Micro-Spherical Sulfur/Graphene Oxide Composite via Spray Drying for High Performance Lithium Sulfur Batteries
Abstract
:1. Introduction
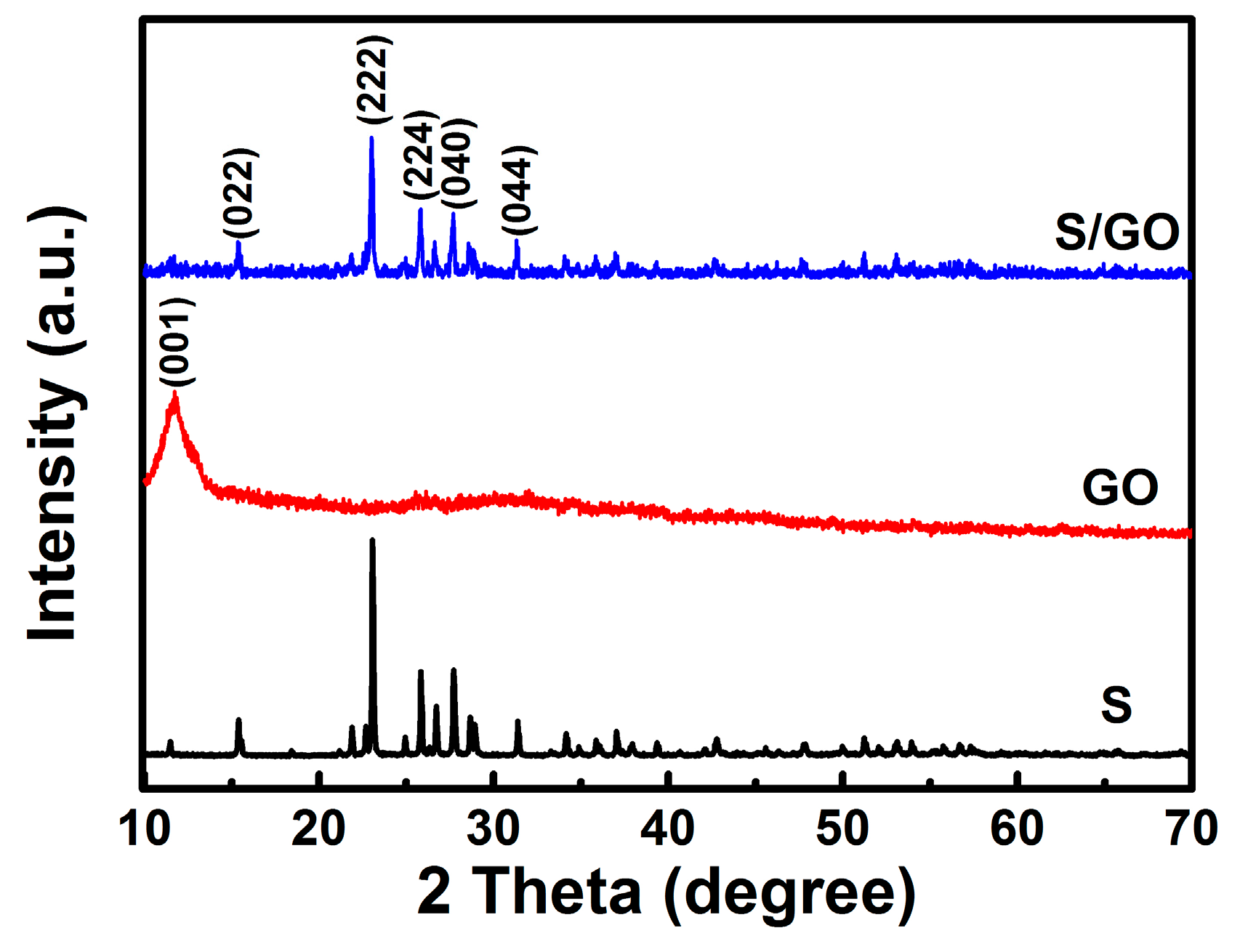
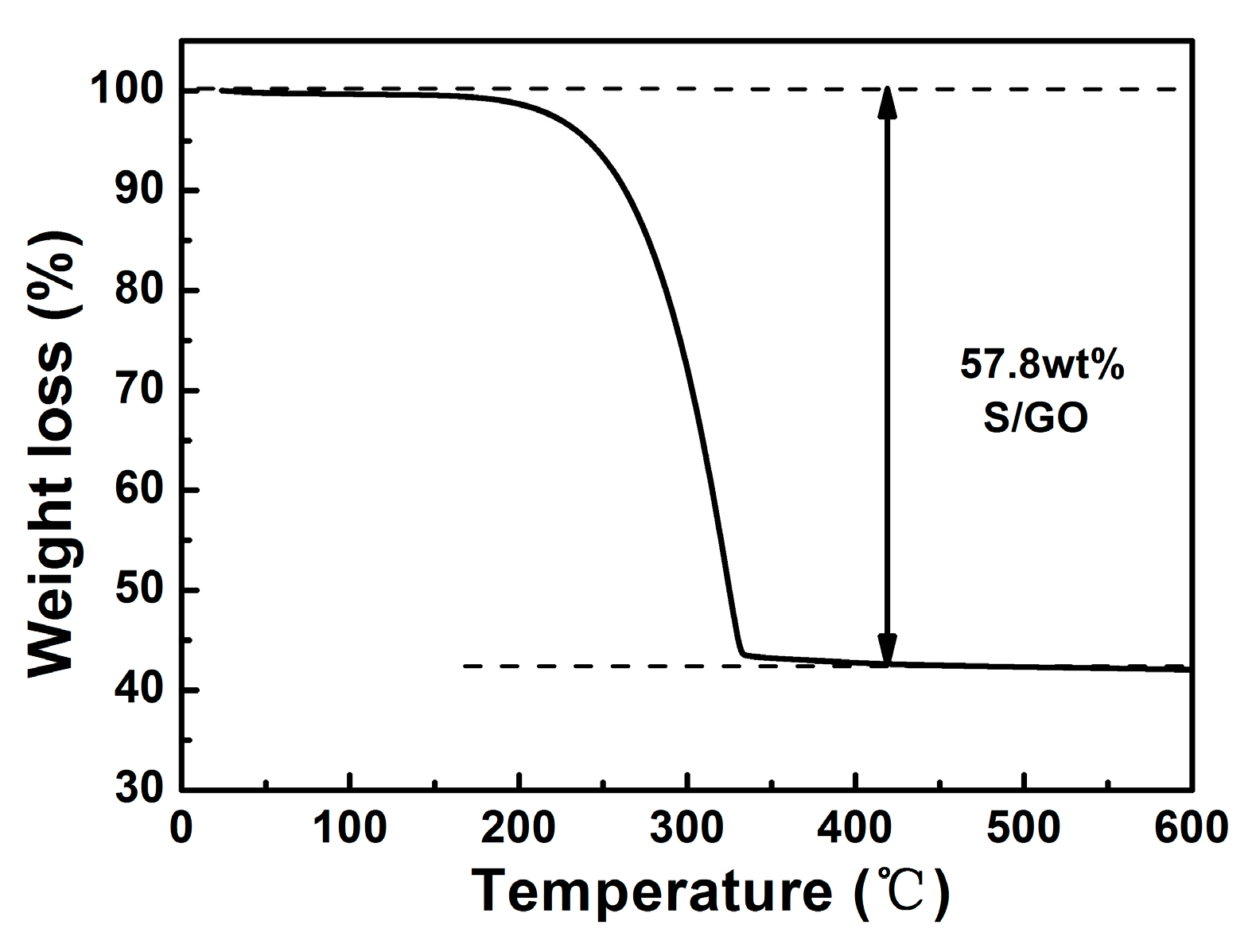
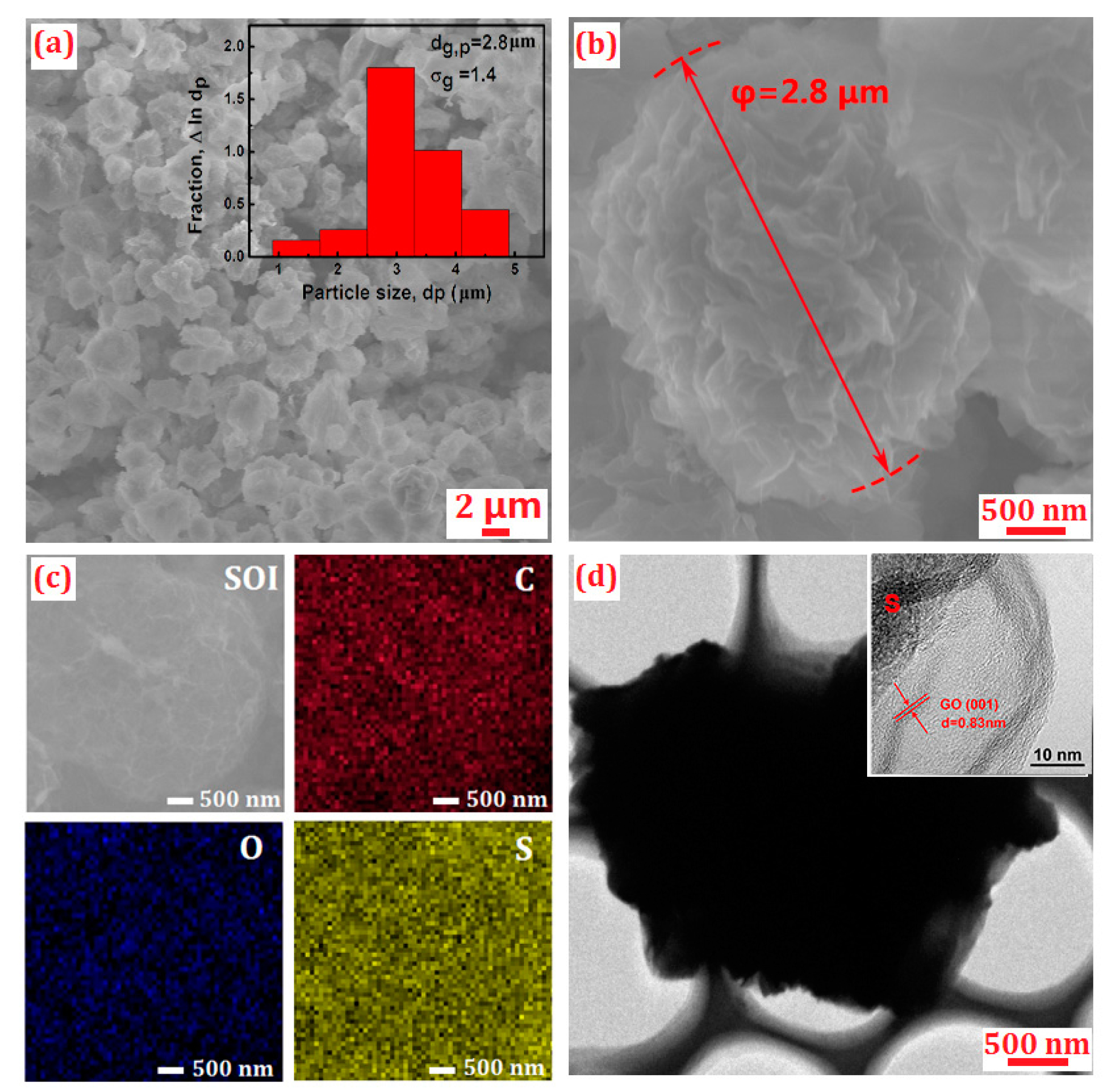
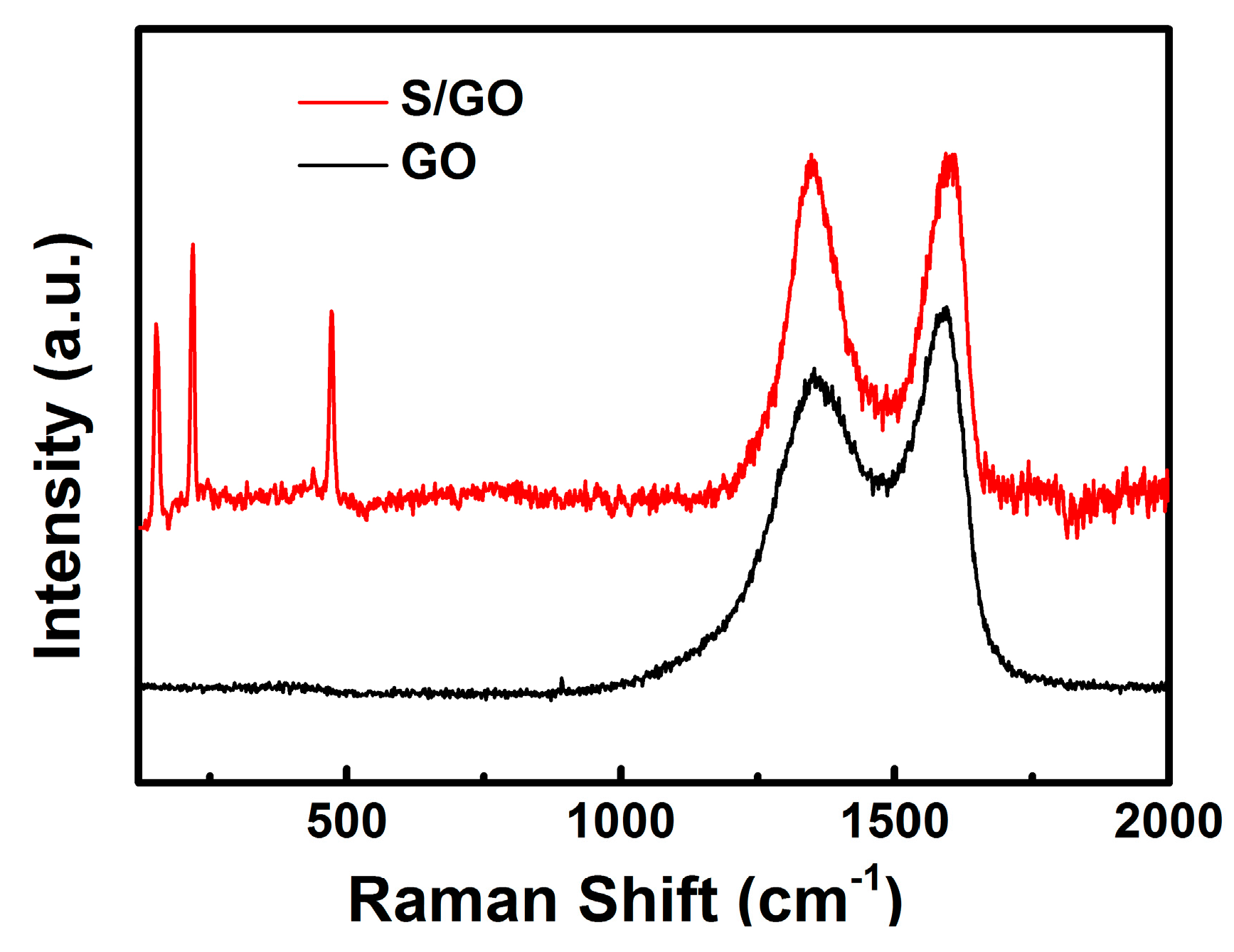
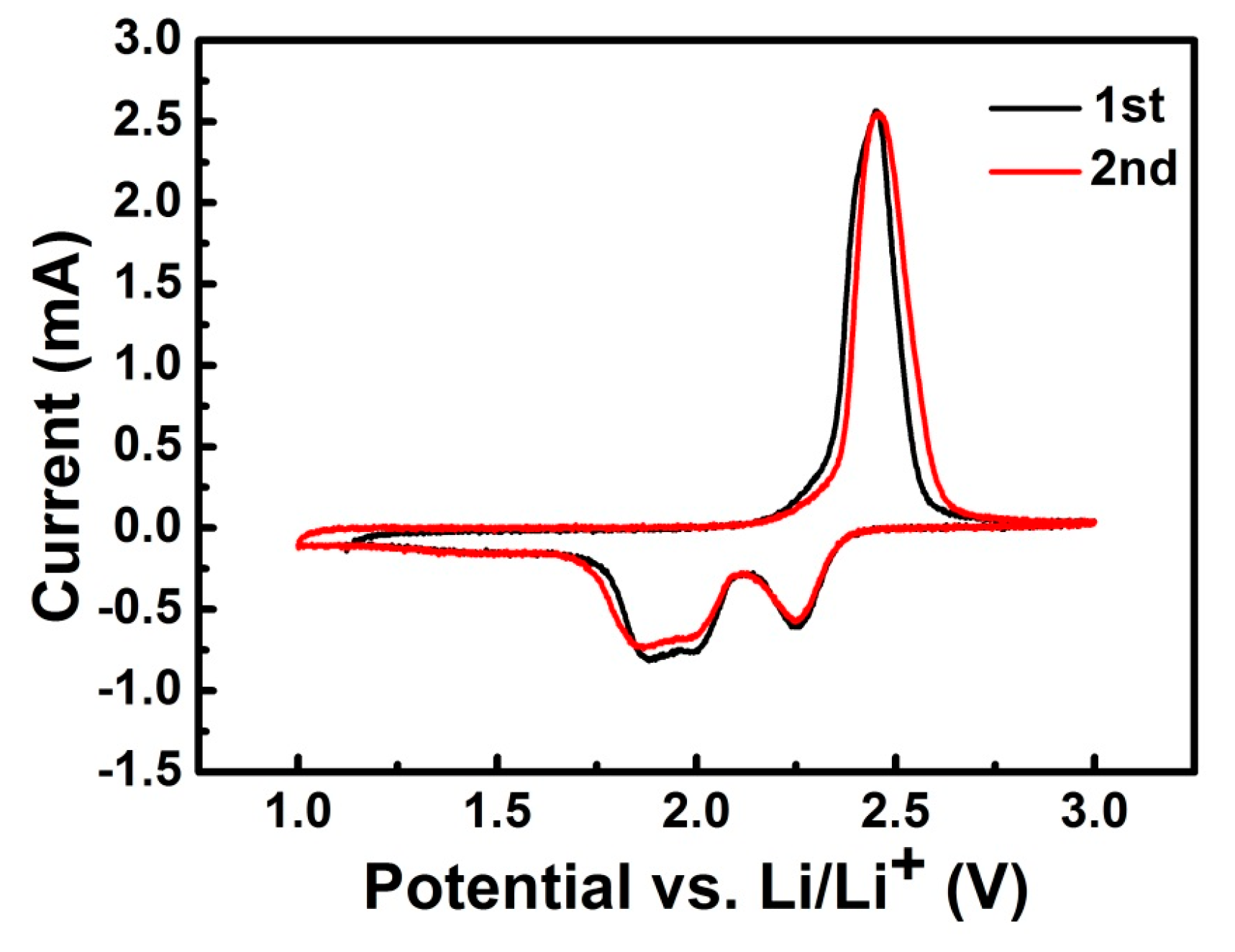
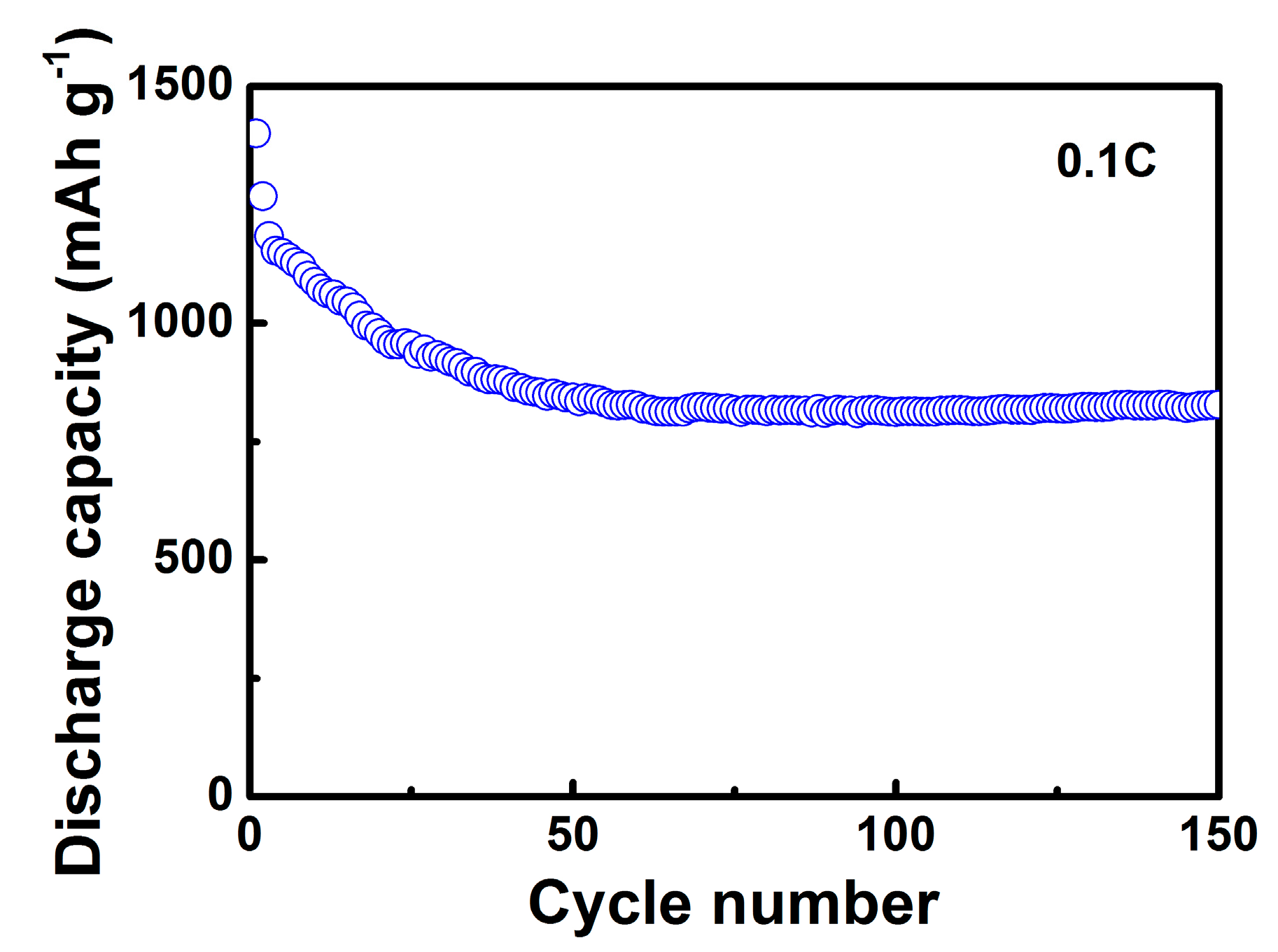
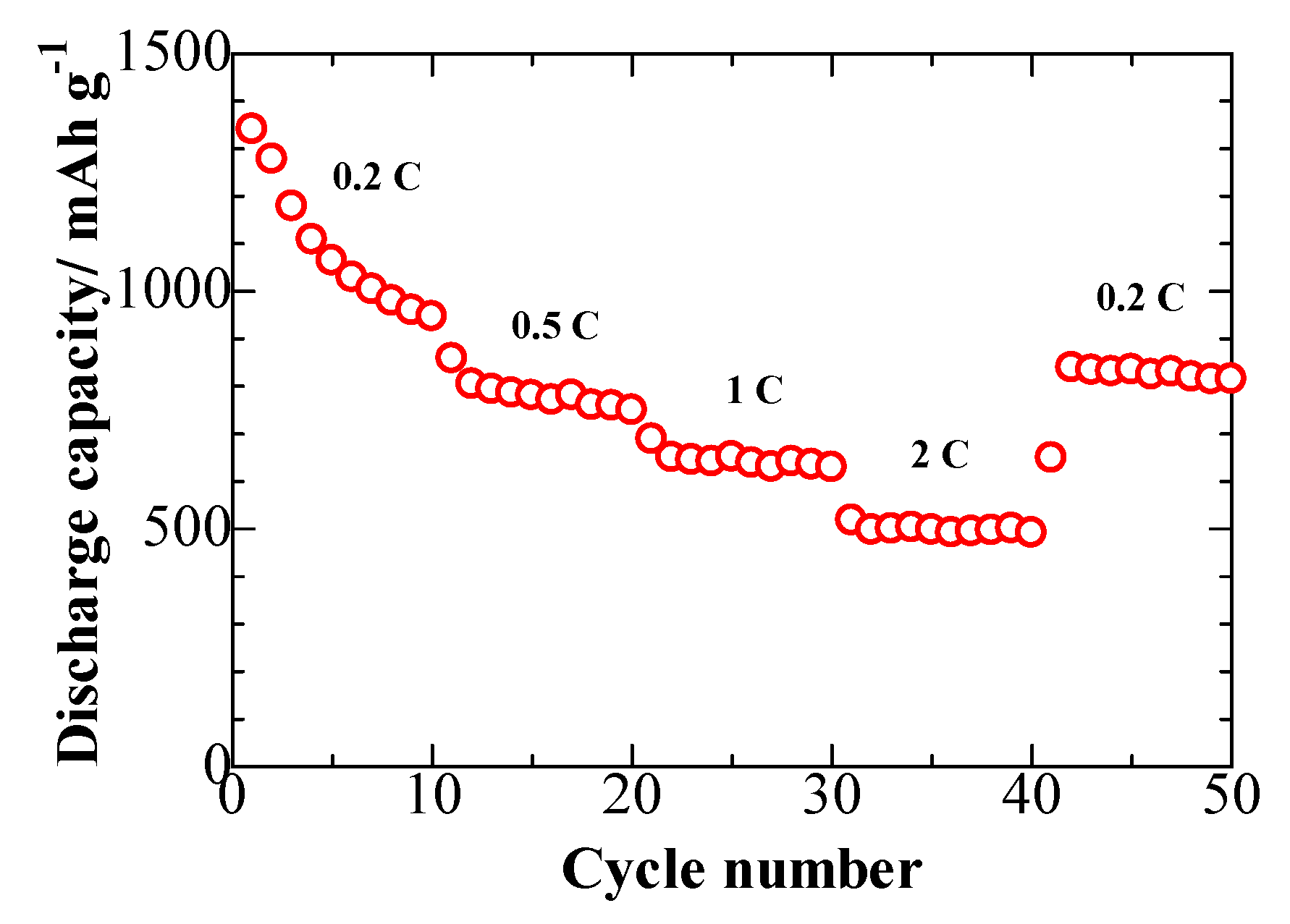
2. Results and Discussion
3. Materials and Methods
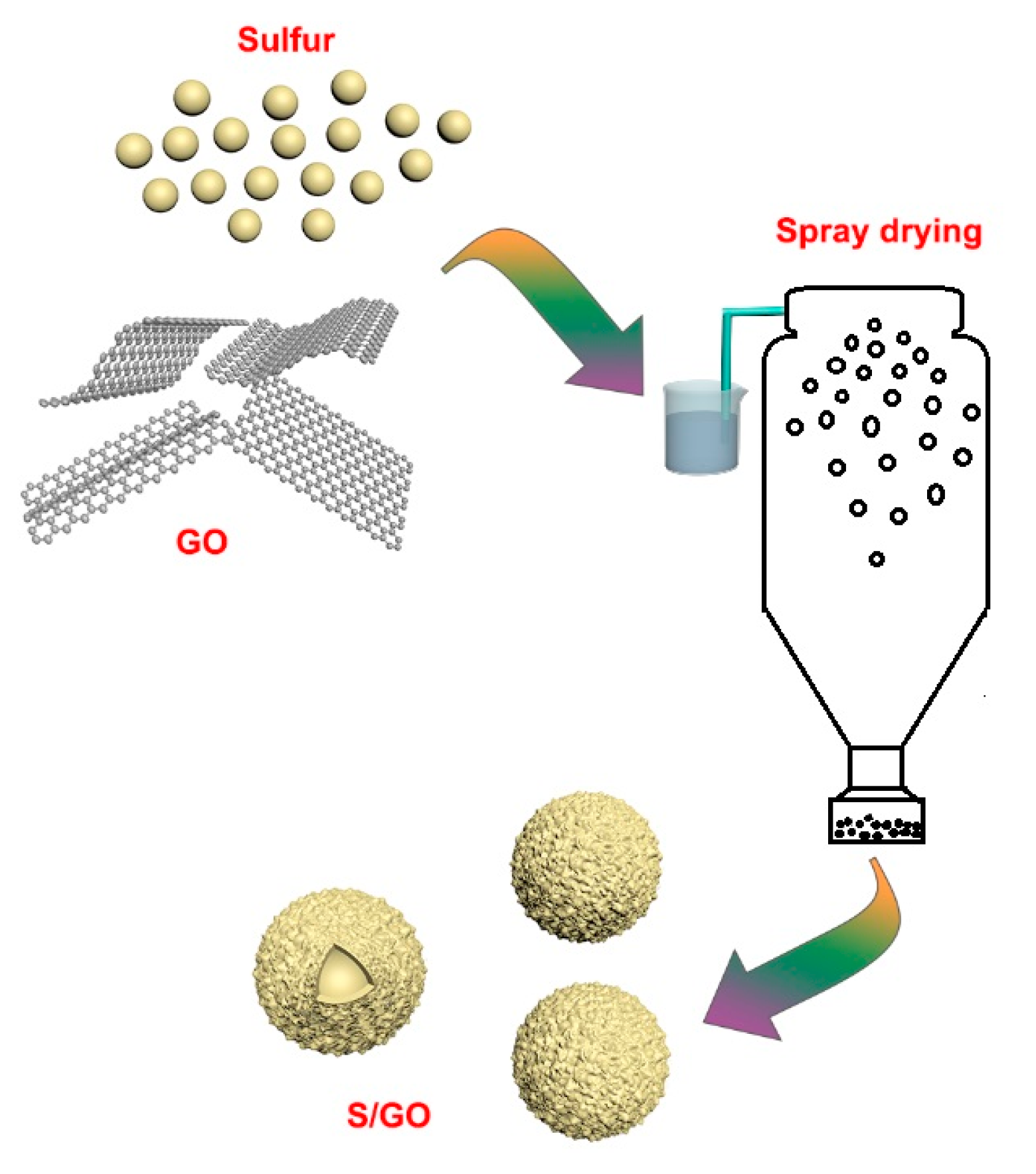
3.1. Material Preparation
3.2. Characterizations
3.3. Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, G.Y.; Lee, S.W.; Liang, Z.; Lee, H.Y.; Yan, K.; Yao, H.B.; Wang, H.T.; Li, W.Y.; Chu, S.; Cui, Y. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 2014, 9, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Chung, S.H.; Zu, C. Lithium sulfur batteries: Progress and prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef] [PubMed]
- Cairns, E.J.; Albertus, P. Batteries for electric and hybrid-electric vehicles. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 299–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Bakenov, Z.; Zhao, Y.; Konarov, A.; Doan, T.N.L.; Sun, K.E.K.; Yermukhambetova, A.; Chen, P. Effect of nanosized Mg0.6Ni0.4O prepared by self-propagating high temperature synthesis on sulfur cathode performance in Li/S batteries. Powder Technol. 2013, 235, 248–255. [Google Scholar] [CrossRef]
- Kang, B.; Ceder, G. Battery materials for ultrafast charging and discharging. Nature 2009, 458, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Gu, L.; Zhao, N.H.; Yin, Y.X.; Zhou, L.J.; Guo, Y.G.; Wan, L.J. Smaller sulfur molecules promise better lithium-sulfur batteries. J. Am. Chem. Soc. 2012, 134, 18510–18513. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Zeng, W.; Yin, Y.X.; Zhang, J.; Yang, C.P.; Zhu, Y.; Guo, Y.G. Hierarchically micro/mesoporous activated graphene with a large surface area for high sulfur loading in Li-S batteries. J. Mater. Chem. A 2015, 3, 4799–4802. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J. Li-O2 and Li-S Batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.R.; Wang, J.; Liang, X.; Liu, H.K.; Dou, S.X. A systematic approach to high and stable discharge capacity for scaling up the lithium-sulfur battery. J. Power Sources 2015, 279, 231–237. [Google Scholar] [CrossRef]
- Wang, J.; Lu, L.; Choucair, M.; Stride, J.A.; Xu, X.; Liu, H.J. Sulfur-graphene composite rechargeable lithium batteries. J. Power Sources 2011, 196, 7030–7034. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.S. Challenges and prospects of lithium-sulfur batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Kim, H.; Magasinski, A.; Lee, J.T.; Lin, H.; Yushin, G. Harnessing steric separation of freshly nucleated Li2S nanoparticles for bottom-up assembly of high-performance cathodes for lithium-sulfur and lithium-ion batteries. Adv. Energy Mater. 2014, 4, 1400196. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhao, Y.; Konarov, A.; Li, Z.; Chen, P. Effect of mesoporous carbon microtube prepared by carbonizing the poplar catkin on sulfur cathode performance in Li/S batteries. J. Alloys Compd. 2015, 619, 298–302. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Bakenov, Z.; Zhao, Y.; Konarov, A.; Doan, T.N.L.; Malik, M.; Paron, T.; Chen, P. One-step synthesis of branched sulfur/polypyrrole nanocomposite cathode for lithium rechargeable batteries. J. Power Sources 2012, 208, 1–8. [Google Scholar] [CrossRef]
- Konarov, A.; Gosselink, D.; Doan, T.N.L.; Zhang, Y.G.; Zhao, Y.; Chen, P. Simple, scalable, and economical preparation of sulfur-PAN composite cathodes for Li/S batteries. J. Power Sources 2014, 259, 183–187. [Google Scholar] [CrossRef]
- Kumar, P.; Wu, F.Y.; Hu, L.H.; Abbsa, S.A.; Ming, J.; Lin, C.N.; Fang, J.; Chu, C.W.; Li, L.J. High-performance graphene/sulphur electrodes for flexible Li-ion batteries using the low-temperature spraying method. Nanoscale 2015, 7, 8093–8100. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.C.; Li, W.F.; Zhao, W. High-rate, ultra long cycle-life lithium sulfur batteries enabled by nitrogen-doped grapheme. Nano Lett. 2014, 14, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yue, S.F.; Sui, Z.Y.; Zhang, X.T.; Hou, S.S.; Cao, G.P.; Yang, Y.S. What is the choice for supercapacitors: Grapheme or grapheme oxide? Energy Environ. Sci. 2011, 4, 2826–2830. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhao, Y.; Bakenov, Z. A simple approach to synthesize nano-sized sulfur/graphene oxide materials for high-performance lithium sulfur batteries. Ionics 2014, 20, 1047–1050. [Google Scholar] [CrossRef]
- Wu, F.; Chen, J.; Chen, R.; Wu, S.; Li, L.; Chen, S.; Zhao, T. Sulfur/polythiophene with a core/shell structure: Synthesis and electrochemical properties of the cathode for rechargeable lithium batteries. J. Phys. Chem. C 2011, 115, 6057–6063. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Shuai, X.; Mao, S.; Yang, H.; Qian, J.; Chen, J.; Yan, J.; Cen, K. Green preparation of reduced graphene oxide for sensing and energy storage applications. Sci. Rep. 2014, 4, 4684–4688. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Ambrosi, A.; Pumera, M. Graphene oxide reduction by standard industrial reducing agent: Thiourea dioxide. J. Mater. Chem. 2012, 22, 11054–11061. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koening, L. Raman spectrum of graphite. J. Phys. Chem. Lett. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of grapheme. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.S.; Jin, H.J. Sulfur-doped, reduced graphene oxide nanoribbons for sodium-ion batteries. Mater. Lett. 2017, 198, 106–109. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, L.; Glans, P.A.; Zhang, Y.; Zhu, J.; Guo, J. Electronic structure and chemical bonding of a graphene oxide-sulfur nanocomposite for use in superior performance lithium-sulfur cells. Phys. Chem. Chem. Phys. 2012, 14, 13670–13675. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Yin, Z.Y.; Zhang, H. Three-dimensional grapheme materials: Preparation, structures and application in supericapacitors. Energy. Environ. Sci. 2014, 7, 1850–1865. [Google Scholar] [CrossRef]
- Deng, Z.F.; Zhang, Z.A.; Lai, Y.Q.; Liu, J.; Li, J.; Liu, Y.X. Electrochemical impedance spectroscopy study of a lithium/sulfur battery: Modeling and analysis of capacity fading. J. Electrochem. Soc. 2013, 160, A553–A558. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhao, Y.; Konarov, A.; Gosselink, D.; Soboleski, H.G.; Chen, P. A novel sulfur/polypyrrole/multi-walled carbon nanotube nanocomposite cathode with core-shell tubular structure for lithium rechargeable batteries. Solid State Ionics 2013, 238, 30–35. [Google Scholar] [CrossRef]
- Canas, N.A.; Hirose, K.; Pascucci, B.; Wagner, N.; Friedrich, K.A.; Hiesgen, R. Investigation of lithium-sulfur batteries using electrochemical impedance spectroscopy. Electrochim. Acta 2013, 97, 42–51. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Zhang, F.; Huang, Y.; Chen, Y.S. Sulfur-infiltrated grapheme-based layered porous carbon cathodes for high-performance lithium-sulfur batteries. ACS Nano 2014, 8, 5208–5215. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Manthiram, A. Nano-cellular carbon current collectors with stable cyclability for Li-S batteries. J. Mater. Chem. 2013, 34, 9590–9596. [Google Scholar] [CrossRef]
- Chung, S.; Manthiram, A. Low-cost, porous carbon current collector with high sulfur loading for lithium-sulfur batteries. Electrochem. Commun. 2014, 38, 91–95. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid electrolyte lithium sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Zhang, S.S.; Tran, D.T. A proof-of-concept lithium sulfur liquid battery with exceptionally high capacity density. J. Power Sources 2012, 211, 169–172. [Google Scholar] [CrossRef]
- Oldham, K.B.; Myland, J.C. Fundamentals of Electrochemical Science; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Zhang, S.L.; Song, Y.H.; Si, D.Y. Performance of reduced graphene oxide-sulfur composite prepared by hydrothermal method. Battery Bimon. 2016, 46, 129–132. [Google Scholar]
- Wang, N.; Xu, H.X.; Wan, Y.H.; Liu, X.M.; Yang, H.; Shen, X.D. Synthesis and characterization of H-GO/S composites as cathode materials for lithium sulfur batteries. J. NanJing Tech Univ. 2016, 38, 17–21. [Google Scholar]
- Liu, F.Y.; Liang, J.Y.; Zhang, C.; Yu, L.; Zhao, J.X.; Liu, C.; Lan, Q.; Chen, S.R.; Cao, Y.C.; Zheng, G. Reduced graphene oxide encapsulated sulfur spheres for the lithium sulfur battery cathode. Results Phys. 2017, 7, 250–255. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, H.; Zhang, Y.; Xiang, M.W.; Zhao, G.; Liu, H.; Zhang, Y. Vesicle-like sulfur/reduced graphene oxide composites for high lithium-sulfur batteries. J. Alloys Compd. 2017, 724, 1007–1013. [Google Scholar] [CrossRef]
- Li, C.; Sui, X.L.; Wang, Z.B.; Wang, Q.; Gu, D.M. 3D N-doped graphene nanomesh foam for long cycle life lithium-sulfur battery. Chem. Eng. J. 2017, 326, 265–272. [Google Scholar] [CrossRef]
- Xu, G.Y.; Yuan, J.R.; Geng, X.M.; Dou, H.; Chen, L.; Yan, X.H.; Zhu, H.L. Caterpillar-like graphene confining sulfur by restacking effect for high performance lithium sulfur batteries. Chem. Eng. J. 2017, 322, 454–462. [Google Scholar] [CrossRef]




| Cathode | Cycle Number | Capacity Remaining (mAh g−1) | Current Density | Reference |
|---|---|---|---|---|
| S/rGO | 20 | 592.4 | 0.2 mA cm−2 | [38] |
| S/GO | 160 | 620 | 0.1 C | [39] |
| S/rGO | 100 | 338 | 0.2 C | [40] |
| S/rGO | 150 | 600 | 2 C | [41] |
| S/3DNG | 500 | 578 | 0.5 C | [42] |
| S/G | 200 | 363 | 1 C | [43] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Sun, Z.; Zhang, Y.; Wang, X.; Bakenov, Z.; Yin, F. Micro-Spherical Sulfur/Graphene Oxide Composite via Spray Drying for High Performance Lithium Sulfur Batteries. Nanomaterials 2018, 8, 50. https://doi.org/10.3390/nano8010050
Tian Y, Sun Z, Zhang Y, Wang X, Bakenov Z, Yin F. Micro-Spherical Sulfur/Graphene Oxide Composite via Spray Drying for High Performance Lithium Sulfur Batteries. Nanomaterials. 2018; 8(1):50. https://doi.org/10.3390/nano8010050
Chicago/Turabian StyleTian, Yuan, Zhenghao Sun, Yongguang Zhang, Xin Wang, Zhumabay Bakenov, and Fuxing Yin. 2018. "Micro-Spherical Sulfur/Graphene Oxide Composite via Spray Drying for High Performance Lithium Sulfur Batteries" Nanomaterials 8, no. 1: 50. https://doi.org/10.3390/nano8010050





