Highly Efficient, Rapid, and Simultaneous Removal of Cationic Dyes from Aqueous Solution Using Monodispersed Mesoporous Silica Nanoparticles as the Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MSNs
2.3. Adsorption Experiments
2.4. Desorption Experiments
2.5. Material Characterization
2.6. Dye Concentration Measurement
3. Results and Discussion
3.1. Characterization of MSNs
3.2. Optimized Adsorption Conditions
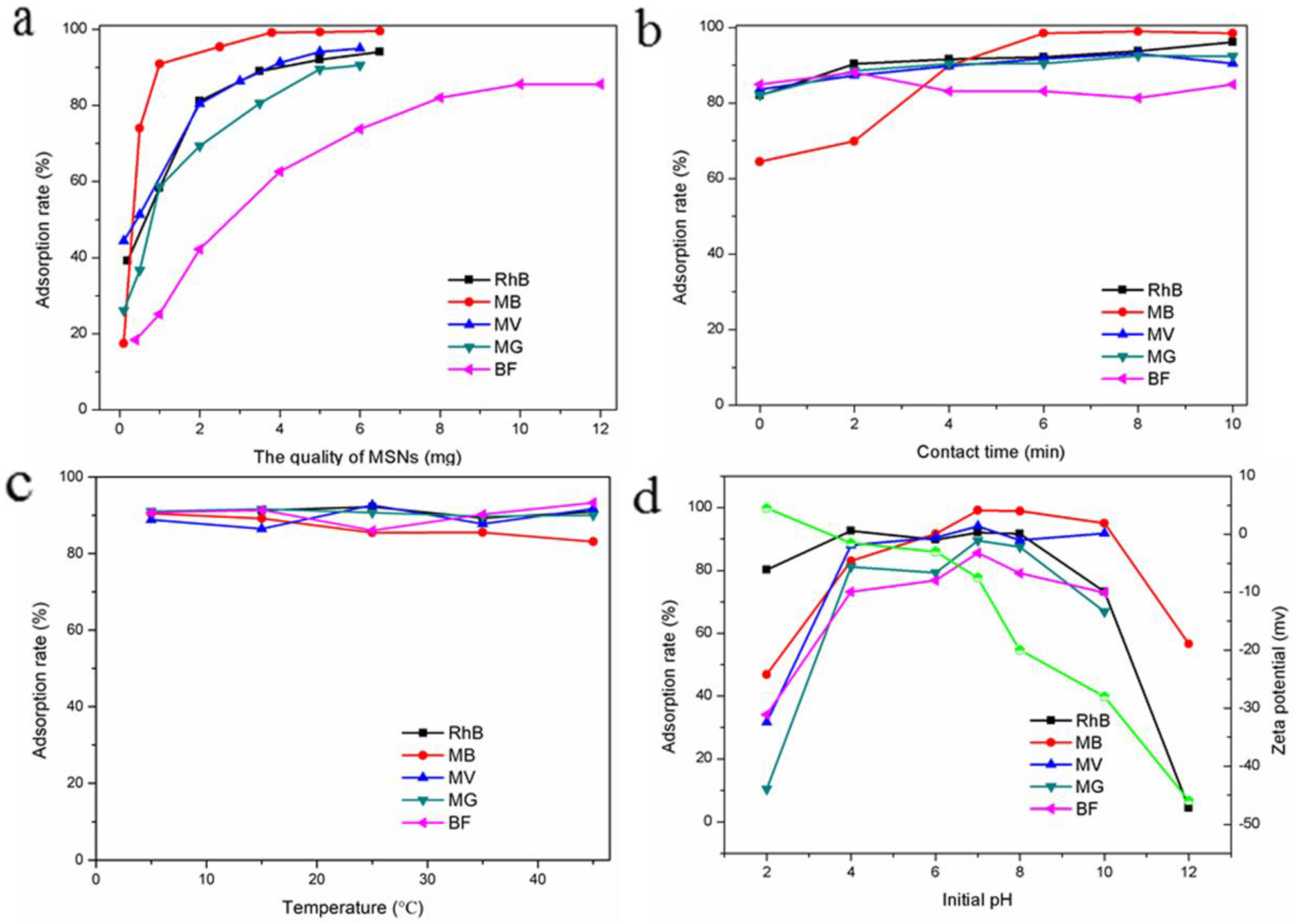
3.2.1. Effect of Adsorbent Mass
3.2.2. Effect of Contact Time
3.2.3. Effect of Temperature
3.2.4. Effect of Initial pH
3.2.5. Adsorption Isotherms
3.2.6. Adsorption Mechanism Prediction
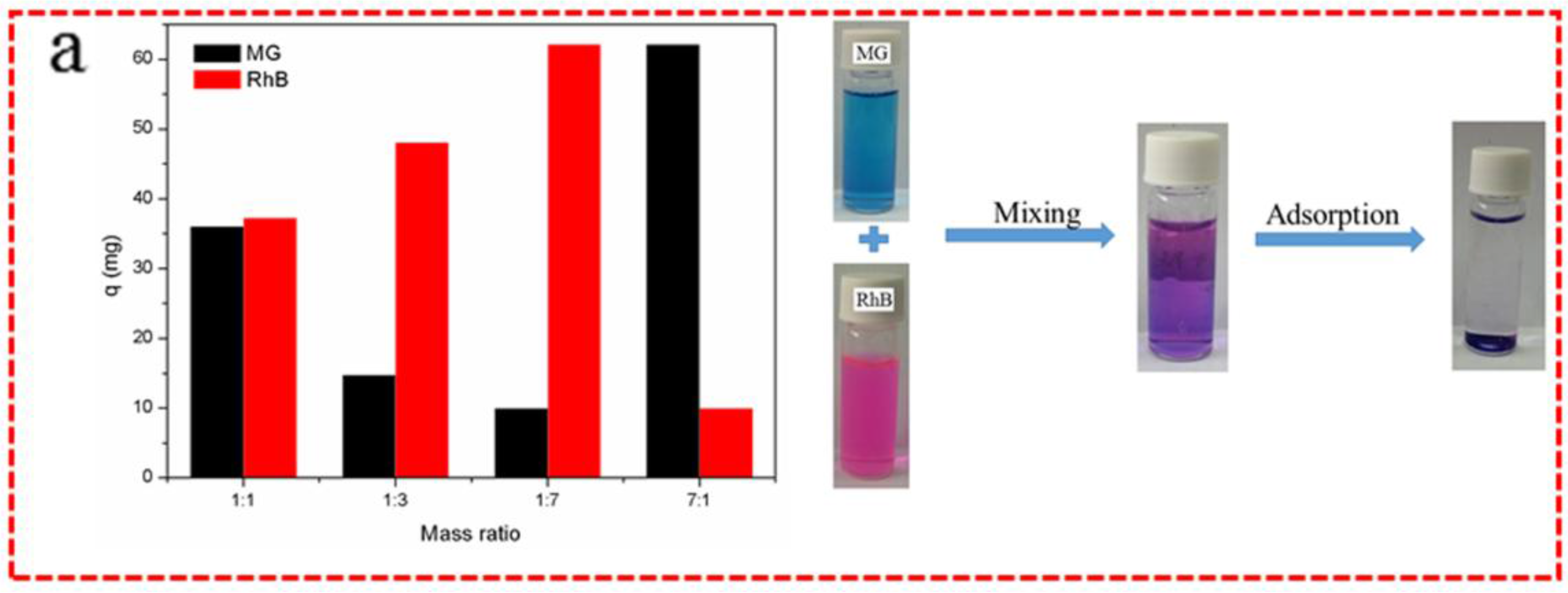
3.3. Adsorption Selectivity
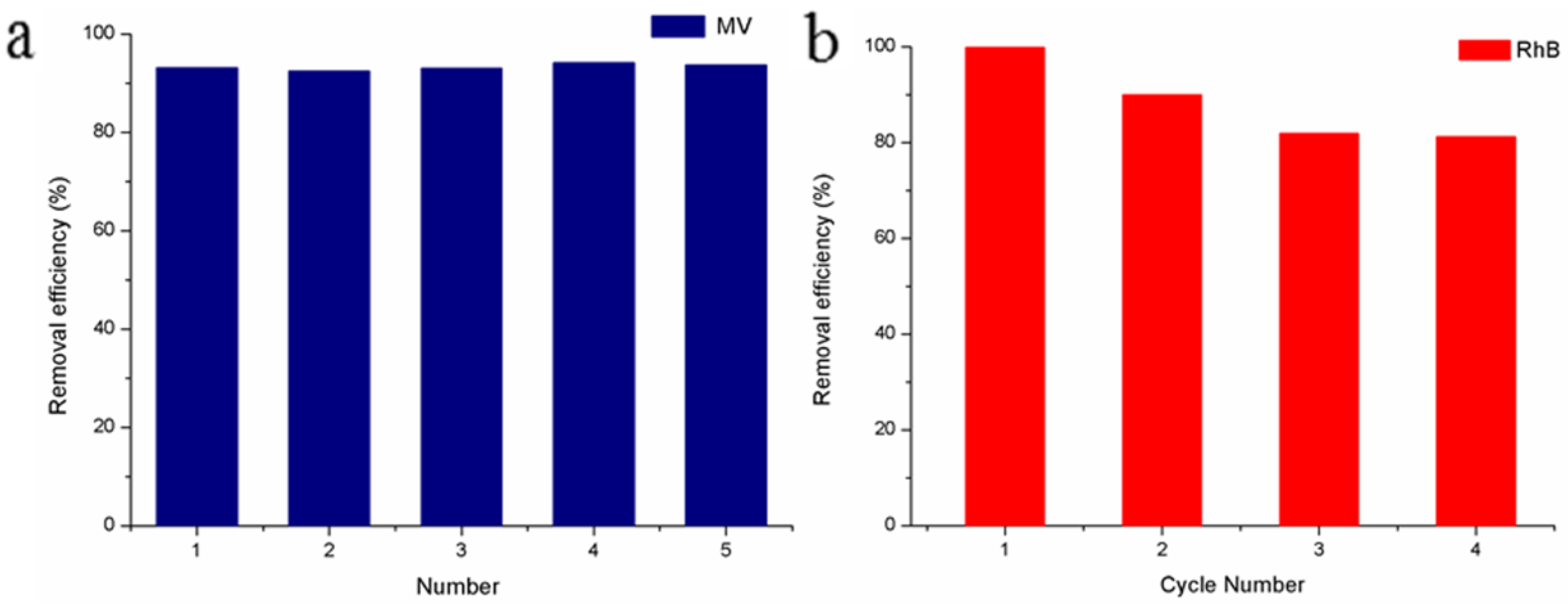
3.4. Repeatability Study
3.5. Reusability Study
3.6. Comparison with Other Absorbents
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MSNs | mesoporous silica nanoparticles |
| RhB | rhodamine B |
| MB | methylene blue |
| MV | methyl violet |
| MG | malachite green |
| BF | basic fuchsin |
| AF | acid fuchsin |
| MO | methyl orange |
| CR | congo red |
| CTAB | cetyltrimethyl ammonium bromide |
| TEOS | tetraethyl orthosilicate |
| THEED | N,N,N,N-Tetrakis (2-hydroxyethyl)ethylenediamine |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
| FT-IR | Fourier-transform infrared |
| BET | Brunauer-Emmett-Teller |
| DFT | Density functional theory |
| R2 | correlation coefficients |
| pHpzc | point of zero charge |
References
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gong, W.; Luo, J.; Zou, C.; Yang, Y.; Yang, S. Selective adsorption of cationic dyes from aqueous solution by polyoxometalate-based metal-organic framework composite. Appl. Surf. Sci. 2016, 362, 517–524. [Google Scholar] [CrossRef]
- Pei, Y.C.; Wang, J.J.; Xuan, X.P.; Fan, J.; Fan, M. Factors affecting ionic liquids based removal of anionic dyes from water. Environ. Sci. Technol. 2007, 41, 5090–5095. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; He, Q.; Lv, M.; Xu, Y.; Yang, H.; Liu, X.; Wei, F. Selective adsorption of cationic dyes by UiO-66-NH2. Appl. Surf. Sci. 2015, 327, 77–85. [Google Scholar] [CrossRef]
- Konicki, W.; Hełminiak, A.; Arabczyk, W.; Mijowska, E. Removal of anionic dyes using magnetic Fe@graphite core-shell nanocomposite as an adsorbent from aqueous solutions. J. Colloid Interface Sci. 2017, 497, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, S.; Mahmoudi, F.; Amini, M.M.; Dusek, M.; Jarosova, M. Synthesis and characterization of a series of novel perovskite-type LaMnO3/Keggin-type polyoxometalate hybrid nanomaterials for fast and selective removal of cationic dyes from aqueous solutions. Dalton Trans. 2017, 46, 3252–3264. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiong, Z.; Zhang, J.; Wu, C. The Strengthening Role of the Amino Group in Metal-Organic Framework MIL-53 (Al) for Methylene Blue and Malachite Green Dye Adsorption. J. Chem. Eng. Data 2015, 60, 3414–3422. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, T.; Wang, S.; Niu, H.; Wang, X.; Cai, Y. One-pot synthesis of C18-functionalized core-shell magnetic mesoporous silica composite as efficient sorbent for organic dye. J. Colloid Interface Sci. 2015, 448, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ren, B.; Jiang, H.; Zhou, B.; Liping, L.V.; Ren, J.; Dong, L.; Liu, Z.; Jing, L. Dual-porosity Mn2O3 cubes for highly efficient dye adsorption. J. Hazard. Mater. 2017, 333, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, H.; Jiang, Z.; Cai, T.; Li, H.; Li, H.; Li, A.; Cheng, R. Flocculation of both anionic and cationic dyes in aqueous solutions by the amphoteric grafting flocculant carboxymethyl chitosan-graft-polyacrylamide. J. Hazard. Mater. 2013, 254, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Bassyouni, D.; Hamad, H.; El-Ashtoukhy, E.; Amin, N.; El-Latif, M.A. Comparative performance of anodic oxidation and electrocoagulation as clean processes for electrocatalytic degradation of diazo dye Acid Brown 14 in aqueous medium. J. Hazard. Mater. 2017, 335, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Yang, N.; Gao, F.; Zhao, J.; Li, L.; Teng, C. Three-dimensional polypyrrole-derived carbon nanotube framework for dye adsorption and electrochemical supercapacitor. Appl. Surf. Sci. 2017, 414, 218–223. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, C.; Chen, J.; Zhang, B.; Zhang, H.; Zhang, Y.; Chen, L. Accelerating biodegradation of a monoazo dye Acid Orange 7 by using its endogenous electron donors. J. Hazard. Mater. 2016, 324, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xiao, P.; Tian, J.; Wang, F.; Yu, J. Phenylamine-Functionalized rGO/TiO2 Photocatalysts: Spatially Separated Adsorption Sites and Tunable Photocatalytic Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 29470–29477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tan, Y.; Wu, F.; Niu, H.; Tang, Z.; Cai, Y.; Giesy, J.P. Cu/Cu2O/CuO loaded on the carbon layer derived from novel precursors with amazing catalytic performance. Sci. Total Environ. 2016, 571, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Fei, T.; Gu, W.; Liu, Z.; Zhao, Y.; An, Z.; Zhe, L.; Teng, Y. A simple post-synthesis conversion approach to Zn(OH)F and the effects of fluorine and hydroxyl on the photodegradation properties of dye wastewater. J. Hazard. Mater. 2017, 333, 250–258. [Google Scholar]
- Huang, Q.; Liu, M.; Mao, L.; Xu, D.; Zeng, G.; Huang, H.; Jiang, R.; Deng, F.; Zhang, X.; Wei, Y. Surface functionalized SiO2 nanoparticles with cationic polymers via the combination of mussel inspired chemistry and surface initiated atom transfer radical polymerization: Characterization and enhanced removal of organic dye. J. Colloid Interface Sci. 2017, 499, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cheng, L.; Zhao, Y.; Zhu, L. Interfacially crosslinked composite porous membranes for ultrafast removal of anionic dyes from water through permeating adsorption. J. Hazard. Mater. 2017, 337, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Hadi, P.; Guo, J.; Barford, J.; Mckay, G. Multilayer Dye Adsorption in Activated Carbons-Facile Approach to Exploit Vacant Sites and Interlayer Charge Interaction. Environ. Sci. Technol. 2016, 50, 5041–5049. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Huang, D.; Odoom-Wubah, T.; Fu, D.; Huang, J.; Qin, Q. Adsorption of anionic and cationic dyes on ferromagnetic ordered mesoporous carbon from aqueous solution: Equilibrium, thermodynamic and kinetics. J. Colloid Interface Sci. 2014, 430, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Wan, Y.; Feng, C.; Shen, Y.; Zhao, D. Highly Efficient Adsorption of bulky dye molecules in wastewater on ordered mesoporous carbons. Chem. Mater. 2009, 21, 706–716. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, G.; Tang, L.; Cai, Y.; Pang, Y.; Zhang, Y.; Yang, G.; Zhou, Y.; He, X.; He, Y. Highly effective adsorption of cationic and anionic dyes on magnetic Fe/Ni nanoparticles doped bimodal mesoporous carbon. J. Colloid Interface Sci. 2015, 448, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Li, Y.; Yu, Y.; Li, Y.; Xia, K.; Wang, Y.; Li, S. Synthesis of mesoporous carbon nanospheres for highly efficient adsorption of bulky dye molecules. J. Colloid Interface Sci. 2016, 51, 7016–7028. [Google Scholar] [CrossRef]
- Machado, F.M.; Ayyappan, C.S.; Lima, E.C.; Dias, S.L.P.; Prola, L.D.T.; Saucier, C.; Jauris, I.M.; Zanella, I.; Fagan, S.B. Adsorption of alizarin red s dye by carbon nanotubes: An experimental and theoretical investigation. J. Phys. Chem. C 2016, 120, 18296–18306. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Liu, J.; Zhang, Y. Facile one-pot synthesis of novel spherical zeolite-reduced graphene oxide composites for cationic dye adsorption. Ind. Eng. Chem. Res. 2014, 53, 13711–13717. [Google Scholar] [CrossRef]
- Li, H.; Hou, J.; Duan, L.; Ji, C.; Zhang, Y.; Chen, V. Graphene oxide-enzyme hybrid nanoflowers for efficient water soluble dye removal. J. Hazard. Mater. 2017, 338, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Karan, C.K.; Bhattacharjee, M. Self-healing and moldable metallogels as the recyclable materials for selective dye adsorption and separation. ACS Appl. Mater. Interfaces 2016, 8, 5526–5535. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ren, G.J.; Li, A.; Zhang, L.Z.; Feng, R.; Zhang, Y.H.; Bu, X.H. Temperature-related synthesis of two anionic metal-organic frameworks with distinct performance in organic dye adsorption. Cryst. Growth Des. 2016, 16, 5593–5597. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Zhi, T.; Niu, H.; Cai, Y.; Wei, M.; Wu, F.; Giesy, J.P. Synthesis of magnetic metal-organic framework (MOF) for efficient removal of organic dyes from water. Sci. Rep. 2015, 5, 11849–11858. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhao, E.; Kim, T.; Wang, J.; Hableel, G.; Pjt, R.; Ananthakrishna, S.J.; Wang, T.; Arconada-Alvarez, S.; Knowles, J.C. Organosilica nanoparticles with an intrinsic secondary amine: An efficient and reusable adsorbent for dyes. ACS Appl. Mater. Interfaces 2017, 9, 15566–15576. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shen, H.; Guo, L.; Wang, C.; Fu, F. Porous-BiOBr/Bi2MoO6 heterostructures for highly selective adsorption of methylene blue. ACS Omega 2016, 1, 566–577. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, L.; Xing, F.; Lin, H. Controlled synthesis of monodispersed mesoporous silica nanoparticles: Particle size tuning and formation mechanism investigation. Microporous Mesoporous Mater. 2016, 225, 238–244. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Durand, J.O. Large pore mesoporous silica nanomaterials for application in delivery of biomolecules. Nanoscale 2015, 7, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yu, M.; Noonan, O.; Zhang, J.; Song, H.; Zhang, H.; Lei, C.; Niu, Y.; Huang, X.; Yang, Y. Core-cone structured monodispersed mesoporous silica nanoparticles with ultra-large cavity for protein delivery. Small 2016, 11, 5949–5955. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Na, H.K.; Kim, Y.K.; Ryoo, S.R.; Cho, H.S.; Lee, K.E.; Jeon, H.; Ryoo, R.; Min, D.H. Facile synthesis of monodispersed mesoporous silica nanoparticles with ultralarge pores and their application in gene delivery. ACS Nano 2011, 5, 3568–3576. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Botella, P.; Corma, A.; Blesa, J.; Dong, L. Monodispersed mesoporous silica nanoparticles with very large pores for enhanced adsorption and release of DNA. J. Phys. Chem. B 2009, 113, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Zhu, Y.; Yang, X.; Li, C. Preparation of monodispersed mesoporous silica spheres with tunable pore size and pore-size effects on adsorption of Au nanoparticles and urease. Mater. Sci. Eng. C 2011, 31, 166–172. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Highly efficient methylene blue dyes removal from aqueous systems by chitosan coated magnetic mesoporous silica nanoparticles. J. Porous Mater. 2015, 22, 1383–1392. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, K.P.; Ou, H.D.; Chiang, Y.C.; Wang, C.F. Adsorption of cationic dyes onto mesoporous silica. Microporous Mesoporous Mater. 2011, 141, 102–109. [Google Scholar] [CrossRef]
- Chaudhuri, H.; Dash, S.; Sarkar, A. Adsorption of different dyes from aqueous solution using Si-MCM-41 having very high surface area. J. Porous Mater. 2016, 23, 1227–1237. [Google Scholar] [CrossRef]
- Chaudhuri, H.; Dash, S.; Ghorai, S.; Pal, S.; Sarkar, A. SBA-16: Application for the removal of neutral, cationic, and anionic dyes from aqueous medium. J. Environ. Chem. Eng. 2016, 4, 157–166. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chang, W.C.; Saikia, D.; Wu, C.E.; Kao, H.M. Functionalization of cubic mesoporous silica SBA-16 with carboxylic acid via one-pot synthesis route for effective removal of cationic dyes. J. Hazard. Mater. 2015, 309, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhao, Z.; Sun, T.; Shi, W.; Cui, F. Enhanced adsorption of the cationic dyes in the spherical CuO/meso-silica nano composite and impact of solution chemistry. J. Colloid Interface Sci. 2017, 485, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, B.; Wang, Y.; Li, M.; Liu, Y.; Gao, L.; Mao, L. Preparation of monodispersed mesoporous silica particles and their applications in adsorption of Au3+ and Hg2+ after mercapto-functionalized treatment. Microporous Mesoporous Mater. 2015, 214, 15–22. [Google Scholar] [CrossRef]
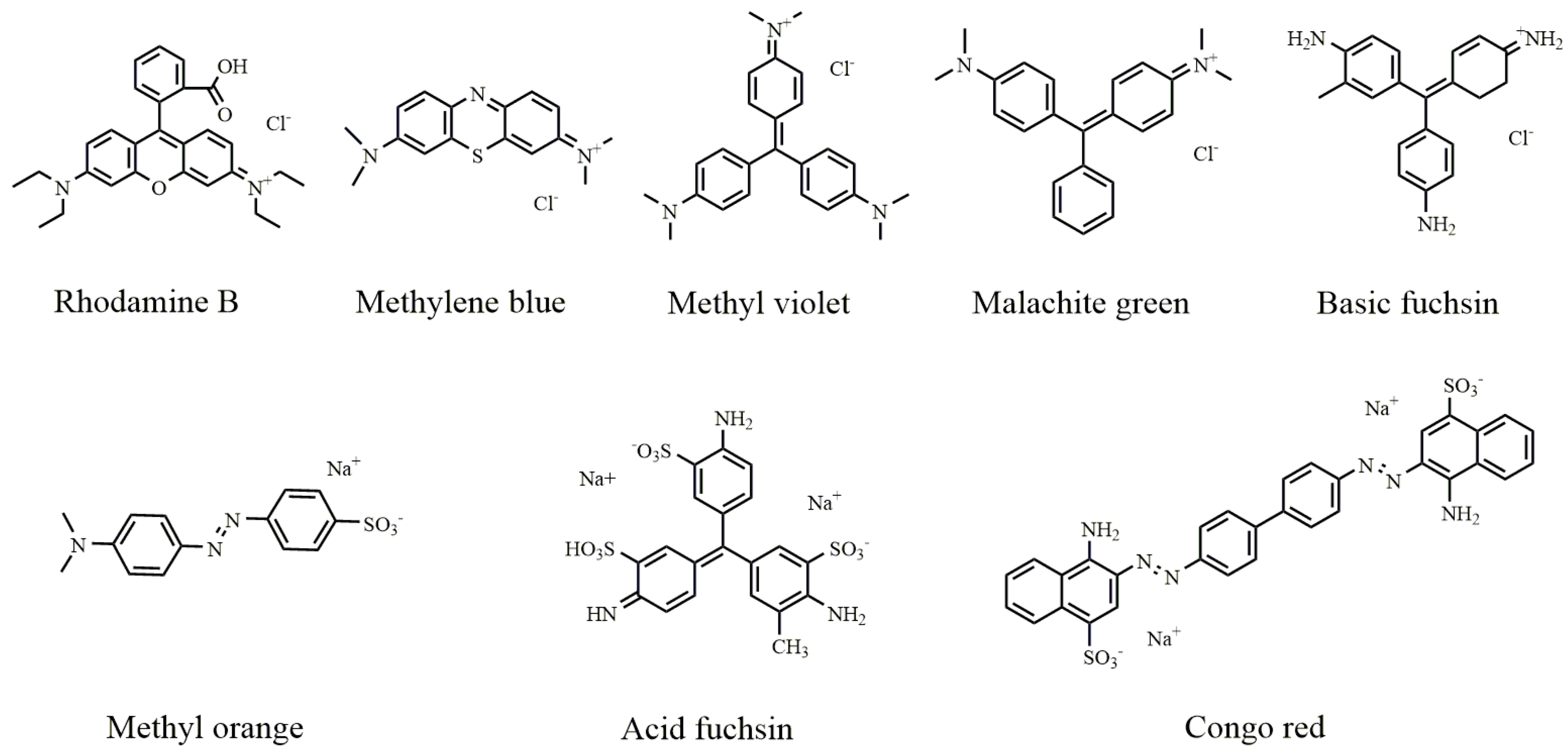


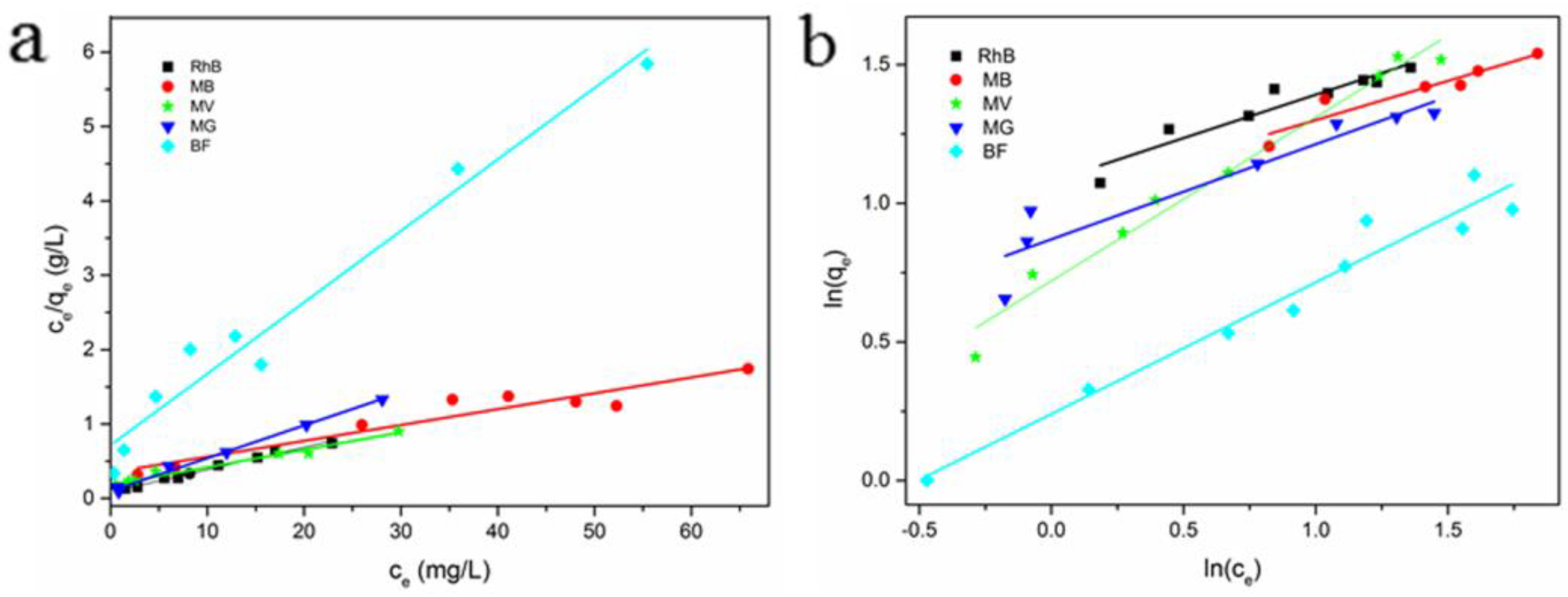
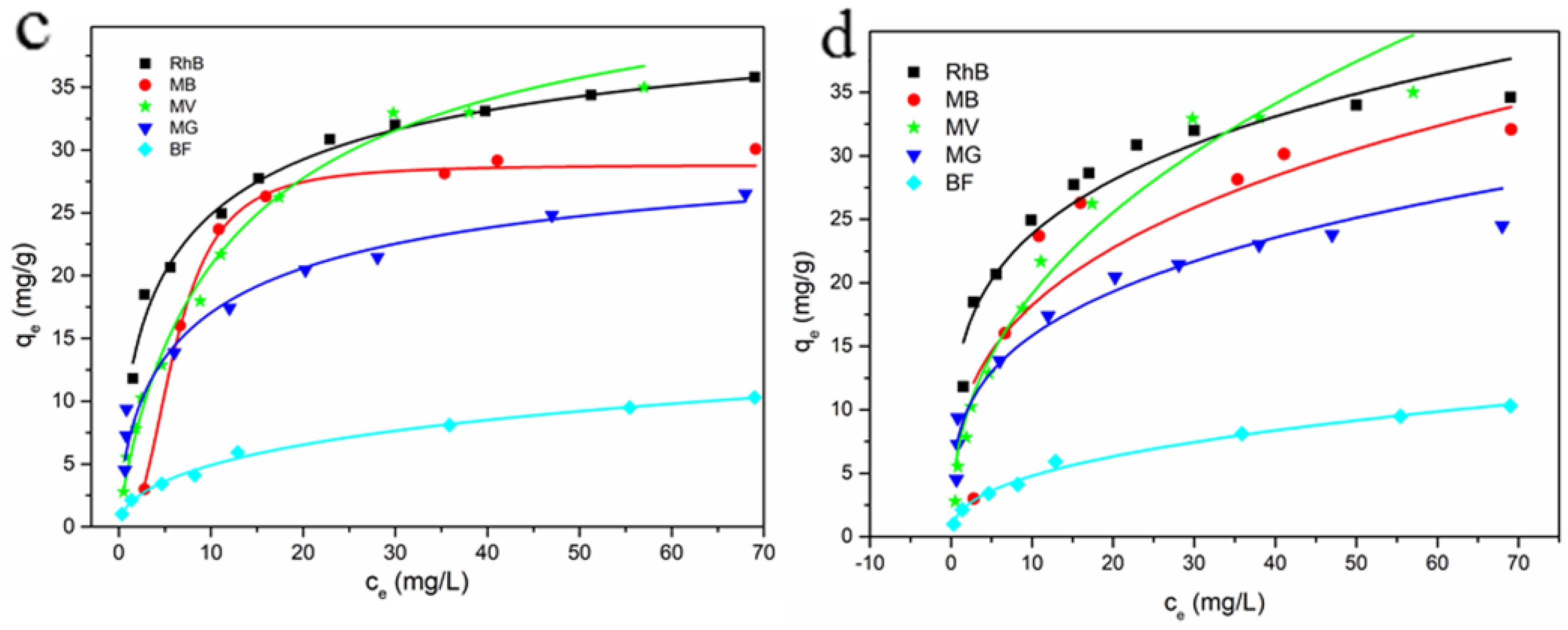

| Dyes | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/g) | R2 | KF (L/g) | 1/n | R2 | |
| RhB | 33.22 | 0.3500 | 0.9890 | 9.330 | 0.6900 | 0.9432 |
| MB | 38.17 | 0.098 | 0.9731 | 10.39 | 0.2800 | 0.8904 |
| MV | 40.65 | 0.1300 | 0.9793 | 5.260 | 0.5800 | 0.9747 |
| MG | 22.68 | 0.444 | 0.9940 | 4.445 | 0.3400 | 0.8874 |
| BF | 10.41 | 0.1350 | 0.9666 | 1.736 | 0.4700 | 0.9544 |
| Adsorbents | Contact Time (min) | Dyes | Qmax (mg/g) | Reference |
|---|---|---|---|---|
| CCMSN | 300 | Methylene blue | 43.03 | [39] |
| SBA-15 | 60 | Methylene blue Janus Green B | 49.26 66.44 | [40] |
| Si-MCM-41 | 30 | Safranin O | 275.5 | [41] |
| SBA-16 | 30 | Safranin O | 240.39 | [42] |
| S16C-30 CuO/MCM-41 | 200 60 | Methylene blue Methylene blue | 561 87.8 | [43] [44] |
| MSNs | 2~6 | Methylene blue Rhodamine B Methyl violet Malachite green Basic fuchsin | 34.23 23.26 20.36 20.10 14.70 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, P.; Yang, Y.; Zhang, X.; Niu, J.; Yang, H.; Tian, S.; Zhu, J.; Lu, M. Highly Efficient, Rapid, and Simultaneous Removal of Cationic Dyes from Aqueous Solution Using Monodispersed Mesoporous Silica Nanoparticles as the Adsorbent. Nanomaterials 2018, 8, 4. https://doi.org/10.3390/nano8010004
Qin P, Yang Y, Zhang X, Niu J, Yang H, Tian S, Zhu J, Lu M. Highly Efficient, Rapid, and Simultaneous Removal of Cationic Dyes from Aqueous Solution Using Monodispersed Mesoporous Silica Nanoparticles as the Adsorbent. Nanomaterials. 2018; 8(1):4. https://doi.org/10.3390/nano8010004
Chicago/Turabian StyleQin, Peige, Yixin Yang, Xiaoting Zhang, Jiahua Niu, Hui Yang, Shufang Tian, Jinhua Zhu, and Minghua Lu. 2018. "Highly Efficient, Rapid, and Simultaneous Removal of Cationic Dyes from Aqueous Solution Using Monodispersed Mesoporous Silica Nanoparticles as the Adsorbent" Nanomaterials 8, no. 1: 4. https://doi.org/10.3390/nano8010004




