005, 109, 24260–24267. [Google Scholar] [CrossRef] [PubMed]
- Colón, G.; Maicu, M.; Hidalgo, M.C.; Navío, J.A.; Kubacka, A.; Fernández-García, M. Gas phase photocatalytic oxidation of toluene using highly active Pt doped TiO2. J. Mol. Catal. A Chem. 2010, 320, 14–18. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Zaleska, A. Ag/Pt-modified TiO2 nanoparticles for toluene photooxidation in the gas phase. Catal. Today 2014, 230, 104–111. [Google Scholar] [CrossRef]
- Pham, T.; Lee, B.; Pham-Cong, D. Advanced removal of toluene in aerosol by adsorption and photocatalytic degradation of silver-doped TiO2/PU under visible light irradiation. RSC Adv. 2016, 6, 25346–25358. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X.; Sun, H.; Huo, M. Photocatalytic degradation of malathion in aqueous solution using an Au-Pd-TiO2 nanotube film. J. Hazard. Mater. 2010, 184, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Grover, I.S.; Prajapat, R.C.; Singh, S.; Pal, B. Highly photoactive Au-TiO2 nanowires for improved photo-degradation of propiconazole fungicide under UV/sunlight irradiation. Sol. Energy 2017, 144, 612–618. [Google Scholar] [CrossRef]
- Pham, T.N.; Shi, D.; Resasco, D.E. Reaction kinetics and mechanism of ketonization of aliphatic carboxylic acids with different carbon chain lengths over Ru/TiO2 catalyst. J. Catal. 3014, 334, 149–158. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Antonopoulou, M.; Papavasiliou, J.; Deligiannakis, Y.; Konstantinou, I. Photocatalytic performance of Pt-TiO2, Pt-N-TiO2 and Pt-N/F-TiO2 towards simultaneous Cr(VI) reduction/benzoic acid oxidation: Insights into photogenerated charge carrier dynamics and catalyst properties. J. Photochem. Photobiol. A Chem. 2017, 349, 25–35. [Google Scholar] [CrossRef]
- Ding, Q.; Chen, S.; Shang, F.; Liang, J.; Liu, C. Cu2O/Ag co-deposited TiO2 nanotube array film prepared by pulse-reversing voltage and photocatalytic properties. Nanotechnology 2016, 27, 485705. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lu, J.; Ye, W.; Yu, C.; Chang, Y. Preparation of Pt/TiO2 hollow nanofibers with highly visible light photocatalytic activity. Appl. Surf. Sci. 2017, 392, 472–480. [Google Scholar] [CrossRef]
- Theurich, J.; Lindner, M.; Bahnemann, D.W. Photocatalytic Degradation of 4-Chlorophenol in Aerated Aqueous Titanium Dioxide Suspensions: A Kinetic and Mechanistic Study. Langmuir 1996, 12, 6368–6376. [Google Scholar] [CrossRef]
- Kim, S.; Choi, W. Visible-light-induced photocatalytic degradation of 4-chlorophenol and phenolic compounds in aqueous suspension of pure titania: Demonstrating the existence of a surface-complex-mediated path. J. Phys. Chem. B 2005, 109, 5143–5149. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.F.; Ivanova, I.; Dillert, R.; Bahnemann, D.W.; Salvador, P.; Peral, J. Catalytic role of surface oxygens in TiO2 photooxidation reactions: Aqueous benzene photooxidation with Ti18O2 under anaerobic conditions. J. Phys. Chem. Lett. 2013, 4, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Tiruvalam, R.; He, Q.; Dimitratos, N.; Kesavan, L.; Hammond, C.; Lopez-Sanchez, J.A.; Bechstein, R.; Kiely, C.J.; Hutchings, G.J.; et al. Promotion of Phenol Photodecomposition over TiO2 Using Au, Pd, and Au-Pd nanoparticles. ACS NANO 2012, 6, 6284–6292. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Salvador, P.A.; Rohrer, G.S. Photocatalysts with internal electric fields. Nanoscale 2014, 6, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Sobczyński, A.; Duczmal, L.; Zmudziński, W. Phenol destruction by photocatalysis on TiO2: An attempt to solve the reaction mechanism. J. Mol. Catal. A Chem. 2004, 213, 225–230. [Google Scholar] [CrossRef]
- Zhang, L.; Kanki, T.; Sano, N.; Toyoda, A. Pathways and kinetics on photocatalytic destruction of aqueous phenol. Environ. Monit. Assess. 2006, 115, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Diesen, V.; Jonsson, M. Comment on the use of phenols as probes for the kinetics of heterogeneous photocatalysis. Appl. Catal. B Environ. 2014, 158–159, 429–431. [Google Scholar] [CrossRef]
- Hui, C.; Shen, C.; Tian, J.; Bao, L.; Ding, H.; Li, C.; Tian, Y.; Shi, X.; Gao, H.-J. Core-shell Fe3O4@SiO2 nanoparticles synthesized with well-dispersed hydrophilic Fe3O4 seeds. Nanoscale 2011, 3, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Belessi, V.; Lambropoulou, D.; Konstantinou, I.; Zboril, R.; Tucek, J.; Jancik, D.; Albanis, T.; Petridis, D. Structure and photocatalytic performance of magnetically separable titania photocatalysts for the degradation of propachlor. Appl. Catal. B Environ. 2009, 87, 181–189. [Google Scholar] [CrossRef]
- Chi, Y.; Yuan, Q.; Li, Y.; Zhao, L.; Li, N.; Li, X.; Yan, W. Magnetically separable Fe3O4@SiO2@TiO2-Ag microspheres with well-designed nanostructure and enhanced photocatalytic activity. J. Hazard. Mater. 2013, 262, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jia, Z.; Ji, S.; Zheng, Y.; Li, M.; Yang, H. Synthesis of TiO2/SiO2@Fe3O4 magnetic microspheres and their properties of photocatalytic degradation dyestuff. Catal. Today 2011, 175, 293–298. [Google Scholar] [CrossRef]
- Xiong, L.; Li, J.; Yang, B.; Yu, Y. Ti3+ in the Surface of Titanium Dioxide: Generation, Properties and Photocatalytic Application. J. Nanomater. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Wang, K.; Rokicka, P.; Endo, M.; Wei, Z.; Ohtani, B.; Morawski, A.W.; Kowalska, E. The effect of anatase and rutile crystallites isolated from titania P25 photocatalyst on growth of selected mould fungi. J. Photochem. Photobiol. B Biol. 2015, 151, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Jurek, A.; Wei, Z.; Wysocka, I.; Szweda, P.; Kowalska, E. The effect of nanoparticles size on photocatalytic and antimicrobial properties of Ag-Pt/TiO2 photocatalysts. Appl. Surf. Sci. 2015, 353, 317–325. [Google Scholar] [CrossRef]
- Grabowska, E.; Zaleska, A.; Sorgues, S.; Kunst, M.; Etcheberry, A.; Colbeau-Justin, C.; Remita, H. Modification of titanium(IV) dioxide with small silver nanoparticles: Application in photocatalysis. J. Phys. Chem. C 2013, 117, 1955–1962. [Google Scholar] [CrossRef]
- Kowalska, E.; Rau, S.; Ohtani, B. Plasmonic titania photocatalysts active under UV and visible-light irradiation: Influence of gold amount, size, and shape. J. Nanotechnol. 2012, 2012, 361853. [Google Scholar] [CrossRef]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Skwarek, E.; Zaleska, A.; Gazda, M.; Hupka, J. Preparation of silver nanoparticles with controlled particle size. Procedia Chem. 2009, 1, 1560–1566. [Google Scholar] [CrossRef]
- Radecka, M.; Rekas, M.; Trenczek-Zajac, A.; Zakrzewska, K. Importance of the band gap energy and flat band potential for application of modified TiO2 photoanodes in water photolysis. J. Power Sources 2008, 181, 46–55. [Google Scholar] [CrossRef]
- Tanabe, I.; Ozaki, Y. Consistent changes in electronic states and photocatalytic activities of metal (Au, Pd, Pt)-modified TiO2 studied by far-ultraviolet spectroscopy. Chem. Commun. 2014, 1, 2117–2119. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Wolf, E.; Kamat, P.V. Semiconductor–Metal Composite Nanostructures. To What Extent Do Metal Nanoparticles Improve the Photocatalytic Activity of TiO2 Films? J. Phys. Chem. B 2001, 105, 11439–11446. [Google Scholar] [CrossRef]
- Rashid, J.; Barakat, M.A.; Ruzmanova, Y.; Chianese, A. Fe3O4/SiO2/TiO2 nanoparticles for photocatalytic degradation of 2-chlorophenol in simulated wastewater. Environ. Sci. Pollut. Res. 2015, 22, 3149–3157. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Guo, X.; Wu, X.; Li, Q.; Ho, W.; Li, M.; Ye, H.; Du, D. Photocatalytic selective oxidation of phenol to produce dihydroxybenzenes in a TiO2/UV system: Hydroxyl radical versus hole. Appl. Catal. B Environ. 2016, 199, 405–411. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Kowalska, E.; Sobczak, J.W.; Lisowski, W.; Ohtani, B.; Zaleska, A. Preparation and characterization of monometallic (Au) and bimetallic (Ag/Au) modified-titania photocatalysts activated by visible light. Appl. Catal. B Environ. 2011, 101, 504–514. [Google Scholar] [CrossRef]
- Kowalska, E.; Janczarek, M.; Rosa, L.; Juodkazis, S.; Ohtani, B. Mono- and bi-metallic plasmonic photocatalysts for degradation of organic compounds under UV and visible light irradiation. Catal. Today 2014, 230, 131–137. [Google Scholar] [CrossRef]
- Kalan, R.E.; Yaparatne, S.; Amirbahman, A.; Tripp, C.P. P25 titanium dioxide coated magnetic particles: Preparation, characterization and photocatalytic activity. Appl. Catal. B Environ. 2016, 187, 249–258. [Google Scholar] [CrossRef]
- Emeline, A.V.; Zhang, X.; Murakami, T.; Fujishima, A. Activity and selectivity of photocatalysts in photodegradation of phenols. J. Hazard. Mater. 2012, 121–212, 54–160. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, E.B.; Aquino Neto, F.R.; Dezotti, M. TiO2-Photocatalyzed degradation of phenol in saline media in an annular reactor: Hydrodynamics, lumped kinetics, intermediates, and acute toxicity. Braz. J. Chem. Eng. 2009, 26, 75–87. [Google Scholar] [CrossRef]
- Montoya, J.F.; Velásquez, J.A.; Salvador, P. The direct-indirect kinetic model in photocatalysis: A reanalysis of phenol and formic acid degradation rate dependence on photon flow and concentration in TiO2 aqueous dispersions. Appl. Catal. B Environ. 2009, 88, 50–58. [Google Scholar] [CrossRef]
- Miyazaki, T.; Katsumura, Y.; Lin, M.; Muroya, Y.; Kudo, H.; Taguchi, M.; Asano, M.; Yoshida, M. Radiolysis of phenol in aqueous solution at elevated temperatures. Radiat. Phys. Chem. 2006, 75, 408–415. [Google Scholar] [CrossRef]
- Murcia, J.J.; Hidalgo, M.C.; Navío, J.A.; Araña, J.; Doña-Rodríguez, J.M. Correlation study between photo-degradation and surface adsorption properties of phenol and methyl orange on TiO2 Vs platinum-supported TiO2. Appl. Catal. B Environ. 2014, 150–151, 107–115. [Google Scholar] [CrossRef]
- Wei, Z.; Rosa, L.; Wang, K.; Endo, M.; Juodkazis, S.; Ohtani, B.; Kowalska, E. Size-controlled gold nanoparticles on octahedral anatase particles as efficient plasmonic photocatalyst. Appl. Catal. B Environ. 2017, 206, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Yustos, P.; Quintanilla, A.; Rodríguez, S.; García-Ochoa, F. Route of the catalytic oxidation of phenol in aqueous phase. Appl. Catal. B Environ. 2002, 39, 97–113. [Google Scholar] [CrossRef]
- Ishibashi, K.I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in TiO2photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
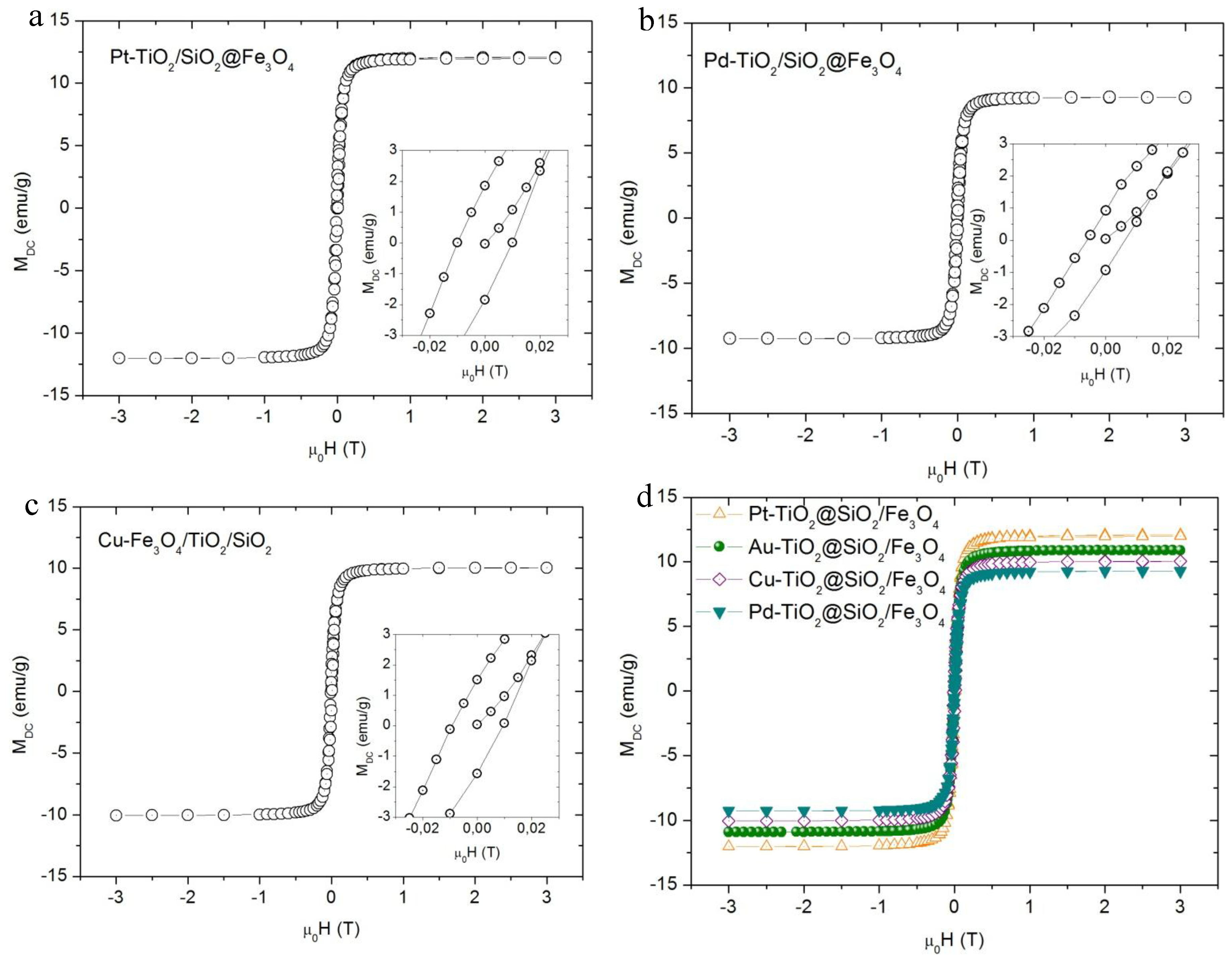

| Photocatalyst | Crystalline Size | Magnetization (emu·g−1) | |||||
|---|---|---|---|---|---|---|---|
| TiO2 | Magnetite | ||||||
| Anatase | Rutile | ||||||
| Size (nm) | Phase Content wt % | Size (nm) | Phase Content wt % | Size (nm) | Phase Content wt % | ||
| TiO2/SiO2@Fe3O4 | 20 | 57 ± 0.5 | 30 | 8 ± 1 | 44.5 | 34 ± 1 | 11 |
| Pt-TiO2/SiO2@Fe3O4 | 19.5 | 63 ± 2.5 | 28 | 6.5 ± 0.2 | 44 | 29 ± 2 | 12 |
| Pd-TiO2/SiO2@Fe3O4 | 19 | 64 ± 3 | 31 | 8 ± 1.4 | 43 | 29 ± 2.5 | 9.5 |
| Cu-TiO2/SiO2@Fe3O4 | 20 | 61 ± 3 | 30 | 7.5 ± 0.5 | 44 | 30 ± 3 | 10 |
| Au-TiO2/SiO2@Fe3O4 | 20 | 60 ± 1 | 31 | 7 ± 0.8 | 42 | 32 ± 2 | 11 |
| Photocatalyst | Content (at.%) | Ti 2p3/2 (%) | C 1s (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti 2p | O 1s | Si 2p | C 1s | Metal | Ti4+ | Ti3+ | C-C | C-O | C=C | C=O | COOH | |
| TiO2/SiO2@Fe3O4 | 8.7 | 60.0 | 20.4 | 10.9 | 0 | 97.0 | 3.0 | 34.0 | 24.5 | 12.8 | 18.1 | 10.5 |
| Pd-TiO2/SiO2@Fe3O4 | 8.3 | 59.4 | 20.5 | 11.6 | 0.2 | 96.0 | 4.0 | 33.9 | 21.5 | 22.0 | 11.5 | 9.0 |
| Cu-TiO2/SiO2@Fe3O4 | 6.7 | 56.5 | 22.6 | 13.4 | 0.8 | 96.0 | 4.0 | 43.1 | 41.2 | 8.4 | 2.9 | 4.4 |
| Au-TiO2/SiO2@Fe3O4 | 9.8 | 57.6 | 19.1 | 13.3 | 0.2 | 97.0 | 3.0 | 31.5 | 43.4 | 18.8 | 3.0 | 3.3 |
| Pt-TiO2/SiO2@Fe3O4 | 8.5 | 59.6 | 22.3 | 9.5 | 0.1 | 97.0 | 3.0 | 41.1 | 35.5 | 7.4 | 15.4 | 0.6 |
| Photocatalyst | Amount of Metal | ||
|---|---|---|---|
| Used for Deposition (mol %) | Deposited | ||
| EDS (wt %) | XPS (at.%) | ||
| TiO2/SiO2@Fe3O4 | 0 | 0 | 0 |
| Pd-TiO2/SiO2@Fe3O4 | 0.5 | 0.5 | 0.2 |
| Cu-TiO2/SiO2@Fe3O4 | 0.5 | 0.7 | 0.8 |
| Au-TiO2/SiO2@Fe3O4 | 0.5 | 0.49 | 0.2 |
| Pt-TiO2/SiO2@Fe3O4 | 0.5 | 0.58 | 0.1 |
| Photocatalysts | Iron Concentration (mg·L−l) | Comment |
|---|---|---|
| TiO2/SiO2@Fe3O4 | 0.55 ± 0.08 | <LOQ |
| Cu-TiO2/SiO2@Fe3O4 | 0.53 ± 0.07 | <LOQ |
| Pd-TiO2/SiO2@Fe3O4 | 0.50 ± 0.08 | <LOQ |
| Pt–TiO2/SiO2@Fe3O4 | 0.53 ± 0.08 | <LOQ |
| Au–TiO2/SiO2@Fe3O4 | 0.55 ± 0.10 | <LOQ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysocka, I.; Kowalska, E.; Trzciński, K.; Łapiński, M.; Nowaczyk, G.; Zielińska-Jurek, A. UV-Vis-Induced Degradation of Phenol over Magnetic Photocatalysts Modified with Pt, Pd, Cu and Au Nanoparticles. Nanomaterials 2018, 8, 28. https://doi.org/10.3390/nano8010028
Wysocka I, Kowalska E, Trzciński K, Łapiński M, Nowaczyk G, Zielińska-Jurek A. UV-Vis-Induced Degradation of Phenol over Magnetic Photocatalysts Modified with Pt, Pd, Cu and Au Nanoparticles. Nanomaterials. 2018; 8(1):28. https://doi.org/10.3390/nano8010028
Chicago/Turabian StyleWysocka, Izabela, Ewa Kowalska, Konrad Trzciński, Marcin Łapiński, Grzegorz Nowaczyk, and Anna Zielińska-Jurek. 2018. "UV-Vis-Induced Degradation of Phenol over Magnetic Photocatalysts Modified with Pt, Pd, Cu and Au Nanoparticles" Nanomaterials 8, no. 1: 28. https://doi.org/10.3390/nano8010028